Post-Concussion Syndrome and Functional Neurological Disorder: Diagnostic Interfaces, Risk Mechanisms, and the Functional Overlay Model
Abstract
1. Introduction
2. Diagnostic Criteria: PCS vs. FND and Functional Cognitive Disorder
2.1. Post-Concussion Syndrome (PCS)
2.2. Functional Neurological Disorder (FND)
- One or more symptoms of altered voluntary motor or sensory function;
- Clinical evidence showing incompatibility between symptoms and recognized neurological disease;
- Symptoms not better explained by another medical or mental disorder;
- Symptoms causing significant distress or impairment.
2.3. Functional Cognitive Disorder (FCD)
- •
- Performance variability: Good real-world functioning but poor test consistency;
- •
- Metacognitive distortion: Over-focusing on minor lapses;
- •
- Symptom improvement with distraction: e.g., fluency on testing improves when anxiety is redirected.
2.4. Cognitive Profiles: FCD vs. PCS
2.5. Comparison and Clinical Implications
3. Personality Traits and Risk Factors
3.1. Post-Concussion Syndrome
3.2. Personality Traits and Psychological Profiles in Functional Neurological Disorder (FND) and Functional Cognitive Disorder (FCD)
3.3. Risk Factors in Functional Neurological Disorder and Post-Concussion Syndrome: A Comparative Perspective
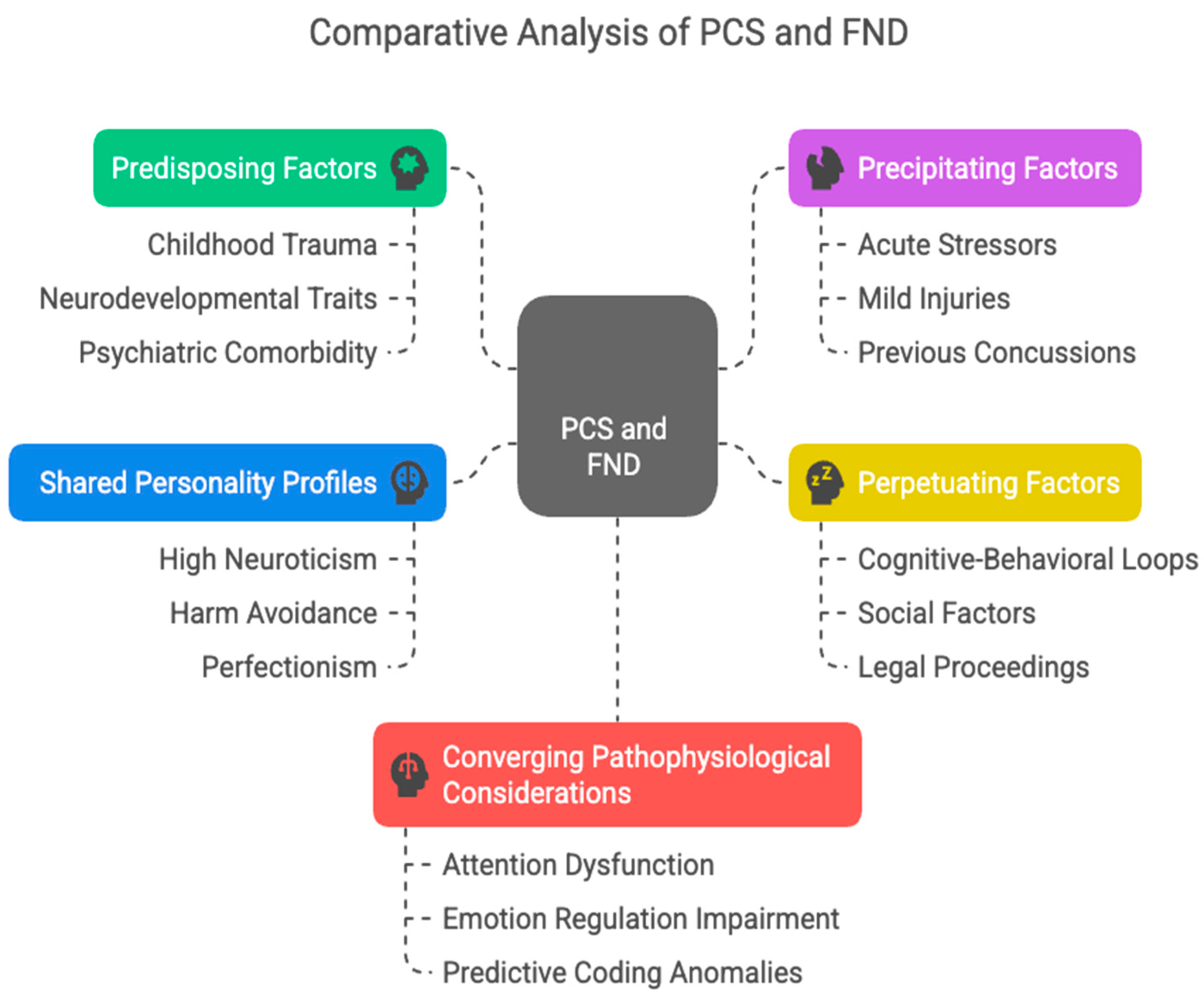
4. Predisposing, Precipitating and Perpetuating Factors
4.1. Predisposing Factors
4.2. Precipitating Factors
4.3. Perpetuating Factors
4.4. Shared Personality Profiles
4.5. Converging Pathophysiological Considerations
5. Neuroimaging in Post-Concussion Syndrome and Functional Neurological Disorder
5.1. Post-Concussion Syndrome
5.2. FND: Disrupted Networks and Functional Correlates
6. Biomarkers and Diagnostic Differentiation in PCS and FND
7. Discussion
Author Contributions
Funding
Conflicts of Interest
Glossary
| PCS | Post-Concussion Syndrome |
| FND | Functional Neurological Disorder |
| FCD | Functional Cognitive Disorder |
| TBI | Traumatic Brain Injury |
| GFAP | Glial Fibrillary Acidic Protein |
| NF-L | Neurofilament Light Chain |
References
- Bazarian, J.J.; Wong, T.; Harris, M.; Leahey, N.; Mookerjee, S.; Dombovy, M. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj. 1999, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Strauss, I.; Savitsky, N. Head injury, Neurologic and psychiatric aspects. Arch. Neurol. Psychiatry 1934, 31, 893. [Google Scholar] [CrossRef]
- Evans, R.W. Persistent post-traumatic headache, postconcussion syndrome, and whiplash injuries, the evidence for a non-traumatic basis with an historical review. Headache 2010, 50, 716. [Google Scholar] [CrossRef] [PubMed]
- Iverson, G.L.; Karr, J.E.; Gardner, A.J.; Silverberg, N.D.; Terry, D.P. Results of scoping review do not support mild traumatic brain injury being associated with a high incidence of chronic cognitive impairment: Commentary on McInnes et al. 2017. PLoS ONE 2019, 14, e0218997. [Google Scholar] [CrossRef]
- Dwyer, B.; Katz, D.I. Postconcussion syndrome. Handb. Clin. Neurol. 2018, 158, 163. [Google Scholar] [CrossRef]
- Haas, D.C. Chronic post-traumatic headaches classified and compared with natural headaches. Cephalalgia 1996, 16, 486. [Google Scholar] [CrossRef]
- McCauley, S.R.; Boake, C.; Pedroza, C.; Brown, S.A.; Levin, H.S.; Goodman, H.S.; Merritt, S.G. Postconcussional disorder, Are the DSM-IV criteria an improvement over the ICD-10? J. Nerv. Ment. Dis. 2005, 193, 540. [Google Scholar] [CrossRef]
- de Kruijk, J.R.; Leffers, P.; Meerhoff, S.; Rutten, J.; Twijnstra, A. Effectiveness of bed rest after mild traumatic brain injury, a randomised trial of no versus six days of bed rest. J. Neurol. Neurosurg. Psychiatry 2002, 73, 167. [Google Scholar] [CrossRef]
- Hughes, D.G.; Jackson, A.; Mason, D.L.; Berry, E.; Hollis, S.; Yates, D.W. Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury, correlation with neuropsychological tests and delayed recovery. Neuroradiology 2004, 46, 550. [Google Scholar] [CrossRef]
- McCauley, S.R.; Boake, C.; Levin, H.S.; Contant, C.F.; Song, J.X. Postconcussional disorder following mild to moderate traumatic brain injury, anxiety, depression, and social support as risk factors and comorbidities. J. Clin. Exp. Neuropsychol. 2001, 23, 792–808. [Google Scholar] [CrossRef]
- Eisenberg, M.A.; Andrea, J.; Meehan, W.; Mannix, R. Time interval between concussions and symptom duration. Pediatrics 2013, 132, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, S.A.; Diamond, C.; Carson, A.; Stone, J. Incidence and prevalence of functional neurological disorder: A systematic review. J. Neurol. Neurosurg. Psychiatry. 2025, 96, 383–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villagrán, A.; Eldøen, G.; Duncan, R.; Aaberg, K.M.; Hofoss, D.; Lossius, M.I. Incidence and prevalence of psychogenic nonepileptic seizures in a Norwegian county: A 10-year population-based study. Epilepsia 2021, 62, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Razvi, S.; Mulhern, S. Newly presenting psychogenic nonepileptic seizures: Incidence, population characteristics, and early outcome from a prospective audit of a first seizure clinic. Epilepsy Behav. 2011, 20, 308–311. [Google Scholar] [CrossRef]
- O’Sullivan, S.S.; Spillane, J.E.; McMahon, E.M.; Sweeney, B.J.; Galvin, R.J.; McNamara, B.; Cassidy, E.M. Clinical characteristics and outcome of patients diagnosed with psychogenic nonepileptic seizures: A 5-year review. Epilepsy Behav. 2007, 11, 77–84. [Google Scholar] [CrossRef]
- Kotsopoulos, I.; de Krom, M.; Kessels, F.; Lodder, J.; Troost, J.; Twellaar, M.; van Merode, T.; Knottnerus, A. Incidence of epilepsy and predictive factors of epileptic and non-epileptic seizures. Seizure 2005, 14, 175–182. [Google Scholar] [CrossRef]
- Sigurdardottir, K.R.; Olafsson, E. Incidence of psychogenic seizures in adults: A population-based study in Iceland. Epilepsia 1998, 39, 749–752. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Ficker, D.M.; Cahill, W.T.; Privitera, M.D. Four-year incidence of psychogenic nonepileptic seizures in adults in Hamilton County, OH. Neurology 2000, 55, 1561–1563. [Google Scholar] [CrossRef]
- Forsgren, L. Prospective incidence study and clinical characterization of seizures in newly referred adults. Epilepsia 1990, 31, 292–301. [Google Scholar] [CrossRef]
- Hansen, A.S.; Rask, C.U.; Rodrigo-Domingo, M.; Pristed, S.G.; Christensen, J.; Nielsen, R.E. Incidence rates and characteristics of pediatric onset psychogenic nonepileptic seizures. Pediatr. Res. 2020, 88, 796–803. [Google Scholar] [CrossRef]
- Lehn, A.; Watson, E.; Ryan, E.G.; Jones, M.; Cheah, V.; Dionisio, S. Psychogenic nonepileptic seizures treated as epileptic seizures in the emergency department. Epilepsia 2021, 62, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Carson, A.; Duncan, R.; Roberts, R.; Warlow, C.; Hibberd, C.; Coleman, R.; Cull, R.; Murray, G.; Pelosi, A.; et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin. Neurol. Neurosurg. 2010, 112, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Carson, A.; Stone, J.; Hibberd, C.; Murray, G.; Duncan, R.; Coleman, R.; Warlow, C.; Roberts, R.; Pelosi, A.; Cavanagh, J.; et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J. Neurol. Neurosurg. Psychiatry 2011, 82, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Gelauff, J.; Stone, J.; Edwards, M.; Carson, A. The prognosis of functional (psychogenic) motor symptoms, a systematic review. J. Neurol. Neurosurg. Psychiatry 2014, 85, 220–226. [Google Scholar] [CrossRef]
- Durrant, J.; Rickards, H.; Cavanna, A.E. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res. Treat. 2011, 2011, 274736. [Google Scholar] [CrossRef]
- Stone, J. The bare essentials, Functional symptoms in neurology. Pract. Neurol. 2009, 9, 179. [Google Scholar] [CrossRef]
- Rosebush, P.I.; Mazurek, M.F. Treatment of conversion disorder in the 21st century, have we moved beyond the couch? Curr. Treat. Options Neurol. 2011, 13, 255. [Google Scholar] [CrossRef]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics, 11th ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Mavroudis, I.; Chatzikonstantinou, S.; Petridis, F.; Palade, O.D.; Ciobica, A.; Balmus, I.M. Functional Overlay Model of Persistent Post-Concussion Syndrome. Brain Sci. 2023, 13, 1028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atif, H.; Morgan, B.; Tuohy, K.; Zukowski, M.; Foster, Z.; Loeffert, A.; Yeates, K.O.; Hicks, S.D. Personality Traits and Social Supports in Adolescents With Persistent Postconcussion Symptoms. J. Head. Trauma. Rehabil. 2022, 37, E71–E79. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.L.; Bigler, E.D.; Tate, R.L. Personality traits and cognitive complaints in post-concussion syndrome. Arch. Clin. Neuropsychol. 2019, 34, 865–878. [Google Scholar]
- Mavroudis, I.; Balmus, I.M.; Ciobica, A.; Hogas, M. A narrative review of risk factors and predictors for poor outcome and prolonged recovery after a mild traumatic brain injury. Int. J. Neurosci. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- King, N.S.; Pernet, C.; Carson, A.; Chamberlain, S.R.; Stamatakis, E.A. Recovery trajectories of patients with mild traumatic brain injury: A prospective cohort study. J. Neurotrauma 2025, 42, 45–58. [Google Scholar]
- Mao, L.; Dumkrieger, G.; Ku, D.; Ross, K.; Berisha, V.; Schwedt, T.J.; Li, J.; Chong, C.D. Developing multivariable models for predicting headache improvement in patients with acute post-traumatic headache attributed to mild traumatic brain injury: A preliminary study. Headache 2023, 63, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Wijenberg, M.; Rauwenhoff, J.; Stapert, S.; Verbunt, J.; van Heugten, C. Do fear and catastrophizing about mental activities relate to fear-avoidance behavior in a community sample? An experimental study. J. Clin. Exp. Neuropsychol. 2021, 43, 66–77. [Google Scholar] [CrossRef]
- Stone, J.; Warlow, C.; Deary, I.J.; Sharpe, M. Predisposing risk factors for functional limb weakness: A case–control study. J. Neurol. Neurosurg. Psychiatry 2020, 32, 50–57. [Google Scholar] [CrossRef]
- De Vroege, L.; Kop, W.J.; van der Feltz-Cornelis, C.M. Personality traits related to cognitive functioning in patients with functional neurological disorder. J. Clin. Exp. Neuropsychol. 2023, 45, 1014–1023. [Google Scholar] [CrossRef]
- Pun, A.L.; Martin, D.M.; McKenzie, J.M.; Anderson, N.E. Psychiatric profiles and trauma exposure in a Functional Neurological Disorder cohort from a multidisciplinary clinic in Australia. Front. Neurol. 2020, 11, 580267. [Google Scholar] [CrossRef]
- Edwards, M.J.; Adams, R.A.; Brown, H.; Pareés, I.; Friston, K.J. A Bayesian account of ‘hysteria’. Brain 2012, 135, 3495–3512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manley, G.; Gardner, A.J.; Schneider, K.J.; Guskiewicz, K.M.; Bailes, J.; Cantu, R.C.; Castellani, R.J.; Turner, M.; Jordan, B.D.; Randolph, C.; et al. A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 2017, 51, 969–977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haider, M.N.; Bezherano, I.; Wertheimer, A.; Siddiqui, A.H.; Horn, E.C.; Willer, B.S.; Leddy, J.J. Exercise for Sport-Related Concussion and Persistent Postconcussive Symptoms. Sports Health. 2021, 13, 154–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sidenius, U.; Corazon, S.S.; Varning Poulsen, D.; Jul Olsen, L.; Kæreby, N. The experiences and perspectives of participating in a nature integrative rehabilitation programme when suffering from post-concussion syndrome: Responses, gains, and impact from using enriched nature environments as a rehabilitation setting and integrating nature as rehabilitation means. Int. J. Qual. Stud. Health Well-Being 2025, 20, 2503604. [Google Scholar] [CrossRef] [PubMed]
- Echlin, H.V.; Rahimi, A.; Wojtowicz, M. Systematic Review of the Long-Term Neuroimaging Correlates of Mild Traumatic Brain Injury and Repetitive Head Injuries. Front. Neurol. 2021, 12, 726425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mavroudis, I.; Kazis, D.; Petridis, F.E.; Balmus, I.M.; Ciobica, A. The Use of Magnetoencephalography in the Diagnosis and Monitoring of Mild Traumatic Brain Injuries and Post-Concussion Syndrome. Brain Sci. 2025, 15, 154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vasilevskaya, A.; Anastassiadis, C.; Thapa, S.; Taghdiri, F.; Khodadadi, M.; Multani, N.; Rusjan, P.; Ozzoude, M.; Tarazi, A.; Mushtaque, A.; et al. 18F-Flortaucipir (AV1451) imaging identifies grey matter atrophy in retired athletes. J. Neurol. 2024, 271, 6068–6079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, Y.; Protas, H.; Luo, J.; Chen, K.; Alosco, M.L.; Adler, C.H.; Balcer, L.J.; Bernick, C.; Au, R.; Banks, S.J.; et al. DIAGNOSE CTE Research Project Investigators. Flortaucipir tau PET findings from former professional and college American football players in the DIAGNOSE CTE research project. Alzheimers Dement. 2024, 20, 1827–1838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirov, I.I.; Whitlow, C.T.; Zamora, C. Susceptibility-Weighted Imaging and Magnetic Resonance Spectroscopy in Concussion. Neuroimaging Clin. N. Am. 2018, 28, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.L.; Nicholson, T.R.; Asadi-Pooya, A.A.; Bègue, I.; Butler, M.; Carson, A.J.; David, A.S.; Deeley, Q.; Diez, I.; Edwards, M.J.; et al. Neuroimaging in Functional Neurological Disorder: State of the Field and Research Agenda. Neuroimage Clin. 2021, 30, 102623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rai, S.; Foster, S.; Griffiths, K.R.; Breukelaar, I.A.; Kozlowska, K.; Korgaonkar, M.S. Altered resting-state neural networks in children and adolescents with functional neurological disorder. Neuroimage Clin. 2022, 35, 103110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weber, S.; Heim, S.; Richiardi, J.; Van De Ville, D.; Serranová, T.; Jech, R.; Marapin, R.S.; Tijssen, M.A.J.; Aybek, S. Multi-centre classification of functional neurological disorders based on resting-state functional connectivity. Neuroimage Clin. 2022, 35, 103090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mavroudis, I.; Petridis, F.; Kazis, D.; Ciobica, A.; Dăscălescu, G.; Petroaie, A.D.; Dobrin, I.; Novac, O.; Vata, I.; Novac, B. The Diagnostic and Prognostic Role of Biomarkers in Mild Traumatic Brain Injury: An Umbrella Meta-Analysis. Brain Sci. 2025, 15, 581. [Google Scholar] [CrossRef]
- Weber, S.; Bühler, J.; Messmer, F.; Bruckmaier, R.; Aybek, S. Cortisol in functional neurological disorders: State, trait and prognostic biomarkers. J. Psychosom. Res. 2024, 179, 111615. [Google Scholar] [CrossRef] [PubMed]
| Domain | PCS | FND/FCD |
|---|---|---|
| Etiology | Biomechanical Brain Trauma (e.g., mTBI) | Functional Brain Network Dysfunction |
| Symptoms | Headache, dizziness, fatigue, cognitive complaints, mood changes | Motor/sensory symptoms, PNES, cognitive complaints, dissociation |
| Objective Findings | Often normal imaging; subtle DTI/fMRI anomalies in some cases | Typically normal imaging; positive functional signs on exam |
| Diagnostic Criteria | Based on symptom constellation post-mTBI; ICD-10/DSM-IV (historical) | Positive signs (DSM-5/ICD-11); symptom incongruence with disease patterns |
| Neuropsychological Profile | May show subtle deficits; often normal in chronic phase | Discrepancy between complaints and test performance; variable consistency |
| Biomarkers | GFAP, NF-L (acute phase); exploratory use of miRNAs, inflammatory markers | No established biomarkers; cortisol explored as a state/trait indicator |
| Risk Factor | PCS | FND/FCD | Shared? |
|---|---|---|---|
| Female sex Prior psychiatric illness Personality traits | High prevalence | High prevalence | ✔ |
| Depression, anxiety | Depression, anxiety, PTSD | ✔ | |
| High neuroticism, somatic anxiety | Neuroticism, perfectionism, alexithymia | ✔ | |
| Trauma history Multiple prior concussions | Psychological and physical trauma | Early life adversity, abuse | ✔ |
| Associated with chronic symptoms | Less directly implicated | Unclear; under-investigated | |
| Positive clinical signs Diagnostic delay Neuroimaging changes Risk Factor | Typically absent | Hoover’s sign, distractibility, variability | ✖ |
| Often overlooked in chronic phase | Frequently misdiagnosed | ✔ | |
| White matter disruption, network dysfunction | Network dysfunction in emotion/agency networks | ✔ | |
| PCS | FND/FCD | Shared? | |
| Female sex Prior psychiatric illness | High prevalence | High prevalence | ✔ |
| Depression, anxiety | Depression, anxiety, PTSD | ✔ |
| Biomarker | Acute PCS | Chronic PCS | FND | Notes |
|---|---|---|---|---|
| GFAP | +(within 24 h) | ±(weeks to months) | - | May persist in some chronic cases |
| NF-L | +(24–72 h) | ± | - | Elevated in axonal injury; low specificity for symptoms |
| Cortisol | ± | ± | ± | May reflect stress/reactivity in both conditions |
| MicroRNAs | ± | Research-phase | Unknown | Promising direction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroudis, I.; Petridis, F.; Karantali, E.; Ciobica, A.; Papagiannopoulos, S.; Kazis, D. Post-Concussion Syndrome and Functional Neurological Disorder: Diagnostic Interfaces, Risk Mechanisms, and the Functional Overlay Model. Brain Sci. 2025, 15, 755. https://doi.org/10.3390/brainsci15070755
Mavroudis I, Petridis F, Karantali E, Ciobica A, Papagiannopoulos S, Kazis D. Post-Concussion Syndrome and Functional Neurological Disorder: Diagnostic Interfaces, Risk Mechanisms, and the Functional Overlay Model. Brain Sciences. 2025; 15(7):755. https://doi.org/10.3390/brainsci15070755
Chicago/Turabian StyleMavroudis, Ioannis, Foivos Petridis, Eleni Karantali, Alin Ciobica, Sotirios Papagiannopoulos, and Dimitrios Kazis. 2025. "Post-Concussion Syndrome and Functional Neurological Disorder: Diagnostic Interfaces, Risk Mechanisms, and the Functional Overlay Model" Brain Sciences 15, no. 7: 755. https://doi.org/10.3390/brainsci15070755
APA StyleMavroudis, I., Petridis, F., Karantali, E., Ciobica, A., Papagiannopoulos, S., & Kazis, D. (2025). Post-Concussion Syndrome and Functional Neurological Disorder: Diagnostic Interfaces, Risk Mechanisms, and the Functional Overlay Model. Brain Sciences, 15(7), 755. https://doi.org/10.3390/brainsci15070755





