Diagnostic, Therapeutic, and Prognostic Applications of Artificial Intelligence (AI) in the Clinical Management of Brain Metastases (BMs)
Abstract
1. Introduction
1.1. Overview of Brain Metastases (BMs)
1.2. Challenges in BM Diagnosis and Treatment
1.3. The Role of Artificial Intelligence (AI) in Neuro-Oncology
1.4. Purpose and Scope of This Review
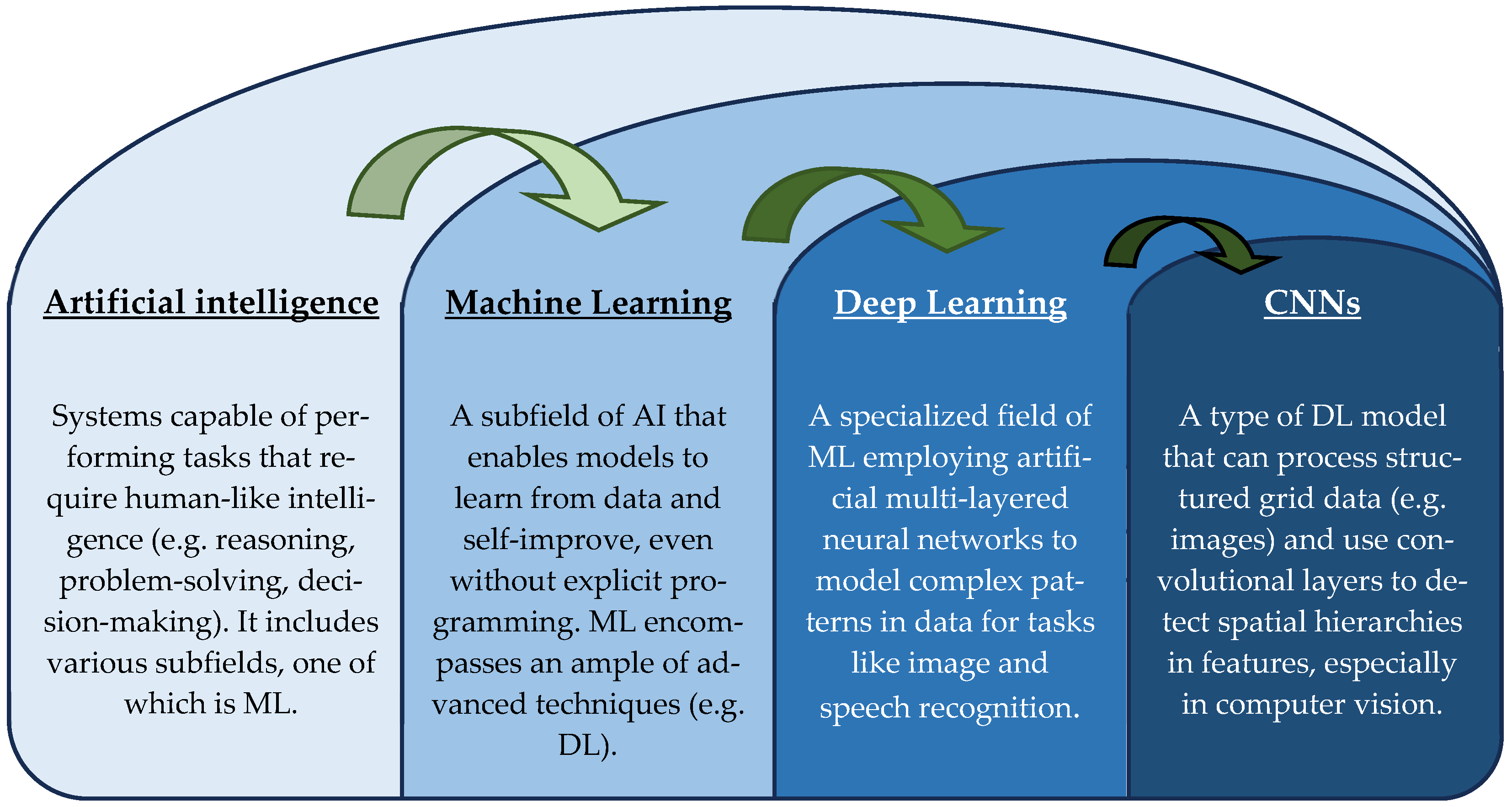
2. Fundamentals of AI in Oncology and Neuro-Oncology
2.1. Overview of AI Technologies
2.2. Radiomics and Radiogenomics
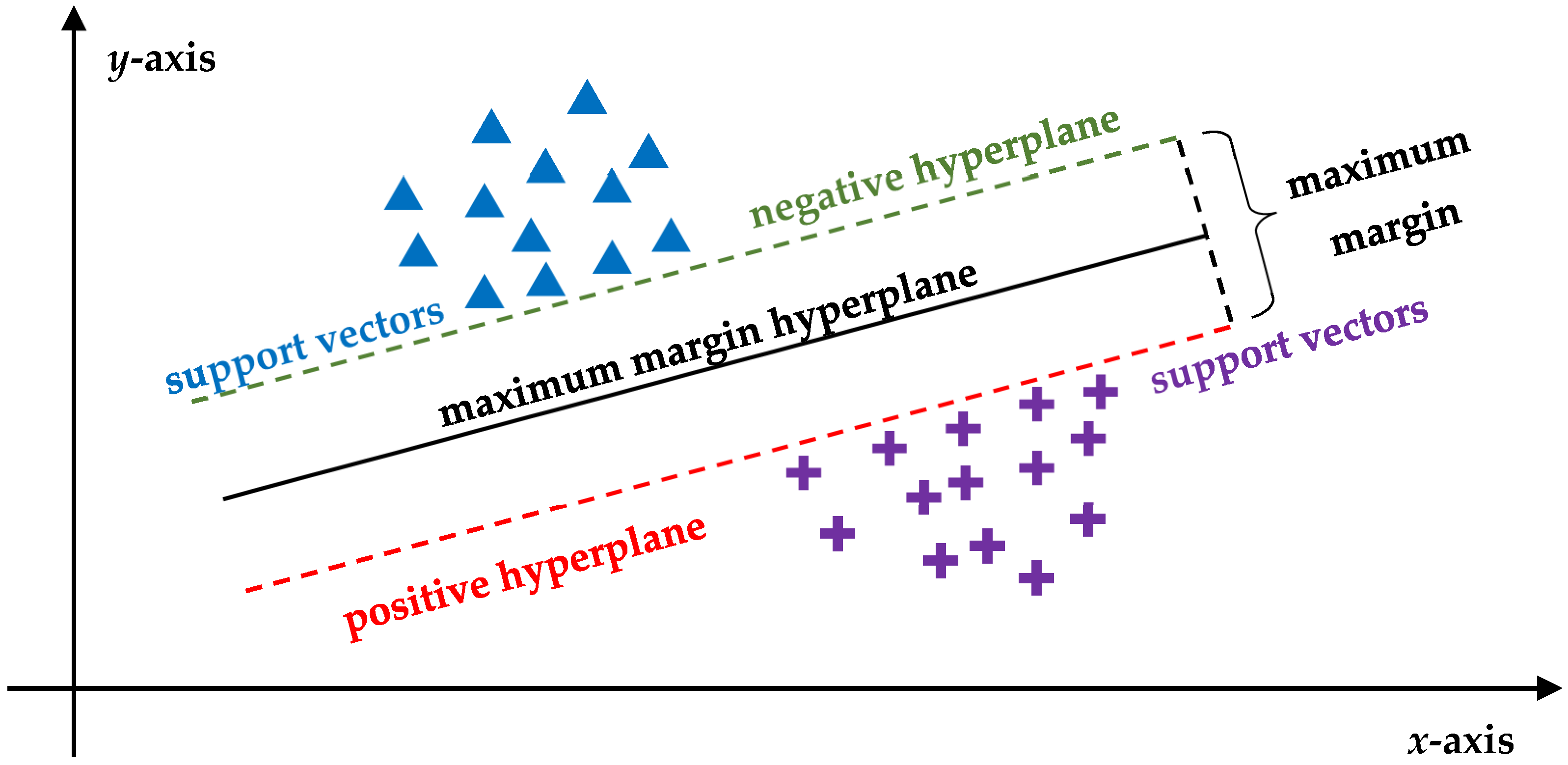
2.3. AI Algorithms in Oncology
3. AI Applications in BM Diagnosis
3.1. Lesion Detection and Diagnostic Imaging Segmentation
3.2. Differential Diagnosis of Brain Metastases
3.3. Non-Invasive Molecular Characterization
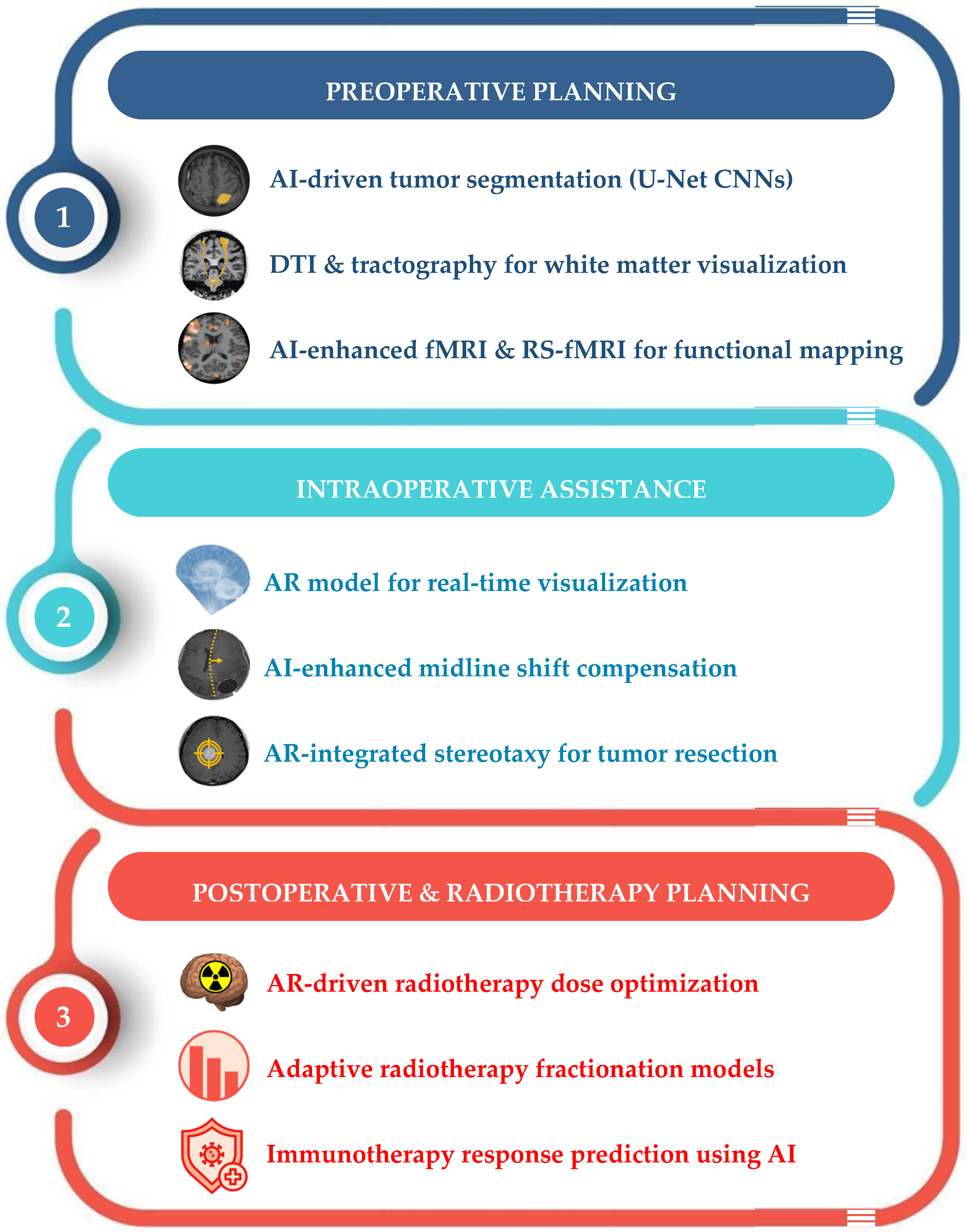
4. AI-Assisted Therapeutic Planning
4.1. Surgical Planning and Intraoperative Assistance
4.2. Radiotherapy Planning Optimization
4.3. AI-Driven Approaches to Systemic Therapy in BMs: Integrating Immunotherapy and Targeted Treatment
4.4. Emerging Approaches in Immunotherapy for BMs
5. AI in BM Prognostic Assessment
5.1. Survival and Recurrence Prediction
5.2. Disease Progression Monitoring
5.3. Longitudinal Follow-Up Strategies
6. Challenges and Limitations of AI in BM Management
6.1. Data Standardization and Availability
6.2. Interpretability and the ‘Black Box’ Issue
6.3. Ethical and Legal Considerations
7. Future Directions and Innovations in AI for BMs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Apparent diffusion coefficient |
| AI | Artificial intelligence |
| AKT | Protein kinase B |
| ALK | Anaplastic lymphoma kinase |
| ALL-IDB | Acute Lymphoblastic Leukemia Image Database |
| ANN | Artificial neural network |
| AR | Augmented reality |
| AREs | Adverse radiation effects |
| AUC | Area under the curve |
| BBB | Blood–brain barrier |
| BM | Brain metastasis |
| BOLD | Blood oxygen level-dependent |
| BraTS-METS | Brain Tumor Segmentation—Metastases |
| CBCT | Cone beam CT |
| CBF | Cerebral blood flow |
| CET | Contrast-enhancing tumor |
| CMRO2 | Cerebral metabolic rate of oxygen |
| CNNs | Convolutional neural networks |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| ctDNA | Circulating tumor DNA |
| DDSM | Digital Database for Screening Mammography |
| DL | Deep learning |
| DMN | Default mode network |
| DNNs | Deep neural networks |
| DSC | Dynamic susceptibility contrast |
| DTI | Diffusion tensor imaging |
| DWI | Diffusion-weighted imaging |
| EGFR | Epidermal growth factor receptor |
| ESP-Unet | Edge Strengthening Parallel Unet |
| FDA | Food and Drug Administration |
| FET | O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET) |
| FLAIR | Fluid-attenuated inversion recovery |
| fMRI | Functional MRI |
| FRT | Fractionated radiotherapy |
| GAN | Generative adversarial networks |
| GPA | Graded Prognostic Assessment |
| HER2 | Human epidermal growth factor receptor 2 |
| HMD | Head-mounted displays |
| HUD | Head-up displays |
| IBM | International Business Machines Corporation |
| ICI | Immune checkpoint inhibitor |
| IQ-OTH | Iraq Oncology Teaching Hospital |
| MHC | Major histocompatibility complex |
| mitPO2 | Tissue oxygen saturation |
| ML | Machine learning |
| MMPs | Matrix metalloproteinases |
| MRI | Magnetic resonance imaging |
| mTOR | Mammalian target of rapamycin |
| NCCD | National Center for Cancer Diseases |
| NET2 | Non-enhancing T2 hyperintense region |
| NLP | Natural language processing |
| NSCLC | Non-small cell lung cancer |
| NYU | New York University |
| NYUMets | NYU Langone Health database |
| OAR | Organ at risk |
| OCT | Optical coherence tomography |
| OEF | Oxygen extraction fraction |
| PCNSL | Primary central nervous system lymphoma |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death ligand 1 |
| PFS | Progression-free survival |
| PI3K | Phosphoinositide 3-kinases |
| PsP | Pseudoprogression |
| PTV | Planning target volume |
| qBOLD | Quantitative blood oxygen level-dependent |
| QSM | Quantitative susceptibility mapping |
| rCBV | Relative cerebral blood volume |
| RDD | Research Data Deposit |
| RPA | Recursive partitioning analysis |
| RSD | Relative standard deviation |
| RS-fMRI | Resting-state fMRI |
| SIR | Score Index for Radiosurgery |
| SRS | Stereotactic radiosurgery |
| SVM | Support vector machines |
| TIL | Tumor-infiltrating lymphocytes |
| TKI | Tyrosine kinase inhibitor |
| TMB | Tumor mutational burden |
| TME | Tumor immune microenvironment |
| TP | True progression |
| UCSF-BMSR | University of California San Francisco Brain Metastases Stereotactic Radiosurgery |
| VEGF | Vascular endothelial growth factor |
| XAI | Explainable AI |
References
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Spallanzani, A.; Fontana, A.; Depenni, R.; Luppi, G. Brain metastases: An overview. CNS Oncol. 2015, 4, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Dhermain, F.; Noël, G.; Antoni, D.; Tallet, A. Role of radiation therapy in brain metastases management. Cancer Radiother. 2020, 24, 463–469. [Google Scholar] [CrossRef]
- Neman, J. Brain metastasis: The next steps for translational research. Cancer Rep. 2022, 5, e1623. [Google Scholar] [CrossRef]
- Izadi, N.; Solár, P.; Hašanová, K.; Zamani, A.; Akbar, M.S.; Mrázová, K.; Bartošík, M.; Kazda, T.; Hrstka, R.; Joukal, M. Breaking boundaries: Role of the brain barriers in metastatic process. Fluids Barriers CNS 2025, 22, 3. [Google Scholar] [CrossRef]
- Tobar, L.E.; Farnsworth, R.H.; Stacker, S.A. Brain Vascular Microenvironments in Cancer Metastasis. Biomolecules 2022, 12, 401. [Google Scholar] [CrossRef]
- Halstead, M.R.; Geocadin, R.G. The Medical Management of Cerebral Edema: Past, Present, and Future Therapies. Neurotherapeutics 2019, 16, 1133–1148. [Google Scholar] [CrossRef]
- Stokum, J.A.; Kurland, D.B.; Gerzanich, V.; Simard, J.M. Mechanisms of astrocyte-mediated cerebral edema. Neurochem. Res. 2015, 40, 317–328. [Google Scholar] [CrossRef]
- Ding, Y.; Xing, Z.; Liu, B.; Lin, X.; Cao, D. Differentiation of primary central nervous system lymphoma from high-grade glioma and brain metastases using susceptibility-weighted imaging. Brain Behav. 2014, 4, 841–849. [Google Scholar] [CrossRef]
- Fordham, A.J.; Hacherl, C.C.; Patel, N.; Jones, K.; Myers, B.; Abraham, M.; Gendreau, J. Differentiating Glioblastomas from Solitary Brain Metastases: An Update on the Current Literature of Advanced Imaging Modalities. Cancers 2021, 13, 2960. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.Y.; Sun, Y.F.; Yin, X.P.; Zhang, Y.; Xing, L.H.; Ma, Z.P.; Xue, L.Y.; Wang, J.N. Using machine learning-based radiomics to differentiate between glioma and solitary brain metastasis from lung cancer and its subtypes. Discov. Oncol. 2023, 14, 224. [Google Scholar] [CrossRef]
- Li, T.; Sun, S.; Li, Y.; Zhang, Y.; Wei, L. Immunotherapy revolutionizing brain metastatic cancer treatment: Personalized strategies for transformative outcomes. Front. Immunol. 2024, 15, 1418580. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Górska, Z.; Duchnowska, R.; Jassem, J. Molecular Profiles of Brain Metastases: A Focus on Heterogeneity. Cancers 2021, 13, 2645. [Google Scholar] [CrossRef]
- Youssef, A.; Sahgal, A.; Das, S. Radioresistance and brain metastases: A review of the literature and applied perspective. Front. Oncol. 2024, 14, 1477448. [Google Scholar] [CrossRef]
- Onofrio, L.; Gaeta, A.; D’Ecclesiis, O.; Cugliari, G.; Gandini, S.; Queirolo, P. Melanoma Brain Metastases: Immunotherapy or Targeted Therapy? A Systematic Review and Meta-Analyses. Appl. Sci. 2024, 14, 2222. [Google Scholar] [CrossRef]
- Kim, M.; Kizilbash, S.H.; Laramy, J.K.; Gampa, G.; Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Barriers to Effective Drug Treatment for Brain Metastases: A Multifactorial Problem in the Delivery of Precision Medicine. Pharm. Res. 2018, 35, 177. [Google Scholar] [CrossRef] [PubMed]
- Dumachi, A.I.; Buiu, C. Applications of Machine Learning in Cancer Imaging: A Review of Diagnostic Methods for Six Major Cancer Types. Electronics 2024, 13, 4697. [Google Scholar] [CrossRef]
- Bhinder, B.; Gilvary, C.; Madhukar, N.S.; Elemento, O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021, 11, 900–915. [Google Scholar] [CrossRef]
- Samani, Z.R.; Parker, D.; Akbari, H.; Wolf, R.L.; Brem, S.; Bakas, S.; Verma, R. Artificial intelligence-based locoregional markers of brain peritumoral microenvironment. Sci. Rep. 2023, 13, 963. [Google Scholar]
- Wu, J.; Mayer, A.T.; Li, R. Integrated imaging and molecular analysis to decipher tumor microenvironment in the era of immunotherapy. Semin. Cancer Biol. 2022, 84, 310–328. [Google Scholar] [CrossRef] [PubMed]
- Obuchowicz, R.; Lasek, J.; Wodziński, M.; Piórkowski, A.; Strzelecki, M.; Nurzynska, K. Artificial Intelligence-Empowered Radiology—Current Status and Critical Review. Diagnostics 2025, 15, 282. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, Y.; Wang, Y.; Liu, W.; Jia, W.; Wang, J. MR-based radiomics predictive modelling of EGFR mutation and HER2 overexpression in metastatic brain adenocarcinoma: A two-centre study. Cancer Imaging 2024, 24, 65. [Google Scholar] [CrossRef]
- Dixon, D.; Sattar, H.; Moros, N.; Kesireddy, S.R.; Ahsan, H.; Lakkimsetti, M.; Fatima, M.; Doshi, D.; Sadhu, K.; Junaid Hassan, M. Unveiling the Influence of AI Predictive Analytics on Patient Outcomes: A Comprehensive Narrative Review. Cureus 2024, 16, e59954. [Google Scholar] [CrossRef]
- Kufel, J.; Bargieł-Łączek, K.; Kocot, S.; Koźlik, M.; Bartnikowska, W.; Janik, M.; Czogalik, Ł.; Dudek, P.; Magiera, M.; Lis, A.; et al. What Is Machine Learning, Artificial Neural Networks and Deep Learning?-Examples of Practical Applications in Medicine. Diagnostics 2023, 13, 2582. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial intelligence assists precision medicine in cancer treatment. Front. Oncol. 2022, 12, 998222. [Google Scholar] [CrossRef]
- Bhandari, A. Revolutionizing Radiology With Artificial Intelligence. Cureus 2024, 16, e72646. [Google Scholar] [CrossRef]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Kulikowski, C.A.; Safir, A. Glaucoma consultation by computer. Comput. Biol. Med. 1978, 8, 25–40. [Google Scholar] [CrossRef]
- Shortliffe, E.H.; Davis, R.; Axline, S.G.; Buchanan, B.G.; Green, C.C.; Cohen, S.N. Computer-based consultations in clinical therapeutics: Explanation and rule acquisition capabilities of the MYCIN system. Comput. Biomed. Res. 1975, 8, 303–320. [Google Scholar] [CrossRef]
- Greenhill, A.E.B. A primer of AI in medicine. Techn. Gastrointest. Endosc. 2020, 22, 85–89. [Google Scholar] [CrossRef]
- Kulikowski, C.A. Beginnings of Artificial Intelligence in Medicine (AIM): Computational Artifice Assisting Scientific Inquiry and Clinical Art-with Reflections on Present AIM Challenges. Yearb. Med. Inf. 2019, 28, 249–256. [Google Scholar] [CrossRef]
- Amisha, M.P.; Pathania, M.; Rathaur, V.K. Overview of artificial intelligence in medicine. J. Fam. Med. Prim. Care 2019, 8, 2328–2331. [Google Scholar] [CrossRef] [PubMed]
- Kaul, V.; Enslin, S.; Gross, S.A. History of artificial intelligence in medicine. Gastrointest. Endosc. 2020, 92, 807–812. [Google Scholar] [CrossRef]
- Ferrucci, D.; Levas, A.; Bagchi, S.; Gondek, D.; Mueller, E.T. Watson: Beyond jeopardy! Artif. Intell. 2013, 199, 93–105. [Google Scholar] [CrossRef]
- Arterys Cardio DL Cloud MRI Analytics Software Receives FDA Clearance. Available online: https://www.dicardiology.com/product/arterys-cardio-dl-cloud-mri-analytics-software-receives-fda-clearance (accessed on 24 March 2025).
- Arif, A.A.; Jiang, S.X.; Byrne, M.F. Artificial intelligence in endoscopy: Overview, applications, and future directions. Saudi J. Gastroenterol. 2023, 29, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kolla, L.; Parikh, R.B. Uses and limitations of artificial intelligence for oncology. Cancer 2024, 130, 2101–2107. [Google Scholar] [CrossRef]
- Knudsen, J.E.; Ghaffar, U.; Ma, R.; Hung, A.J. Clinical applications of artificial intelligence in robotic surgery. J. Robot. Surg. 2024, 18, 102. [Google Scholar] [CrossRef]
- Zhou, M.; Scott, J.; Chaudhury, B.; Hall, L.; Goldgof, D.; Yeom, K.W.; Iv, M.; Ou, Y.; Kalpathy-Cramer, J.; Napel, S.; et al. Radiomics in Brain Tumor: Image Assessment, Quantitative Feature Descriptors, and Machine-Learning Approaches. AJNR Am. J. Neuroradiol. 2018, 39, 208–216. [Google Scholar] [CrossRef]
- Alobaidli, S.; McQuaid, S.; South, C.; Prakash, V.; Evans, P.; Nisbet, A. The role of texture analysis in imaging as an outcome predictor and potential tool in radiotherapy treatment planning. Br. J. Radiol. 2014, 87, 20140369. [Google Scholar] [CrossRef]
- Cao, Y. The promise of dynamic contrast-enhanced imaging in radiation therapy. Semin. Radiat. Oncol. 2011, 21, 147–156. [Google Scholar] [CrossRef] [PubMed]
- White, N.S.; McDonald, C.; Farid, N.; Kuperman, J.; Karow, D.; Schenker-Ahmed, N.M.; Bartsch, H.; Rakow-Penner, R.; Holland, D.; Shabaik, A.; et al. Diffusion-weighted imaging in cancer: Physical foundations and applications of restriction spectrum imaging. Cancer Res. 2014, 74, 4638–4652. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Duan, T.; Zhang, Y.; Weng, S.; Xu, H.; Ren, Y.; Zhang, Z.; Han, X. Radiogenomics: A key component of precision cancer medicine. Br. J. Cancer 2023, 129, 741–753. [Google Scholar] [CrossRef]
- Bitencourt, A.G.V.; Gibbs, P.; Rossi Saccarelli, C.; Daimiel, I.; Lo Gullo, R.; Fox, M.J.; Thakur, S.; Pinker, K.; Morris, E.A.; Morrow, M.; et al. MRI-based machine learning radiomics can predict HER2 expression level and pathologic response after neoadjuvant therapy in HER2 overexpressing breast cancer. EBioMedicine 2020, 61, 103042. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, P.; Guo, X.; Zhang, X.; Gao, Q.; Zhang, J.; Zhao, G.; Zheng, J. Identification of EGFR mutation status in male patients with non-small-cell lung cancer: Role of 18F-FDG PET/CT and serum tumor markers CYFRA21-1 and SCC-Ag. EJNMMI Res. 2023, 13, 27. [Google Scholar] [CrossRef]
- Rutman, A.M.; Kuo, M.D. Radiogenomics: Creating a link between molecular diagnostics and diagnostic imaging. Eur. J. Radiol. 2009, 70, 232–241. [Google Scholar] [CrossRef]
- Rajakumari, R.; Kalaivani, L. Breast Cancer Detection and Classification Using Deep CNN Techniques. Intell. Autom. Soft Comput. 2022, 32, 1089–1107. [Google Scholar] [CrossRef]
- Al-Yasriy, H.F.; AL-Husieny, M.S.; Mohsen, F.Y.; Khalil, E.A.; Hassan, Z.S. Diagnosis of lung cancer based on CT scans using CNN. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 022035. [Google Scholar] [CrossRef]
- Mondal, C.; Hasan, M.K.; Ahmad, M.; Awal, M.A.; Jawad, M.T.; Dutta, A.; Islam, M.R.; Moni, M.A. Ensemble of Convolutional Neural Networks to diagnose Acute Lymphoblastic Leukemia from microscopic images. Inf. Med. Unlocked 2021, 27, 100794. [Google Scholar] [CrossRef]
- Kather, J.N.; Krisam, J.; Charoentong, P.; Luedde, T.; Herpel, E.; Weis, C.A.; Gaiser, T.; Marx, A.; Valous, N.A.; Ferber, D.; et al. Predicting survival from colorectal cancer histology slides using deep learning: A retrospective multicenter study. PLoS Med. 2019, 16, e1002730. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Jin, P.F.; Bao, J.; Jiang, Q.; Wang, X. Thyroid ultrasound image classification using a convolutional neural network. Ann. Transl. Med. 2021, 9, 1526. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Nosato, H.; Kochi, Y.; Kojima, T.; Kawai, K.; Sakanashi, H.; Murakawa, M.; Nishiyama, H. Support System of Cystoscopic Diagnosis for Bladder Cancer Based on Artificial Intelligence. J. Endourol. 2020, 34, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.A.; Hussain, L.; Awan, I.A.; Abbasi, I.; Majid, A.; Nadeem, M.S.A.; Chaudhary, Q.A. Detecting prostate cancer using deep learning convolution neural network with transfer learning approach. Cogn. Neurodyn. 2020, 14, 523–533. [Google Scholar] [CrossRef]
- Schwartz, D.; Sawyer, T.W.; Thurston, N.; Barton, J.; Ditzler, G. Ovarian Cancer Detection Using Optical Coherence Tomography and Convolutional Neural Networks. Neural Comput. Appl. 2022, 34, 8977–8987. [Google Scholar] [CrossRef]
- Napte, K.M.; Mahajan, A.; Urooj, S. Automatic Liver Cancer Detection Using Deep Convolution Neural Network. IEEE Access 2023, 11, 94852–94862. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhang, X.Y.; Cheng, Y.T.; Li, B.; Teng, X.Z.; Zhang, J.; Lam, S.; Zhou, T.; Ma, Z.R.; Sheng, J.B.; et al. Artificial intelligence-driven radiomics study in cancer: The role of feature engineering and modeling. Mil. Med. Res. 2023, 10, 22. [Google Scholar] [CrossRef]
- Noble, W.S. What is a support vector machine. Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cai, N.; Pacheco, P.P.; Narrandes, S.; Wang, Y.; Xu, W. Applications of Support Vector Machine (SVM) Learning in Cancer Genomics. Cancer Genom. Proteom. 2018, 15, 41–51. [Google Scholar]
- Bilal, A.; Imran, A.; Baig, T.I.; Liu, X.; Nasr, E.A.; Long, H. Breast cancer diagnosis using support vector machine optimized by improved quantum inspired grey wolf optimization. Sci. Rep. 2024, 14, 10714. [Google Scholar] [CrossRef]
- Singh, K.K.; Singh, A. A study of image segmentation algorithms for different types of images. Int. J. Comput. Sci. Issues 2010, 7, 414. [Google Scholar]
- Wang, C.; Mahbod, A.; Ellinger, I.; Galdran, A.; Gopalakrishnan, S.; Niezgoda, J.; Yu, Z. FUSeg: The Foot Ulcer Segmentation Challenge. Information 2024, 15, 140. [Google Scholar] [CrossRef]
- Ramakrishnan, D.; Jekel, L.; Chadha, S.; Janas, A.; Moy, H.; Maleki, N.; Sala, M.; Kaur, M.; Petersen, G.C.; Merkaj, S.; et al. A large open access dataset of brain metastasis 3D segmentations on MRI with clinical and imaging information. Sci. Data 2024, 11, 254. [Google Scholar] [CrossRef]
- Link, K.E.; Schnurman, Z.; Liu, C.; Kwon, Y.J.F.; Jiang, L.Y.; Nasir-Moin, M.; Neifert, S.; Alzate, J.D.; Bernstein, K.; Qu, T.; et al. Longitudinal deep neural networks for assessing metastatic brain cancer on a large open benchmark. Nat. Commun. 2024, 15, 8170. [Google Scholar] [CrossRef]
- Rudie, J.D.; Saluja, R.; Weiss, D.A.; Nedelec, P.; Calabrese, E.; Colby, J.B.; Laguna, B.; Mongan, J.; Braunstein, S.; Hess, C.P.; et al. The University of California San Francisco Brain Metastases Stereotactic Radiosurgery (UCSF-BMSR) MRI Dataset. Radiol. Artif. Intell. 2024, 6, e230126. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yang, Y.; Yin, S.; Li, H.; Shao, Y.; Zheng, D.; Li, X.; Li, J.; Fan, W.; Li, J.; et al. Automated segmentation of brain metastases with deep learning: A multi-center, randomized crossover, multi-reader evaluation study. Neuro Oncol. 2024, 26, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Bauknecht, H.C.; Romano, V.C.; Rogalla, P.; Klingebiel, R.; Wolf, C.; Bornemann, L.; Hamm, B.; Hein, P.A. Intra- and interobserver variability of linear and volumetric measurements of brain metastases using contrast-enhanced magnetic resonance imaging. Invest. Radiol. 2010, 45, 49–56. [Google Scholar] [CrossRef]
- Rudie, J.D.; Rauschecker, A.M.; Bryan, R.N.; Davatzikos, C.; Mohan, S. Emerging Applications of Artificial Intelligence in Neuro-Oncology. Radiology 2019, 290, 607–618. [Google Scholar] [CrossRef]
- Pereira, S.; Pinto, A.; Alves, V.; Silva, C.A. Brain Tumor Segmentation Using Convolutional Neural Networks in MRI Images. IEEE Trans. Med. Imaging 2016, 35, 1240–1251. [Google Scholar] [CrossRef]
- Xue, J.; Wang, B.; Ming, Y.; Liu, X.; Jiang, Z.; Wang, C.; Liu, X.; Chen, L.; Qu, J.; Xu, S.; et al. Deep learning-based detection and segmentation-assisted management of brain metastases. Neuro Oncol. 2020, 22, 505–514. [Google Scholar] [CrossRef]
- Kim, M.; Wang, J.-Y.; Lu, W.; Jiang, H.; Stojadinovic, S.; Wardak, Z.; Dan, T.; Timmerman, R.; Wang, L.; Chuang, C.; et al. Where Does Auto-Segmentation for Brain Metastases Radiosurgery Stand Today? Bioengineering 2024, 11, 454. [Google Scholar] [CrossRef]
- Gállego Pérez-Larraya, J.; Hildebrand, J. Brain metastases. Handb. Clin. Neurol. 2014, 121, 1143–1157. [Google Scholar] [PubMed]
- Barajas, R.F.; Cha, S. Metastasis in Adult Brain Tumors. Neuroimaging Clin. N. Am. 2016, 26, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Porto, L.; You, S.-J.; Attarbaschi, A.; Cario, G.; Döring, M.; Moser, O.; Mücke, U.; Poyer, F.; Temme, C.; Voigt, S.; et al. Invasive Mold Infection of the Central Nervous System in Immunocompromised Children. J. Fungi 2020, 6, 226. [Google Scholar] [CrossRef]
- Ortiz-Ramón, R.; Ruiz-España, S.; Mollá-Olmos, E.; Moratal, D. Glioblastomas and brain metastases differentiation following an MRI texture analysis-based radiomics approach. Phys. Med. 2020, 76, 44–54. [Google Scholar] [CrossRef]
- Artzi, M.; Bressler, I.; Ben Bashat, D. Differentiation between glioblastoma, brain metastasis and subtypes using radiomics analysis. J. Magn. Reson. Imaging 2019, 50, 519–528. [Google Scholar] [CrossRef]
- Qian, Z.; Li, Y.; Wang, Y.; Li, L.; Li, R.; Wang, K.; Li, S.; Tang, K.; Zhang, C.; Fan, X.; et al. Differentiation of glioblastoma from solitary brain metastases using radiomic machine-learning classifiers. Cancer Lett. 2019, 451, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Jekel, L.; Brim, W.R.; von Reppert, M.; Staib, L.; Cassinelli Petersen, G.; Merkaj, S.; Subramanian, H.; Zeevi, T.; Payabvash, S.; Bousabarah, K.; et al. Machine Learning Applications for Differentiation of Glioma from Brain Metastasis-A Systematic Review. Cancers 2022, 14, 1369. [Google Scholar] [CrossRef]
- Bathla, G.; Dhruba, D.D.; Soni, N.; Liu, Y.; Larson, N.B.; A Kassmeyer, B.; Mohan, S.; Roberts-Wolfe, D.; Rathore, S.; Le, N.H.; et al. AI-based classification of three common malignant tumors in neuro-oncology: A multi-institutional comparison of machine learning and deep learning methods. J. Neuroradiol. 2024, 51, 258–264. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Heinz, G.; Marhold, F.; Meyer-Bäse, A.; Ganslandt, O.; Buchfelder, M.; Oberndorfer, S. Differentiation of Glioblastoma and Brain Metastases by MRI-Based Oxygen Metabolomic Radiomics and Deep Learning. Metabolites 2022, 12, 1264. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Marhold, F.; Oberndorfer, S.; Heinz, G.; Zimmermann, M.; Buchfelder, M.; Heynold, E.; Kinfe, T.M. Metabolic Tumor Microenvironment Characterization of Contrast Enhancing Brain Tumors Using Physiologic MRI. Metabolites 2021, 11, 668. [Google Scholar] [CrossRef]
- Baazaoui, H.; Hubertus, S.; Maros, M.E.; Mohamed, S.A.; Förster, A.; Schad, L.R.; Wenz, H. Artificial Neural Network-Derived Cerebral Metabolic Rate of Oxygen for Differentiating Glioblastoma and Brain Metastasis in MRI: A Feasibility Study. Appl. Sci. 2021, 11, 9928. [Google Scholar] [CrossRef]
- Riche, M.; Amelot, A.; Peyre, M.; Capelle, L.; Carpentier, A.; Mathon, B. Complications after frame-based stereotactic brain biopsy: A systematic review. Neurosurg. Rev. 2021, 44, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kniep, H.C.; Madesta, F.; Schneider, T.; Hanning, U.; Schönfeld, M.H.; Schön, G.; Fiehler, J.; Gauer, T.; Werner, R.; Gellissen, S. Radiomics of Brain MRI: Utility in Prediction of Metastatic Tumor Type. Radiology 2019, 290, 479–487. [Google Scholar] [CrossRef]
- Lyu, Q.; Namjoshi, S.V.; McTyre, E.; Topaloglu, U.; Barcus, R.; Chan, M.D.; Cramer, C.K.; Debinski, W.; Gurcan, M.N.; Lesser, G.J.; et al. A transformer-based deep-learning approach for classifying brain metastases into primary organ sites using clinical whole-brain MRI images. Patterns 2022, 3, 100613. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ramón, R.; Larroza, A.; Ruiz-España, S.; Arana, E.; Moratal, D. Classifying brain metastases by their primary site of origin using a radiomics approach based on texture analysis: A feasibility study. Eur. Radiol. 2018, 28, 4514–4523. [Google Scholar] [CrossRef]
- Strotzer, Q.D.; Wagner, T.; Angstwurm, P.; Hense, K.; Scheuermeyer, L.; Noeva, E.; Dinkel, J.; Stroszczynski, C.; Fellner, C.; Riemenschneider, M.J.; et al. Limited capability of MRI radiomics to predict primary tumor histology of brain metastases in external validation. Neurooncol. Adv. 2024, 6, vdae060. [Google Scholar] [CrossRef]
- Abu Mhanna, H.Y.; Omar, A.F.; Radzi, Y.M.; Oglat, A.A.; Akhdar, H.F.; Al Ewaidat, H.; Almahmoud, A.; Bani Yaseen, A.B.; Al Badarneh, L.; Alhamad, O.; et al. Systematic review of functional magnetic resonance imaging (fMRI) applications in the preoperative planning and treatment assessment of brain tumors. Heliyon 2025, 11, e42464. [Google Scholar] [CrossRef]
- Holtkamp, M.; Parmar, V.; Hosch, R.; Salhöfer, L.; Styczen, H.; Li, Y.; Opitz, M.; Glas, M.; Guberina, N.; Wrede, K.; et al. AI-guided virtual biopsy: Automated differentiation of cerebral gliomas from other benign and malignant MRI findings using deep learning. Neurooncol. Adv. 2025, 7, vdae225. [Google Scholar] [CrossRef]
- Rafanan, J.; Ghani, N.; Kazemeini, S.; Nadeem-Tariq, A.; Shih, R.; Vida, T.A. Modernizing Neuro-Oncology: The Impact of Imaging, Liquid Biopsies, and AI on Diagnosis and Treatment. Int. J. Mol. Sci. 2025, 26, 917. [Google Scholar] [CrossRef]
- Nandakumar, N.; Manzoor, K.; Agarwal, S.; Pillai, J.J.; Gujar, S.K.; Sair, H.I.; Venkataraman, A. Automated eloquent cortex localization in brain tumor patients using multi-task graph neural networks. Med. Image Anal. 2021, 74, 102203. [Google Scholar] [CrossRef]
- Luckett, P.H.; Park, K.Y.; Lee, J.J.; Lenze, E.J.; Wetherell, J.L.; Eyler, L.T.; Snyder, A.Z.; Ances, B.M.; Shimony, J.S.; Leuthardt, E.C. Data-efficient resting-state functional magnetic resonance imaging brain mapping with deep learning. J. Neurosurg. 2023, 139, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Johnston, J.M.; Fox, M.D.; Leuthardt, E.C.; Grubb, R.L.; Chicoine, M.R.; Smyth, M.D.; Snyder, A.Z.; Raichle, M.E.; Shimony, J.S. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: Initial experience. Neurosurgery 2009, 65, 226–236. [Google Scholar] [CrossRef]
- Alam Khan, K.; Jain, S.K.; Sinha, V.D.; Sinha, J. Preoperative Diffusion Tensor Imaging: A Landmark Modality for Predicting the Outcome and Characterization of Supratentorial Intra-Axial Brain Tumors. World Neurosurg. 2019, 124, e540–e551. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Kataria, R.; Sinha, V.D. Role of Diffusion Tensor Imaging in Brain Tumor Surgery. Asian J. Neurosurg. 2018, 13, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Zhang, J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 2006, 51, 527–539. [Google Scholar] [CrossRef]
- Samani, Z.R.; Parker, D.; Wolf, R.; Hodges, W.; Brem, S.; Verma, R. Distinct tumor signatures using deep learning-based characterization of the peritumoral microenvironment in glioblastomas and brain metastases. Sci. Rep. 2021, 11, 14469. [Google Scholar] [CrossRef]
- Khalighi, S.; Reddy, K.; Midya, A.; Pandav, K.B.; Madabhushi, A.; Abedalthagafi, M. Artificial intelligence in neuro-oncology: Advances and challenges in brain tumor diagnosis, prognosis, and precision treatment. Npj Precis. Oncol. 2024, 8, 80. [Google Scholar] [CrossRef]
- Mut, M.; Zhang, M.; Gupta, I.; Fletcher, P.T.; Farzad, F.; Nwafor, D. Augmented surgical decision-making for glioblastoma: Integrating AI tools into education and practice. Front. Neurol. 2024, 15, 1387958. [Google Scholar] [CrossRef]
- Khan, M.F.; Iftikhar, A.; Anwar, H.; Ramay, S.A. Brain Tumor Segmentation and Classification using Optimized Deep Learning. J. Comput. Biomed. Inform. 2024, 7, 1. [Google Scholar]
- Rasheed, Z.; Ma, Y.K.; Ullah, I.; Ghadi, Y.Y.; Khan, M.Z.; Khan, M.A.; Abdusalomov, A.; Alqahtani, F.; Shehata, A.M. Brain Tumor Classification from MRI Using Image Enhancement and Convolutional Neural Network Techniques. Brain Sci. 2023, 13, 1320. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, A.K. Brain tumor segmentation with deep learning: Current approaches and future perspectives. J. Neurosci. Methods 2025, 418, 110424. [Google Scholar] [CrossRef] [PubMed]
- Molchanova, N.; Raina, V.; Malinin, A.; Rosa, F.; Depeursinge, A.; Gales, M.; Granziera, C.; Müller, H.; Graziani, M.; Cuadra, M.B. Structural-based uncertainty in deep learning across anatomical scales: Analysis in white matter lesion segmentation. Comput. Biol. Med. 2025, 184, 109336. [Google Scholar] [CrossRef]
- Kos, T.M.; Colombo, E.; Bartels, L.W.; Robe, P.A.; van Doormaal, T.P.C. Evaluation Metrics for Augmented Reality in Neurosurgical Preoperative Planning, Surgical Navigation, and Surgical Treatment Guidance: A Systematic Review. Oper Neurosurg 2023, 26, 491–501. [Google Scholar] [CrossRef]
- Fick, T.; van Doormaal, J.A.M.; Tosic, L.; van Zoest, R.J.; Meulstee, J.W.; Hoving, E.W.; van Doormaal, T.P.C. Fully automatic brain tumor segmentation for 3D evaluation in augmented reality. Neurosurg. Focus 2021, 51, E14. [Google Scholar] [CrossRef]
- Tabrizi, L.B.; Mahvash, M. Augmented reality-guided neurosurgery: Accuracy and intraoperative application of an image projection technique. J. Neurosurg. 2015, 123, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Gurses, M.E.; Gökalp, E.; Spatz, J.; Bahadir, S.; Himic, V.; Komotar, R.J.; Ivan, M.E. Augmented reality in cranial surgery: Surgical planning and maximal safety in resection of brain tumors via head-mounted fiber tractography. Clin. Neurol. Neurosurg. 2025, 251, 108820. [Google Scholar] [CrossRef]
- Huang, C.H.; Hsieh, C.H.; Lee, J.D.; Huang, W.C.; Lee, S.T.; Wu, C.T.; Sun, Y.N.; Wu, Y.T. A CT-ultrasound-coregistered augmented reality enhanced image-guided surgery system and its preliminary study on brain-shift estimation. J. Instrum. 2012, 7, P08016. [Google Scholar] [CrossRef]
- Carbone, M.; Montemurro, N.; Cattari, N.; Autelitano, M.; Cutolo, F.; Ferrari, V.; Cigna, E.; Condino, S. Targeting accuracy of neuronavigation: A comparative evaluation of an innovative wearable AR platform vs. traditional EM navigation. Front. Digit. Health 2024, 6, 1500677. [Google Scholar] [CrossRef] [PubMed]
- Scherschinski, L.; McNeill, I.T.; Schlachter, L.; Shuman, W.H.; Oemke, H.; Yaeger, K.A.; Bederson, J.B. Augmented reality-assisted microsurgical resection of brain arteriovenous malformations: Illustrative case. J. Neurosurg. Case Lessons 2022, 3, CASE21135. [Google Scholar] [CrossRef]
- Abe, Y.; Sato, S.; Kato, K.; Hyakumachi, T.; Yanagibashi, Y.; Ito, M.; Abumi, K. A novel 3D guidance system using augmented reality for percutaneous vertebroplasty: Technical note. J. Neurosurg. Spine 2013, 19, 492–501. [Google Scholar] [CrossRef]
- Khalsa, S.S.S.; Mummaneni, P.V.; Chou, D.; Park, P. Present and Future Spinal Robotic and Enabling Technologies. Oper. Neurosurg. 2021, 21, S48–S56. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Xiao, F.R.; Cheng, J.C.; Yang, W.C.; Cheng, Y.H.; Chang, Y.C.; Lin, J.Y.; Liang, C.H.; Lu, J.T.; Chen, Y.F.; et al. Randomized multi-reader evaluation of automated detection and segmentation of brain tumors in stereotactic radiosurgery with deep neural networks. Neuro Oncol. 2021, 23, 1560–1568. [Google Scholar] [CrossRef]
- Shapey, J.; Wang, G.; Dorent, R.; Dimitriadis, A.; Li, W.; Paddick, I.; Kitchen, N.; Bisdas, S.; Saeed, S.R.; Ourselin, S.; et al. An artificial intelligence framework for automatic segmentation and volumetry of vestibular schwannomas from contrast-enhanced T1-weighted and high-resolution T2-weighted MRI. J. Neurosurg. 2021, 134, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Awuah, W.A.; Adebusoye, F.T.; Wellington, J.; David, L.; Salam, A.; Weng Yee, A.L.; Lansiaux, E.; Yarlagadda, R.; Garg, T.; Abdul-Rahman, T.; et al. Recent Outcomes and Challenges of Artificial Intelligence, Machine Learning, and Deep Learning in Neurosurgery. World Neurosurg. X 2024, 23, 100301. [Google Scholar] [CrossRef] [PubMed]
- Dohm, A.; Tang, J.; Mills, M.; Perez, B.; Robinson, T.; Creelan, B.; Gray, J.; Etame, A.; Vogelbaum, M.; Forsyth, P.; et al. Clinical outcomes of non-small cell lung cancer brain metastases treated with stereotactic radiosurgery and immune checkpoint inhibitors, EGFR tyrosine kinase inhibitors, chemotherapy and immune checkpoint inhibitors, or chemotherapy alone. J. Neurosurg. 2023, 138, 1600–1607. [Google Scholar] [CrossRef]
- Han, E.Y.; Wang, H.; Luo, D.; Li, J.; Wang, X. Dosimetric comparison of fractionated radiosurgery plans using frameless Gamma Knife ICON and CyberKnife systems with linear accelerator-based radiosurgery plans for multiple large brain metastases. J. Neurosurg. 2020, 132, 1473–1479. [Google Scholar] [CrossRef]
- Staartjes, V.E.; Seevinck, P.R.; Vandertop, W.P.; van Stralen, M.; Schröder, M.L. Magnetic resonance imaging-based synthetic computed tomography of the lumbar spine for surgical planning: A clinical proof-of-concept. Neurosurg. Focus 2021, 50, E13. [Google Scholar] [CrossRef]
- Conti, A.; Pontoriero, A.; Ricciardi, G.K.; Granata, F.; Vinci, S.; Angileri, F.F.; Pergolizzi, S.; Alafaci, C.; Rizzo, V.; Quartarone, A.; et al. Integration of functional neuroimaging in CyberKnife radiosurgery: Feasibility and dosimetric results. Neurosurg. Focus 2013, 34, E5. [Google Scholar] [CrossRef]
- Mert, A.; Kiesel, B.; Wöhrer, A.; Martínez-Moreno, M.; Minchev, G.; Furtner, J.; Knosp, E.; Wolfsberger, S.; Widhalm, G. Introduction of a standardized multimodality image protocol for navigation-guided surgery of suspected low-grade gliomas. Neurosurg. Focus 2015, 38, E4. [Google Scholar] [CrossRef]
- Press, R.H.; Shu, H.G.; Shim, H.; Mountz, J.M.; Kurland, B.F.; Wahl, R.L.; Jones, E.F.; Hylton, N.M.; Gerstner, E.R.; Nordstrom, R.J.; et al. The Use of Quantitative Imaging in Radiation Oncology: A Quantitative Imaging Network (QIN) Perspective. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1219–1235. [Google Scholar] [CrossRef]
- Oermann, E.K.; Kress, M.A.; Todd, J.V.; Collins, B.T.; Hoffman, R.; Chaudhry, H.; Collins, S.P.; Morris, D.; Ewend, M.G. The impact of radiosurgery fractionation and tumor radiobiology on the local control of brain metastases. J. Neurosurg. 2013, 119, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Jian, A.; Liu, S.; Di Ieva, A. Artificial Intelligence for Survival Prediction in Brain Tumors on Neuroimaging. Neurosurgery 2022, 91, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Li, Y.; Fang, S.; Jiang, T. Role of molecular biomarkers in glioma resection: A systematic review. Chin. Neurosurg. J. 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, K.; Nakasu, Y.; Kurakane, T.; Hayashi, N.; Harada, H.; Nozaki, K. Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of worse survival after resection in patients with brain metastasis. J. Neurosurg. 2017, 127, 433–437. [Google Scholar] [CrossRef]
- Brown, M.H.; Marcrom, S.R.; Patel, M.P.; Popple, R.A.; Travis, R.L.; McDonald, A.M.; Riley, K.O.; Markert, J.M.; Willey, C.D.; Bredel, M.; et al. Understanding the Effect of Prescription Isodose in Single-Fraction Stereotactic Radiosurgery on Plan Quality and Clinical Outcomes for Solid Brain Metastases. Neurosurgery 2023, 93, 1313–1318. [Google Scholar] [CrossRef]
- Liu, Q.; Tong, X.; Wang, J. Management of brain metastases: History and the present. Chin. Neurosurg. J. 2019, 5, 1. [Google Scholar] [CrossRef]
- Harel, R.; Kaisman-Elbaz, T.; Emch, T.; Elson, P.; Chao, S.T.; Suh, J.H.; Angelov, L. A quantitative and comparative evaluation of stereotactic spine radiosurgery local control: Proposing a consistent measurement methodology. Neurosurg. Focus 2022, 53, E10. [Google Scholar] [CrossRef]
- Vaca, S.D.; Connolly, I.D.; Ho, C.; Neal, J.; Hayden Gephart, M. Commentary: Treatment Considerations for Patients With Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer Brain Metastases in the Era of Tyrosine Kinase Inhibitors. Neurosurgery 2018, 82, E6–E14. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Ahluwalia, M.S.; Gurewitz, J.; Bernstein, K.; Kondziolka, D.; Niranjan, A.; Wei, Z.; Lunsford, L.D.; Fakhoury, K.R.; Rusthoven, C.G.; et al. Imaging-defined necrosis after treatment with single-fraction stereotactic radiosurgery and immune checkpoint inhibitors and its potential association with improved outcomes in patients with brain metastases: An international multicenter study of 697 patients. J. Neurosurg. 2023, 138, 1178–1187. [Google Scholar]
- Richardson, L.G.; Miller, J.J.; Kitagawa, Y.; Wakimoto, H.; Choi, B.D.; Curry, W.T. Implications of IDH mutations on immunotherapeutic strategies for malignant glioma. Neurosurg. Focus 2022, 52, E6. [Google Scholar] [CrossRef]
- Karsy, M.; Azab, M.A.; Abou-Al-Shaar, H.; Guan, J.; Eli, I.; Jensen, R.L.; Ormond, D.R. Clinical potential of meningioma genomic insights: A practical review for neurosurgeons. Neurosurg. Focus 2018, 44, E10. [Google Scholar] [CrossRef] [PubMed]
- Noch, E.K.; Ramakrishna, R.; Magge, R. Challenges in the Treatment of Glioblastoma: Multisystem Mechanisms of Therapeutic Resistance. World Neurosurg. 2018, 116, 505–517. [Google Scholar] [CrossRef]
- Aquilanti, E.; Brastianos, P.K. Immune Checkpoint Inhibitors for Brain Metastases: A Primer for Neurosurgeons. Neurosurgery 2020, 87, E281–E288. [Google Scholar] [CrossRef]
- Zhang, M.; Rodrigues, A.J.; Pollom, E.L.; Gibbs, I.C.; Soltys, S.G.; Hancock, S.L.; Neal, J.W.; Padda, S.K.; Ramchandran, K.J.; Wakelee, H.A.; et al. Improved survival and disease control following pembrolizumab-induced immune-related adverse events in high PD-L1 expressing non-small cell lung cancer with brain metastases. J. Neurooncol. 2021, 152, 125–134. [Google Scholar] [CrossRef]
- Chen, D.; Yao, J.; Hu, B.; Kuang, L.; Xu, B.; Liu, H.; Dou, C.; Wang, G.; Guo, M. New biomarker: The gene HLA-DRA associated with low-grade glioma prognosis. Chin. Neurosurg. J. 2022, 8, 12. [Google Scholar] [CrossRef]
- Shi, D.D.; Arnaout, O.; Bi, W.L.; Buchbinder, E.I.; Cagney, D.N.; Insco, M.L.; Liu, D.; Schoenfeld, J.D.; Aizer, A.A. Severe Radiation Necrosis Refractory to Surgical Resection in Patients with Melanoma and Brain Metastases Managed with Ipilimumab/Nivolumab and Brain-Directed Stereotactic Radiation Therapy. World Neurosurg. 2020, 139, 226–231. [Google Scholar] [CrossRef]
- Raju, B.; Jumah, F.; Ashraf, O.; Narayan, V.; Gupta, G.; Sun, H.; Hilden, P.; Nanda, A. Big data, machine learning, and artificial intelligence: A field guide for neurosurgeons. J. Neurosurg. 2021, 135, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Farooqi, H.A.; Nabi, R.; Hasan, H. Advancing aneurysm management: The potential of AI and machine learning in enhancing safety and predictive accuracy. Neurosurg. Rev. 2024, 47, 432. [Google Scholar] [CrossRef] [PubMed]
- Laufer, I.; Bilsky, M.H. Advances in the treatment of metastatic spine tumors: The future is not what it used to be. J. Neurosurg. Spine 2019, 30, 299–307. [Google Scholar] [CrossRef]
- Senders, J.T.; Staples, P.; Mehrtash, A.; Cote, D.J.; Taphoorn, M.J.B.; Reardon, D.A.; Gormley, W.B.; Smith, T.R.; Broekman, M.L.; Arnaout, O. An Online Calculator for the Prediction of Survival in Glioblastoma Patients Using Classical Statistics and Machine Learning. Neurosurgery 2020, 86, E184–E192. [Google Scholar] [CrossRef]
- Gilbride, L.; Siker, M.; Bovi, J.; Gore, E.; Schultz, C.; Hall, W.A. Current Predictive Indices and Nomograms To Enable Personalization of Radiation Therapy for Patients With Secondary Malignant Neoplasms of the Central Nervous System: A Review. Neurosurgery 2018, 82, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Kano, H.; Morales-Restrepo, A.; Iyer, A.; Weiner, G.M.; Mousavi, S.H.; Kirkwood, J.M.; Tarhini, A.A.; Flickinger, J.C.; Lunsford, L.D. Comparison of prognostic indices in patients who undergo melanoma brain metastasis radiosurgery. J. Neurosurg. 2018, 128, 14–22. [Google Scholar] [CrossRef]
- Jin, M.C.; Parker, J.J.; Prolo, L.M.; Wu, A.; Halpern, C.H.; Li, G.; Ratliff, J.K.; Han, S.S.; Skirboll, S.L.; Grant, G.A. An integrated risk model stratifying seizure risk following brain tumor resection among seizure-naive patients without antiepileptic prophylaxis. Neurosurg. Focus 2022, 52, E3. [Google Scholar] [CrossRef] [PubMed]
- Hollon, T.C.; Parikh, A.; Pandian, B.; Tarpeh, J.; Orringer, D.A.; Barkan, A.L.; McKean, E.L.; Sullivan, S.E. A machine learning approach to predict early outcomes after pituitary adenoma surgery. Neurosurg. Focus 2018, 45, E8. [Google Scholar] [CrossRef] [PubMed]
- London, D.; Patel, D.N.; Donahue, B.; Navarro, R.E.; Gurewitz, J.; Silverman, J.S.; Sulman, E.; Bernstein, K.; Palermo, A.; Golfinos, J.G.; et al. The incidence and predictors of new brain metastases in patients with non-small cell lung cancer following discontinuation of systemic therapy. J. Neurosurg. 2022, 137, 544–554. [Google Scholar] [CrossRef]
- Roh, H.; Lee, S.Y.; Lee, J.; Hwang, S.Y.; Kim, J.H. Use of thyroid transcription factor 1 and napsin A to predict local failure and survival after Gamma Knife radiosurgery in patients with brain metastases from lung adenocarcinoma. J. Neurosurg. 2023, 138, 663–673. [Google Scholar] [CrossRef]
- Bunevicius, A.; Pikis, S.; Kondziolka, D.; Patel, D.N.; Bernstein, K.; Sulman, E.P.; Lee, C.C.; Yang, H.C.; Delabar, V.; Mathieu, D.; et al. Stereotactic radiosurgery for glioblastoma considering tumor genetic profiles: An international multicenter study. J. Neurosurg. 2022, 137, 42–50. [Google Scholar] [CrossRef]
- Sneed, P.K.; Mendez, J.; Vemer-van den Hoek, J.G.; Seymour, Z.A.; Ma, L.; Molinaro, A.M.; Fogh, S.E.; Nakamura, J.L.; McDermott, M.W. Adverse radiation effect after stereotactic radiosurgery for brain metastases: Incidence, time course, and risk factors. J. Neurosurg. 2015, 123, 373–386. [Google Scholar] [CrossRef]
- Karabacak, M.; Jagtiani, P.; Shrivastava, R.K.; Margetis, K. Personalized Prognosis with Machine Learning Models for Predicting In-Hospital Outcomes Following Intracranial Meningioma Resections. World Neurosurg. 2024, 182, e210–e230. [Google Scholar] [CrossRef]
- Feng, R.; Badgeley, M.; Mocco, J.; Oermann, E.K. Deep learning guided stroke management: A review of clinical applications. J. Neurointerv. Surg. 2018, 10, 358–362. [Google Scholar] [CrossRef]
- Taha, B.; Boley, D.; Sun, J.; Chen, C.C. State of Radiomics in Glioblastoma. Neurosurgery 2021, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Ren, J.X.; Yu, Z.P.; Peng, Y.D.; Yu, C.W.; Deng, D.; Xie, Y.; He, Z.Q.; Duan, H.; Wu, B.; et al. Predicting glioblastoma molecular subtypes and prognosis with a multimodal model integrating convolutional neural network, radiomics, and semantics. J. Neurosurg. 2023, 139, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.E.; Miller, H.A.; Chen, J.; Williams, B.J.; Frieboes, H.B. Metabolomic differentiation of tumor core versus edge in glioma. Neurosurg. Focus 2023, 54, E4. [Google Scholar] [CrossRef]
- Young, J.S.; Al-Adli, N.; Scotford, K.; Cha, S.; Berger, M.S. Pseudoprogression versus true progression in glioblastoma: What neurosurgeons need to know. J. Neurosurg. 2023, 139, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, C.; Siempis, T.; Alexiou, G.A.; Zikou, A.; Sioka, C.; Voulgaris, S.; Argyropoulou, M.I. Differentiation Between True Tumor Progression of Glioblastoma and Pseudoprogression Using Diffusion-Weighted Imaging and Perfusion-Weighted Imaging: Systematic Review and Meta-analysis. World Neurosurg. 2020, 144, e100–e109. [Google Scholar] [CrossRef]
- Reuter, G.; Moïse, M.; Roll, W.; Martin, D.; Lombard, A.; Scholtes, F.; Stummer, W.; Suero Molina, E. Conventional and advanced imaging throughout the cycle of care of gliomas. Neurosurg. Rev. 2021, 44, 2493–2509. [Google Scholar] [CrossRef]
- Scherer, M.; Jungk, C.; Götz, M.; Kickingereder, P.; Reuss, D.; Bendszus, M.; Maier-Hein, K.; Unterberg, A. Early postoperative delineation of residual tumor after low-grade glioma resection by probabilistic quantification of diffusion-weighted imaging. J. Neurosurg. 2019, 130, 2016–2024. [Google Scholar] [CrossRef]
- Ghaith, A.K.; Ghanem, M.; Zamanian, C.; Bon-Nieves, A.A.; Bhandarkar, A.; Nathani, K.; Bydon, M.; Quiñones-Hinojosa, A. Using machine learning to predict 30-day readmission and reoperation following resection of supratentorial high-grade gliomas: An ACS NSQIP study involving 9418 patients. Neurosurg. Focus 2023, 54, E12. [Google Scholar] [CrossRef]
- Rao, A.; Rao, G.; Gutman, D.A.; Flanders, A.E.; Hwang, S.N.; Rubin, D.L.; Colen, R.R.; Zinn, P.O.; Jain, R.; Wintermark, M.; et al. A combinatorial radiographic phenotype may stratify patient survival and be associated with invasion and proliferation characteristics in glioblastoma. J. Neurosurg. 2016, 124, 1008–1017. [Google Scholar] [CrossRef]
- Moawad, A.W.; Janas, A.; Baid, U.; Ramakrishnan, D.; Saluja, R.; Ashraf, N.; Maleki, N.; Jekel, L.; Yordanov, N.; Fehringer, P.; et al. The Brain Tumor Segmentation-Metastases (BraTS-METS) Challenge 2023: Brain Metastasis Segmentation on Pre-treatment MRI. arxiv 2024, arXiv:2306.00838v3. [Google Scholar]
- Zhao, J.; Vaios, E.; Yang, Z.; Lu, K.; Floyd, S.; Yang, D.; Ji, H.; Reitman, Z.J.; Lafata, K.J.; Fecci, P.; et al. Radiogenomic explainable AI with neural ordinary differential equation for identifying post-SRS brain metastasis radionecrosis. Med. Phys. 2025, 52, 2661–2674. [Google Scholar] [CrossRef]
- Uggerly, A.S.V.; Cummins, D.D.; Nguyen, M.P.; Saggi, S.; Goldschmidt, E.; Chang, E.F.; McDermott, M.W.; Berger, M.S.; Theodosopoulos, P.V.; Hervey-Jumper, S.L.; et al. Genomic alterations associated with rapid progression of brain metastases. Neurosurg. Focus 2023, 55, E15. [Google Scholar] [CrossRef] [PubMed]
- Sanmillan, J.L.; Fernández-Coello, A.; Fernández-Conejero, I.; Plans, G.; Gabarrós, A. Functional approach using intraoperative brain mapping and neurophysiological monitoring for the surgical treatment of brain metastases in the central region. J. Neurosurg. 2017, 126, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Lazar, A.; Duriseti, S.; Raleigh, D.R.; Hess, C.P.; Fogh, S.E.; Barani, I.J.; Nakamura, J.L.; Larson, D.A.; Theodosopoulos, P.; et al. Discovery of additional brain metastases on the day of stereotactic radiosurgery: Risk factors and outcomes. J. Neurosurg. 2017, 126, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Steiger, H.J.; Vollmer, K.; Rogers, S.; Schwyzer, L. State of affairs regarding targeted pharmacological therapy of cancers metastasized to the brain. Neurosurg. Rev. 2022, 45, 3119–3138. [Google Scholar] [CrossRef]
- Tunthanathip, T.; Duangsuwan, J.; Wattanakitrungroj, N.; Tongman, S.; Phuenpathom, N. Comparison of intracranial injury predictability between machine learning algorithms and the nomogram in pediatric traumatic brain injury. Neurosurg. Focus 2021, 51, E7. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Yondorf, M.Z.; Peng, L.; Trichter, S.; Nedialkova, L.; Sabbas, A.; Kulidzhanov, F.; Parashar, B.; Nori, D.; Chao, K.S.C.; et al. Phase I/II study of resection and intraoperative cesium-131 radioisotope brachytherapy in patients with newly diagnosed brain metastases. J. Neurosurg. 2014, 121, 338–348. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evangelou, K.; Zemperligkos, P.; Politis, A.; Lani, E.; Gutierrez-Valencia, E.; Kotsantis, I.; Velonakis, G.; Boviatsis, E.; Stavrinou, L.C.; Kalyvas, A. Diagnostic, Therapeutic, and Prognostic Applications of Artificial Intelligence (AI) in the Clinical Management of Brain Metastases (BMs). Brain Sci. 2025, 15, 730. https://doi.org/10.3390/brainsci15070730
Evangelou K, Zemperligkos P, Politis A, Lani E, Gutierrez-Valencia E, Kotsantis I, Velonakis G, Boviatsis E, Stavrinou LC, Kalyvas A. Diagnostic, Therapeutic, and Prognostic Applications of Artificial Intelligence (AI) in the Clinical Management of Brain Metastases (BMs). Brain Sciences. 2025; 15(7):730. https://doi.org/10.3390/brainsci15070730
Chicago/Turabian StyleEvangelou, Kyriacos, Panagiotis Zemperligkos, Anastasios Politis, Evgenia Lani, Enrique Gutierrez-Valencia, Ioannis Kotsantis, Georgios Velonakis, Efstathios Boviatsis, Lampis C. Stavrinou, and Aristotelis Kalyvas. 2025. "Diagnostic, Therapeutic, and Prognostic Applications of Artificial Intelligence (AI) in the Clinical Management of Brain Metastases (BMs)" Brain Sciences 15, no. 7: 730. https://doi.org/10.3390/brainsci15070730
APA StyleEvangelou, K., Zemperligkos, P., Politis, A., Lani, E., Gutierrez-Valencia, E., Kotsantis, I., Velonakis, G., Boviatsis, E., Stavrinou, L. C., & Kalyvas, A. (2025). Diagnostic, Therapeutic, and Prognostic Applications of Artificial Intelligence (AI) in the Clinical Management of Brain Metastases (BMs). Brain Sciences, 15(7), 730. https://doi.org/10.3390/brainsci15070730






