Response to Training in Emotion Recognition Function for Mild TBI/PTSD Survivors: Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Intervention
- Discrete units of expression (such as eyes only) progressed to combined units of expression (e.g., eyes and mouth).
- Static stimuli (e.g., photo) progressed to dynamic (video); or single stimulus (vocal pitch) progressed to multiple stimuli (pitch and loudness).
- Intensity of expression progressed from mild intensity to highly intense (face or voice).
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Primary Measure
3.3. Exploratory Measures
3.4. Individual Differences
3.4.1. Three Most Impaired Subjects at Baseline (Four to Seven FAB Subdomains Impaired (Table 4): S1, S5, S7)
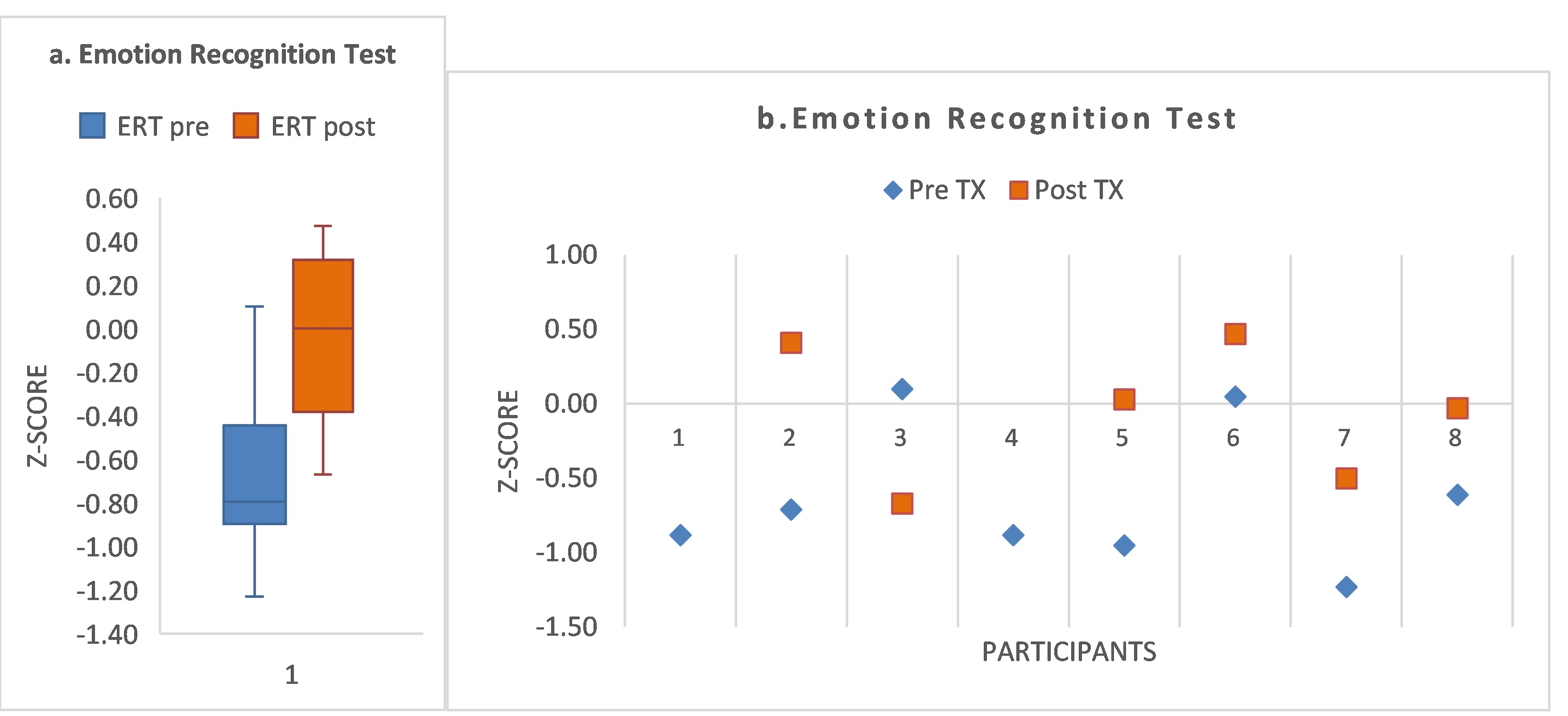
- S5 had the most impaired FAB domains (seven impaired at baseline), with recovery to normal range for five of those domains by post-treatment. S5 showed baseline impairment in attention (score, 0.9th percentile), with a post-treatment increase in score, but only to the 16th percentile (Supplementary Table S1). According to accuracy of speed recognition of facial affect, S5 showed baseline impairment (−0.95) and recovery to within normal range at post-treatment (+0.03; Supplementary Table S2).
- S1 had five impaired FAB domains at baseline, with recovery of three FAB domains to normal range by post-treatment.
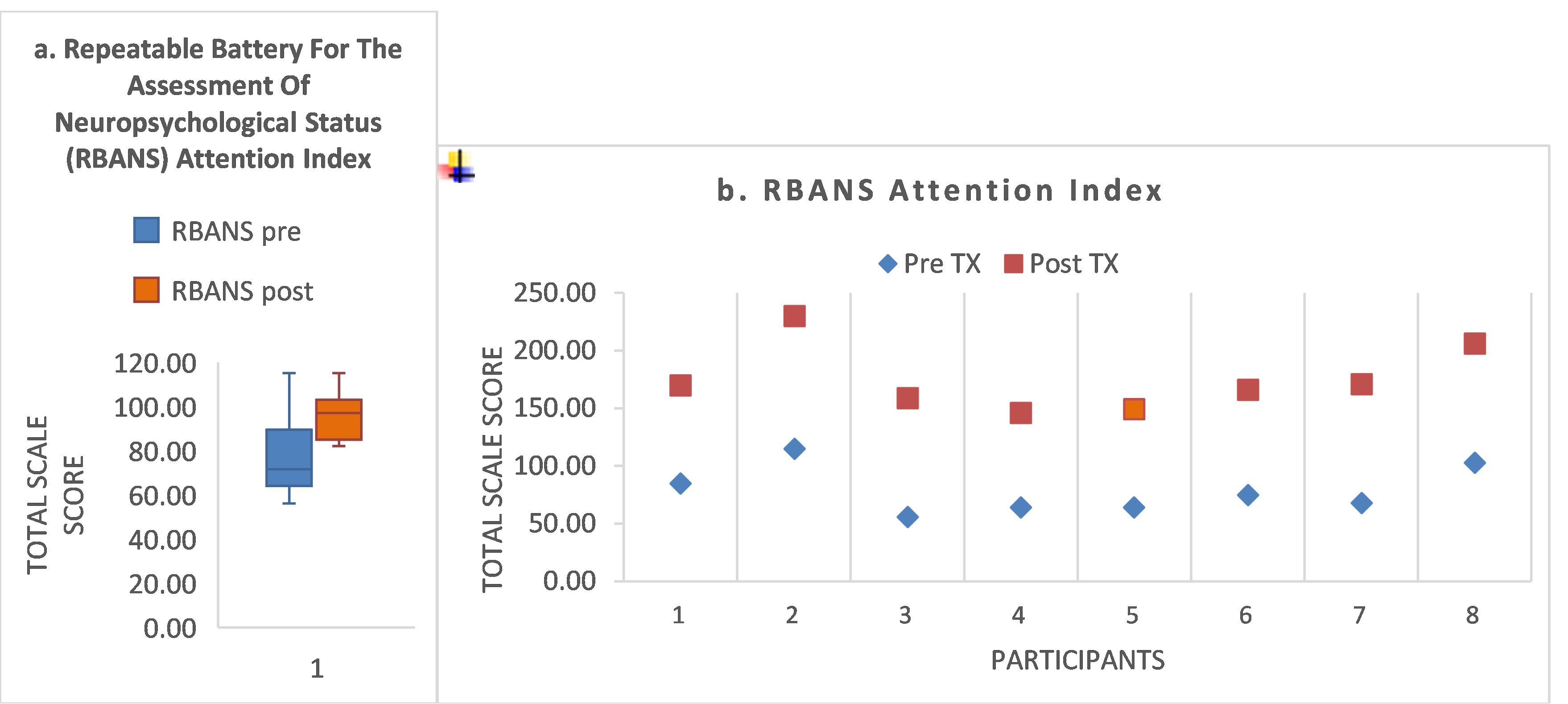
- For the Attention Index, S1 remained unchanged at the impaired level of the 16th percentile throughout.
- S7 had four impaired FAB domains at baseline, with recovery to normal range for three domains by post-treatment. S7 showed impaired baseline attention at the 1.6th percentile, improving by post-treatment to within the normal range (57th percentile). Speed of recognition accuracy improved from the impaired level (−1.23) to −0.5 by post-treatment.
3.4.2. Subjects with Two Impaired FAB Domains at Baseline (S3, S4, S6)
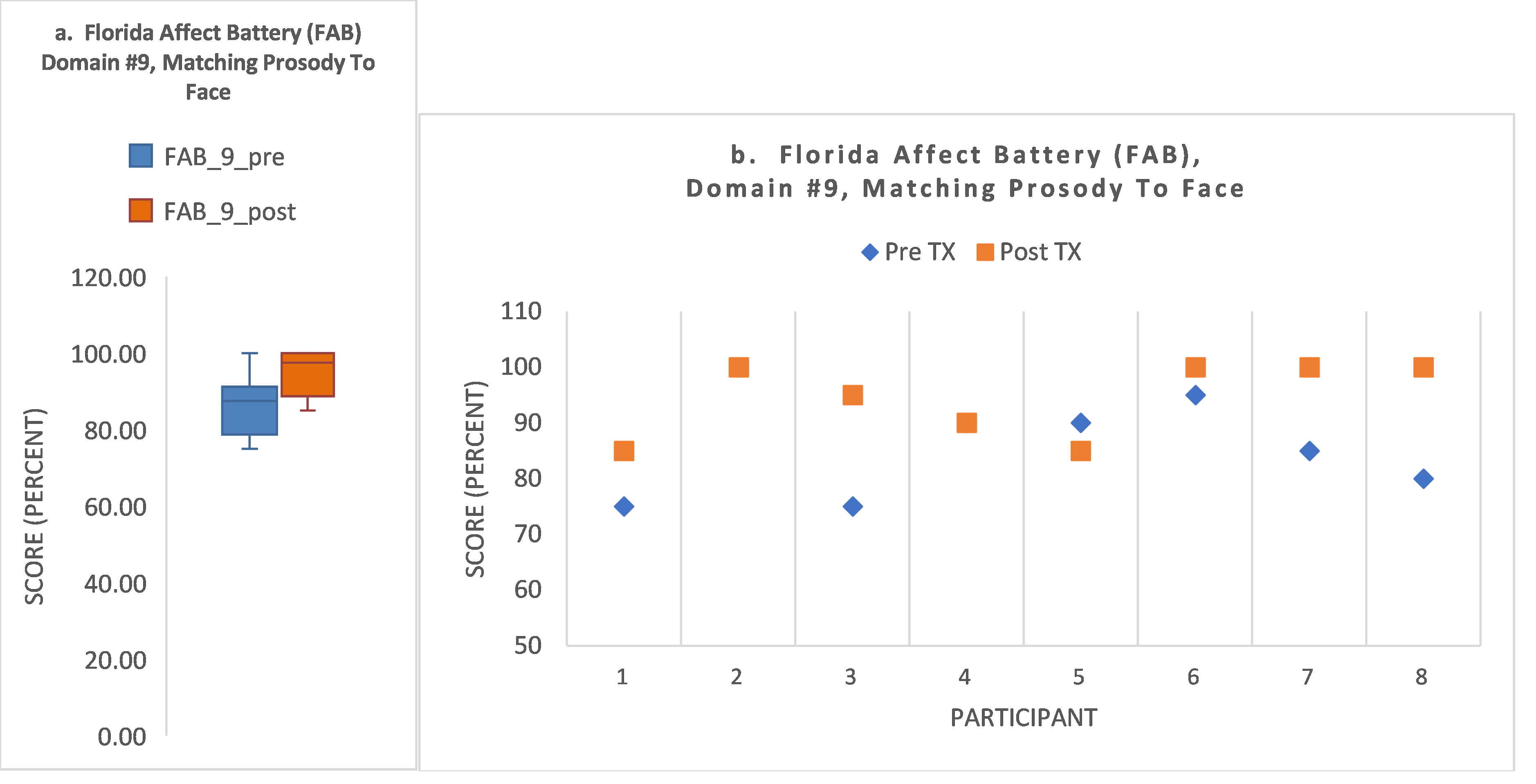
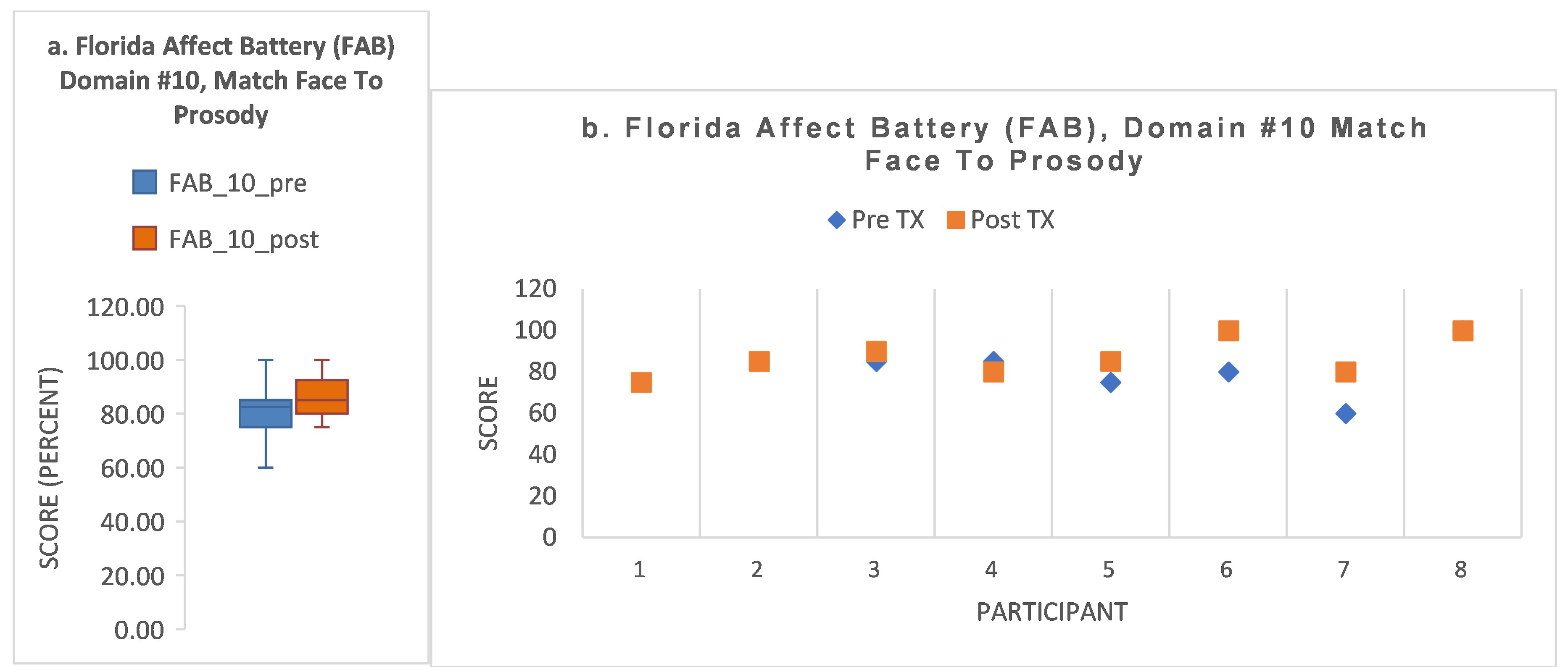
- S3 showed baseline impairment in the two FAB domains requiring attention and encoding of both facial affect and prosody (domains #9, #10), improving to normal range by post-treatment. His baseline attention score was <1.0th percentile, but his attention score improved to normal range by post-treatment (57th percentile). In contrast to his gains in these measures, his ERT score declined from 0.1 to −0.67.
- S6 recovered to within normal range for domains #4 and #10. S6 showed a mixed response to treatment. Though he recovered the two impaired FAB domains (#4, #10) to within normal range, domains #2 and #8b1 showed a decline. His attention score improved 22 percentile points, impaired at the 5th percentile at baseline, improving to the 27th percentile by post-treatment. His speed of recognition improved from baseline (+0.05) to post-treatment (+0.47; a gain of 0.42 z-score points).
- These two participants, S3 and S6, who had only two impaired FAB domains at baseline, one of which was domain #10, recovered to normal range at baseline for domain #10. This finding is in contrast to those subjects who had a greater number of impaired FAB domains at baseline and did not improve to normal in domain #10 (previous subsection on S5, S1, S7).
- S4 was a ‘non-responder’ according to FAB domains #4 and #10. He showed impaired attention at baseline (<1st percentile), improving to only the 16th percentile by post-treatment.
- Summarizing the above, we can note that two subjects had recovery of 2/2 FAB domains, including domain #10. For these two subjects, one had a notable improvement in attention and the other had a notable improvement in speed of accurate recognition. A third subject was a non-responder, with two baseline-impaired FAB domains unchanged at post-treatment and no notable change in other measures.
3.4.3. Subjects with Impairment in Only One FAB Domain at Baseline (S2, S8)
- S2 showed no post-treatment change in domain #10. Attention score was within normal range throughout. However, S2 had an improvement of z-score 1.12 points in speed of facial recognition (from pre-treatment −0.71 to post-treatment (+0.41)).
- S8 improved to within normal limits at post-treatment (domain #9). S8 showed an attention score within normal limits throughout. For speed of accurate facial recognition, S8 showed baseline impairment (−0.61) and improved to normal range by post-treatment (−0.03; z-score gain of 0.58).
4. Discussion
4.1. Successful Administration of a New Multimodal Treatment Protocol, the MMART, That Targets Deficits in Emotion Recognition in Those with mTBI/PTSD
4.2. Statistically and Clinically Significant Improvement, for Those with Mild TBI/PTSD, in Primary and Secondary Measures in Response to Treatment Targeting Impaired Emotion Recognition
4.3. Individual Differences and Precision Neurorehabilitation (Customized Care)
4.4. The Florida Affect Battery Performance
4.5. Study Limitations
5. Conclusions/Impact
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, C.L.; Milanak, M.E.; Judah, M.R.; Berenbaum, H. The association between PTSD and facial affect recognition. Psychiatry Res. 2018, 265, 298–302. [Google Scholar] [CrossRef]
- Marshall, A.D.; Robinson, L.R.; Azar, S.T. Cognitive and emotional contributors to intimate partner violence perpetration following trauma. J. Trauma Stress 2011, 24, 586–590. [Google Scholar] [CrossRef]
- Bornhofen, C.; McDonald, S. Comparing strategies for treating emotion perception deficits in traumatic brain injury. J. Head Trauma Rehabil. 2008, 23, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.L.; Williams, C.; Lewis, R. Role of alexithymia in suicide ideation after traumatic brain injury. J. Int. Neuropsychol. Soc. 2010, 16, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Biszak, A.M.; Babbage, D.R. Facial affect recognition difficulties in traumatic brain injury rehabilitation services. Brain Inj. 2014, 28, 97–104. [Google Scholar] [CrossRef]
- Murphy, J.M.; Bennett, J.M.; de la Piedad Garcia, X.; Willis, M.L. Emotion Recognition and Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2022, 32, 520–536. [Google Scholar] [CrossRef]
- Plana, I.; Lavoie, M.A.; Battaglia, M.; Achim, A.M. A meta-analysis and scoping review of social cognition performance in social phobia, posttraumatic stress disorder and other anxiety disorders. J. Anxiety Disord. 2014, 28, 169–177. [Google Scholar] [CrossRef]
- Passardi, S.; Peyk, P.; Rufer, M.; Plichta, M.M.; Mueller-Pfeiffer, C.; Wingenbach, T.S.H.; Hassanpour, K.; Schnyder, U.; Pfaltz, M.C. Impaired Recognition of Positive Emotions in Individuals with Posttraumatic Stress Disorder, Cumulative Traumatic Exposure, and Dissociation. Psychother. Psychosom. 2018, 87, 118–120. [Google Scholar] [CrossRef]
- Ryan-Gonzalez, C.; Kimbrel, N.A.; Meyer, E.C.; Gordon, E.M.; DeBeer, B.B.; Gulliver, S.B.; Elliott, T.R.; Morissette, S.B. Differences in Post-Traumatic Stress Disorder Symptoms among Post-9/11 Veterans with Blast- and Non-Blast Mild Traumatic Brain Injury. J. Neurotrauma 2019, 36, 1584–1590. [Google Scholar] [CrossRef]
- Troyanskaya, M.; Pastorek, N.J.; Scheibel, R.S.; Petersen, N.J.; McCulloch, K.; Wilde, E.A.; Henson, H.K.; Levin, H.S. Combat exposure, PTSD symptoms, and cognition following blast-related traumatic brain injury in OEF/OIF/OND service members and Veterans. Mil. Med. 2015, 180, 285–289. [Google Scholar] [CrossRef]
- MacDonald, C.L.; Johnson, A.M.; Nelson, E.C.; Werner, N.J.; Fang, R.; Flaherty, S.F.; Brody, D.L. Functional status after blast-plus-impact complex concussive traumatic brain injury in evacuated United States military personnel. J. Neurotrauma 2014, 31, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Sayer, N.A. Traumatic brain injury and its neuropsychiatric sequelae in war veterans. Annu. Rev. Med. 2012, 63, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.C.; Hagel, E.M.; Carlson, K.F.; Cifu, D.X.; Cutting, A.; Bidelspach, D.E.; Sayer, N.A. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med. Care 2012, 50, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kapoor, R. Multi-modal Expression Detection (MED): A cutting-edge review of current trends, challenges and solutions. Eng. Appl. Artif. Intell. 2023, 125, 106661. [Google Scholar] [CrossRef]
- Radice-Neumann, D.; Zupan, B.; Tomita, M.; Willer, B. Training emotional processing in persons with brain injury. J. Head Trauma Rehabil. 2009, 24, 313–323. [Google Scholar] [CrossRef]
- Neumann, D.; Babbage, D.R.; Zupan, B.; Willer, B. A randomized controlled trial of emotion recognition training after traumatic brain injury. J. Head Trauma Rehabil. 2015, 30, E12–E23. [Google Scholar] [CrossRef]
- Vallat-Azouvi, C.; Azouvi, P.; Le-Bornec, G.; Brunet-Gouet, E. Treatment of social cognition impairments in patients with traumatic brain injury: A critical review. Brain Inj. 2019, 33, 87–93. [Google Scholar] [CrossRef]
- Adolphs, R.; Tranel, D.; Damasio, A.R. Dissociable neural systems for recognizing emotions. Brain Cogn. 2003, 52, 61–69. [Google Scholar] [CrossRef]
- Adolphs, R.; Tranel, D. Amygdala damage impairs emotion recognition from scenes only when they contain facial expressions. Neuropsychologia 2003, 41, 1281–1289. [Google Scholar] [CrossRef]
- Neumann, D.; Sander, A.M.; Perkins, S.M.; Bhamidipalli, S.S.; Hammond, F.M. Negative Attribution Bias and Related Risk Factors After Brain Injury. J. Head Trauma Rehabil. 2021, 36, E61–E70. [Google Scholar] [CrossRef]
- Bowers, D.; Blonder, L.X.; Heilman, K.M. The Florida Affect Battery, Revised ed.; University of Florida: Gainesville, FL, USA, 1989. [Google Scholar]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.; Casey, J.E.; Wertheimer, J.; Fichtenberg, N.L. Reliability and validity of the RBANS in a traumatic brain injured sample. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2007, 22, 91–98. [Google Scholar] [CrossRef]
- Phillips, R.; Qi, G.; Collinson, S.L.; Ling, A.; Feng, L.; Cheung, Y.B.; Ng, T.P. The Minimum Clinically Important Difference in the Repeatable Battery for the Assessment of Neuropsychological Status. Clin. Neuropsychol. 2015, 29, 905–923. [Google Scholar] [CrossRef] [PubMed]
- Siew, S.K.H.; Han, M.F.Y.; Mahendran, R.; Yu, J. Regression-Based Norms and Validation of the Cambridge Neuropsychological Test Automated Battery among Community-Living Older Adults in Singapore. Arch. Clin. Neuropsychol. 2022, 37, 457–472. [Google Scholar] [CrossRef]
- Klekociuk, S.Z.; Summers, J.J.; Vickers, J.C.; Summers, M.J. Reducing false positive diagnoses in mild cognitive impairment: The importance of comprehensive neuropsychological assessment. Eur. J. Neurol. 2014, 21, 1330-e83. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.L.; Summers, M.J. Attention and working memory deficits in mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2010, 32, 350–357. [Google Scholar] [CrossRef]
- Strijkert, F.; Huitema, R.B.; Spikman, J.M. Measuring emotion recognition: Added value in diagnosing dementia of the Alzheimer’s disease type. J. Neuropsychol. 2022, 16, 263–282. [Google Scholar] [CrossRef]
- Neumann, D.; Malec, J.F.; Hammond, F.M. Negative Attribution Bias and Anger After Traumatic Brain Injury. J. Head Trauma Rehabil. 2017, 32, 197–204. [Google Scholar] [CrossRef]
- Ponsford, J.; Velikonja, D.; Janzen, S.; Harnett, A.; McIntyre, A.; Wiseman-Hakes, C.; Togher, L.; Teasell, R.; Kua, A.; Patsakos, E.; et al. INCOG 2.0 Guidelines for Cognitive Rehabilitation Following Traumatic Brain Injury, Part II: Attention and Information Processing Speed. J. Head Trauma Rehabil. 2023, 38, 38–51. [Google Scholar] [CrossRef]
- Sohlberg, M.M.; Turkstra, L. Optimizing Cognitive Rehabilitation; The Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Cicerone, K.D.; Goldin, Y.; Ganci, K.; Rosenbaum, A.; Wethe, J.V.; Langenbahn, D.M.; Malec, J.F.; Bergquist, T.F.; Kingsley, K.; Nagele, D.; et al. Evidence-Based Cognitive Rehabilitation: Systematic Review of the Literature From 2009 Through 2014. Arch. Phys. Med. Rehabil. 2019, 100, 1515–1533. [Google Scholar] [CrossRef]
- Williamson, J.; Isaki, E. Facial Affect Recognition Training Through Telepractice: Two Case Studies of Individuals with Chronic Traumatic Brain Injury. Int. J. Telerehabilitation 2015, 7, 13–20. [Google Scholar] [CrossRef]
- Guercio, J.M.; Podolska-Schroeder, H.; Rehfeldt, R.A. Using stimulus equivalence technology to teach emotion recognition to adults with acquired brain injury. Brain Inj. 2004, 18, 593–601. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.; Togher, L.; Tate, R.; Randall, R.; English, T.; Gowland, A. A randomised controlled trial evaluating a brief intervention for deficits in recognising emotional prosody following severe ABI. Neuropsychol. Rehabil. 2013, 23, 267–286. [Google Scholar] [CrossRef]
- Babbage, D.R.; Yim, J.; Zupan, B.; Neumann, D.; Tomita, M.R.; Willer, B. Meta-analysis of facial affect recognition difficulties after traumatic brain injury. Neuropsychology 2011, 25, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, H.; McDonald, S.; Dethier, M.; Kessels, R.P.; Westbrook, R.F. Facial emotion recognition deficits following moderate-severe Traumatic Brain Injury (TBI): Re-examining the valence effect and the role of emotion intensity. J. Int. Neuropsychol. Soc. 2014, 20, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Cowie, E.; Cox, C.; Martin, J.C.; Devillers, L.; Cowie, R.; Sneddon, I.; McRorie, M.; Pelachaud, C.; Peters, C.; Lowry, O. Cowie , R., Pelachaud, C., Petta, P., Eds.; The HUMAINE Database. In Emotion-Oriented Systems. Cognitive Technologies; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Lin, D.J.; Backus, D.; Chakraborty, S.; Liew, S.L.; Valero-Cuevas, F.J.; Patten, C.; Cotton, R.J. Transforming modeling in neurorehabilitation: Clinical insights for personalized rehabilitation. J. Neuroeng. Rehabil. 2024, 21, 18. [Google Scholar] [CrossRef]
- Mateer, C.A.; Sira, C.S.; O’Connell, M.E. Putting Humpty Dumpty together again: The importance of integrating cognitive and emotional interventions. J. Head Trauma Rehabil. 2005, 20, 62–75. [Google Scholar] [CrossRef]
- Perez-Marcos, D.; Bieler-Aeschlimann, M.; Serino, A. Virtual Reality as a Vehicle to Empower Motor-Cognitive Neurorehabilitation. Front Psychol. 2018, 9, 2120. [Google Scholar] [CrossRef]
- Neumann, D.; Malec, J.F.; Hammond, F.M. Reductions in Alexithymia and Emotion Dysregulation After Training Emotional Self-Awareness Following Traumatic Brain Injury: A Phase I Trial. J. Head Trauma Rehabil. 2017, 32, 286–295. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Maisto, M.; Montana, J.I.; Mavrodiev, P.A.; Baglio, F.; Rossetto, F.; Mantovani, F.; Riva, G.; Realdon, O. The Role of Engagement in Teleneurorehabilitation: A Systematic Review. Front. Neurol. 2020, 11, 354. [Google Scholar] [CrossRef]
- MacCann, C.; Roberts, R.D. New paradigms for assessing emotional intelligence: Theory and data. Emotion 2008, 8, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Banziger, T.; Mortillaro, M.; Scherer, K.R. Introducing the Geneva Multimodal expression corpus for experimental research on emotion perception. Emotion 2012, 12, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, K.; Scherer, K.R. Introducing a short version of the Geneva Emotion Recognition Test (GERT-S): Psychometric properties and construct validation. Behav. Res. Methods 2016, 48, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.E.; Ciarleglio, M.M.; Aslan, M.; Marx, B.P.; Ko, J.; Concato, J.; Proctor, S.P.; Vasterling, J.J. Associations Among Increases in Posttraumatic Stress Symptoms, Neurocognitive Performance, and Long-Term Functional Outcomes in U.S. Iraq War Veterans. J. Trauma. Stress 2021, 34, 628–640. [Google Scholar] [CrossRef]
- Pieper, J.; Chang, D.G.; Mahasin, S.Z.; Swan, A.R.; Quinto, A.A.; Nichols, S.L.; Diwakar, M.M.; Huang, C.; Swan, J.; Lee, R.; et al. Brain Amygdala Volume Increases in Veterans and Active-Duty Military Personnel With Combat-Related Posttraumatic Stress Disorder and Mild Traumatic Brain Injury. J. Head Trauma Rehabil. 2020, 35, E1–E9. [Google Scholar] [CrossRef]
- Vasterling, J.J.; Aslan, M.; Lee, L.O.; Proctor, S.P.; Ko, J.; Jacob, S.; Concato, J. Longitudinal Associations among Posttraumatic Stress Disorder Symptoms, Traumatic Brain Injury, and Neurocognitive Functioning in Army Soldiers Deployed to the Iraq War. J. Int. Neuropsychol. Soc. 2018, 24, 311–323. [Google Scholar] [CrossRef]
- Hardy, M.S.; Kennedy, J.E.; Cooper, D.B. Patient Attribution of Posttraumatic Symptoms to Brain Injury Versus PTSD in Military-Related Mild TBI. J. Neuropsychiatry Clin. Neurosci. 2020, 32, 252–258. [Google Scholar] [CrossRef]
| Domain (Number of Items) | Domain Description |
|---|---|
| Affect (facial emotion recognition tasks) | |
| 1 (20) | Identify whether two faces with neutral emotions are the same or different people. |
| 2 (20) | Identify whether two different people are facially expressing the same or different emotion. |
| 3 (20) | Identify whether the facial expression of a face is ‘happy’, ‘sad’, ‘angry’, ‘frightened’, or ‘neutral’. |
| 4 (20) | From five faces expressing different emotions, identify which one is expressing the target emotion given to you. |
| 5 (20) | Identify the facial emotion on the left facial photo, then select the corresponding facial emotion from among five photos on the right side of the screen. |
| Prosody (verbal/vocal emotion recognition tasks) | |
| 6 (16) | Listen to two different spoken sentences and identify whether the two sentences are the same (i.e., both a question or both a statement) or different (one is a question and one is a statement). |
| 7 (20) | Listen to two different spoken sentences and identify if they are both expressing the same or different emotions. |
| 8a (20) | For a given spoken sentence, identify the emotion expressed by the speaker (happy, sad, frightened, angry, or neutral). |
| 8b | For a given spoken sentence, listen to how the sentence is said, not what is said. Identify the emotion expressed by the speaker (happy, sad, frightened, angry, or neutral). |
| 8b1 ** (12) | Congruent items match emotion tone with content. |
| 8b2 ** (24) | Incongruent items do not match emotion tone with content. |
| Combined Affect and Prosody (facial and verbal/vocal combined emotion recognition tasks) | |
| 9 | Listen to a sentence spoken in an emotional tone of voice and then select the facial expression (from three facial photos) that corresponds to the spoken sentence. |
| 10 | Inspect a photo showing a facial expression of emotion, then select from among three different spoken sentences, the one sentence that expresses the same emotion as the face. |
| Characteristics | Age | Race | Vocational Status | Years of Education | Number of mTBI | Years from Last mTBI | PCL-5 Total Score | BDI-II Total Score |
|---|---|---|---|---|---|---|---|---|
| S1 | 50 | African/ American | Unemployed | Some College | 8 | 7 | 60 | 45 Severe |
| S2 | 50 | African/ American | Volunteer 4 days/month | Master’s Degree | 3 | 9 | 66 | 38 Severe |
| S3 | 48 | African/ American | Unemployed | Some College | 3 | 9 | 54 | 28 Moderate |
| S4 | 44 | African/ American | Unemployed | High School | 3 | 12 | 47 | 35 Severe |
| S5 | 40 | African/ American | Unemployed | Some College | 3 | 11 | 79 | 53 Severe |
| S6 | 38 | Caucasion | Full-time | Master’s Degree | 2 | 13 | 26 | 16 Mild |
| S7 | 53 | Caucasion | Unemployed | Bachelor’s degree | 1 | 16 | 28 | 16 Mild |
| S8 | 31 | Caucasion | Part-time | Some College | 2 | 10 | 57 | 37 Severe |
| Participant Number | Participant Report |
|---|---|
| 1 | “I think this is helping. I was taught from the age of 19 to kill a man, so this is a big departure from what I have done for many years. I only have given my family the angry facial expression. Now I try to use the happy facial and voice expression and my son hugged me out the blue this weekend. He hasn’t done that since my last deployment.” |
| 2 | “The role you play in society changes your behavior. I have become more involved in my church leadership and find that I need to have a more approachable demeaner. This is helping me become more aware of that.” |
| 3 | “I have the most difficulty expressing fear because we are taught as a soldier to get angry and aggressive when we feel fear. After training I have started to look at peoples’ emotions more carefully. I used to just look for their angry expression in order to avoid them.” |
| 4 | “I still have trouble controlling my emotions when I get mad, but now I am more aware of my expressions when I do get mad.” |
| 5 | “…confused about the emotions I am feeling after training. Now I notice the emotions I am expressing but don’t know how they got there. Have started to ask myself what I should be feeling.” |
| 6 | “…have become aware that my speech is more halted when I am out in public, even with people I know.” |
| 7 | “I feel like I have always been good at distinguishing facial expressions but now I am more aware of the tone of voice people are expressing. I am more aware of how I am expressing myself to others with both my facial and voice expressions. My wife says that I rarely shared my emotions before and now I state how I am feeling. She said my facial expressions were flat and now I seem more aware and use a greater variety of facial expressions.” |
| 8 | “I have been having difficulty with relationships with family and friends. Now I step back and try to process what they are expressing.” |
| A. Subject | B. Number of Domains Impaired at Baseline (Domain Number #) | C. Number of “Baseline-Impaired” | D. Number of ‘Normal Baseline’ Domains, Declined (Domain Number #) | |
|---|---|---|---|---|
| Domains Improved (Domain Number #) | Domains, No Change (Domain Number #) | |||
| S1 | 5 (#’s 5, 6, 8a, 9, 10) | 3 (#’s 5, 8a, 9) | 2 #’s (6, 10) | 1 (#3) |
| S2 | 1 (#10) | 0 | 1 (# 10) | 0 |
| S3 | 2 (#’s 9, 10) | 2 (#’s 9, 10) | 0 | 0 |
| S4 | 2 (#’s 4, 10) | 0 | 2 (#’s 4, 10) | 0 |
| S5 | 7 (#s 1, 3, 4, 5, 6, 8a,10) | 5 (#s 1, 3, 5, 6, 8a) | 2 (#’s 4, 10) | 0 |
| S6 | 2 (#’s 4, 10) | 2 (#’s 4, 10) | 0 | 2 (#’s 2, 8b1) |
| S7 | 4 (#’s 7, 8a, 8b1, 10) | 3 (#’s 7, 8a, 8b1) | 1 (#10) | 1 (#5) |
| S8 | 1 (# 9) | 1 (# 9) | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waid-Ebbs, J.K.; Lewandowski, K.; Zhang, Y.; Graham, S.; Daly, J.J. Response to Training in Emotion Recognition Function for Mild TBI/PTSD Survivors: Pilot Study. Brain Sci. 2025, 15, 728. https://doi.org/10.3390/brainsci15070728
Waid-Ebbs JK, Lewandowski K, Zhang Y, Graham S, Daly JJ. Response to Training in Emotion Recognition Function for Mild TBI/PTSD Survivors: Pilot Study. Brain Sciences. 2025; 15(7):728. https://doi.org/10.3390/brainsci15070728
Chicago/Turabian StyleWaid-Ebbs, J. Kay, Kristen Lewandowski, Yi Zhang, Samantha Graham, and Janis J. Daly. 2025. "Response to Training in Emotion Recognition Function for Mild TBI/PTSD Survivors: Pilot Study" Brain Sciences 15, no. 7: 728. https://doi.org/10.3390/brainsci15070728
APA StyleWaid-Ebbs, J. K., Lewandowski, K., Zhang, Y., Graham, S., & Daly, J. J. (2025). Response to Training in Emotion Recognition Function for Mild TBI/PTSD Survivors: Pilot Study. Brain Sciences, 15(7), 728. https://doi.org/10.3390/brainsci15070728






