Quercetin and Tranylcypromine Improve Memory, Behavioral Performance, and Cholinergic Function in Male Rats Subjected to Chronic Restraint Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Equipment
2.2. Animals and Ethical Approval
2.3. Experimental Protocol
2.3.1. Chronic Restraint Stress (CRS) Model
2.3.2. Treatments
2.3.3. Experimental Design
2.4. Sample Collection
2.5. Body Weight and Water Consumption
2.6. Behavioral Tasks
2.6.1. Sucrose Preference Test (SPT)
2.6.2. Open Field Test (OFT)
2.6.3. Novel Object Recognition (NOR) Test
2.6.4. Elevated Plus Maze (EPM)
2.6.5. Forced Swim Test (FST)
2.7. Enzymatic Assays
Acetylcholinesterase (AChE) Enzymatic Activity
2.8. Molecular Assays
2.8.1. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8.2. Immunoblotting
2.9. Statistical Analysis
3. Results
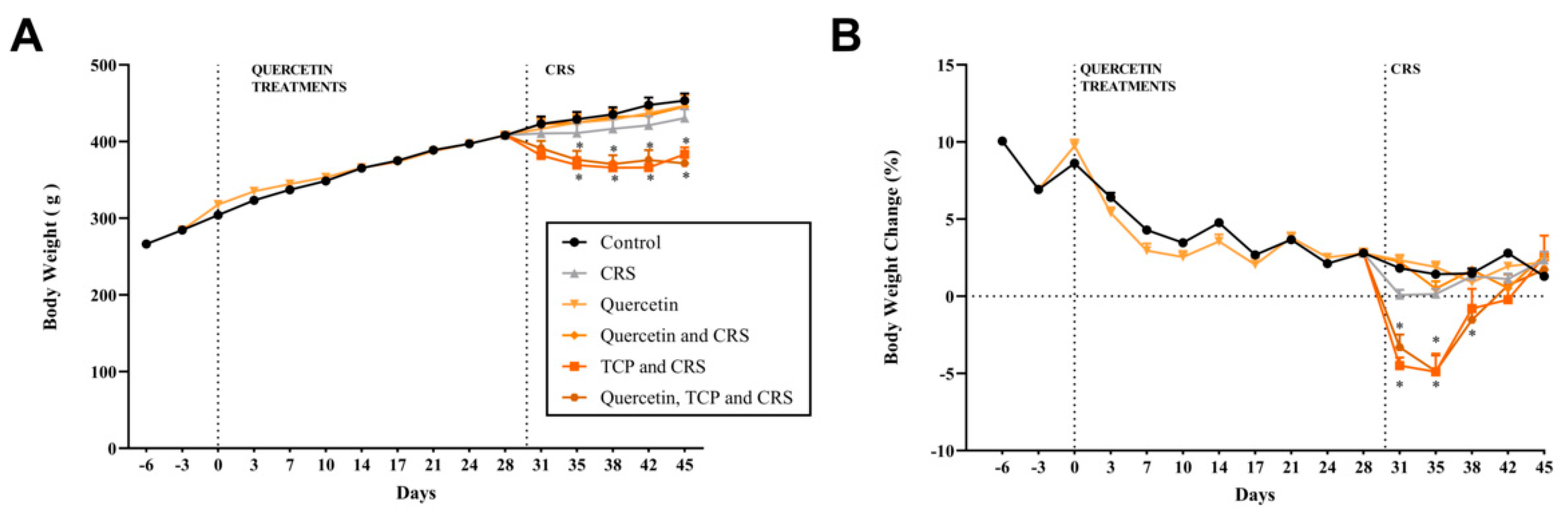
3.1. Alterations in Body Weight, Water Consumption, and Sucrose Preference in Animals Subjected to CRS
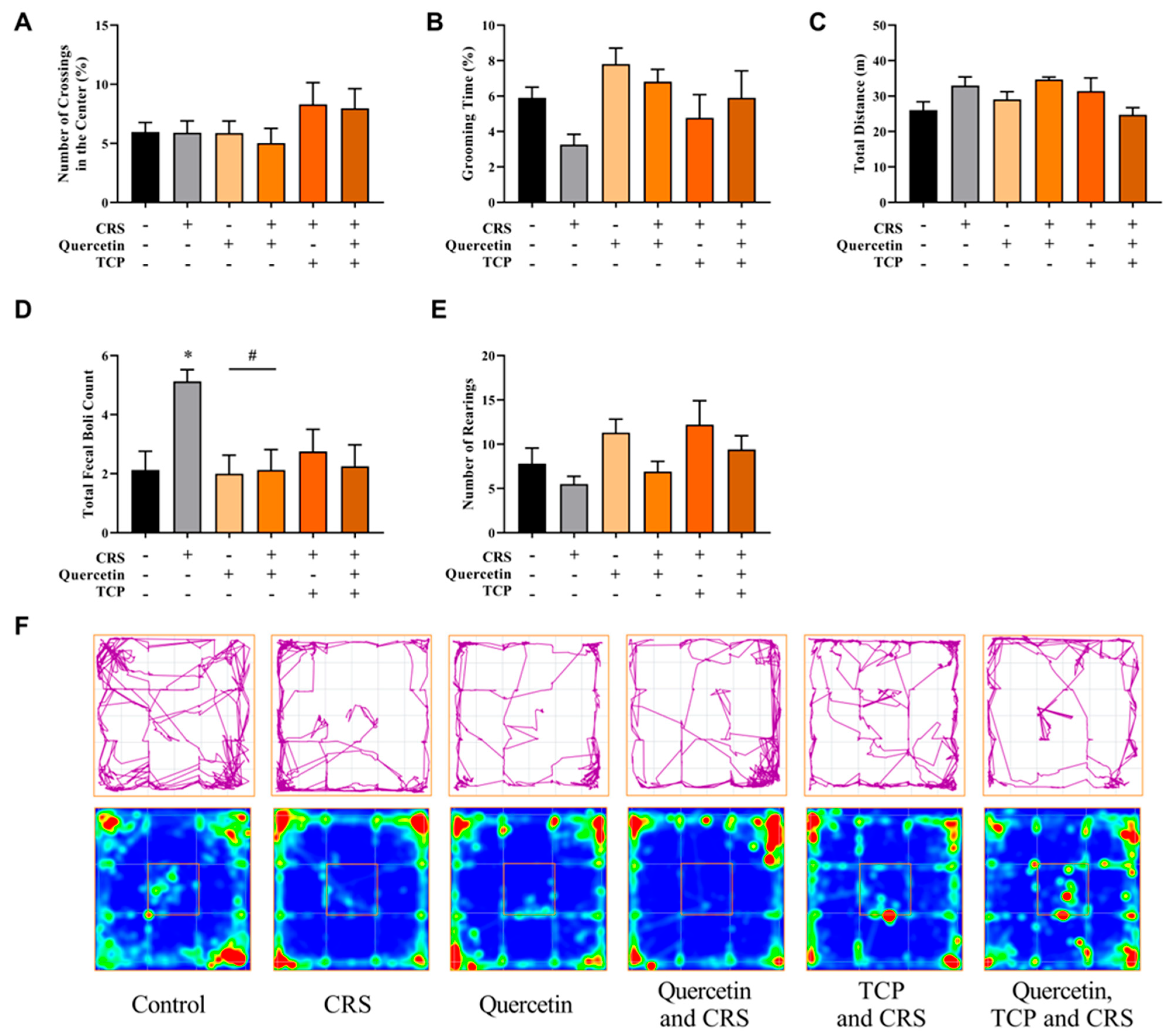
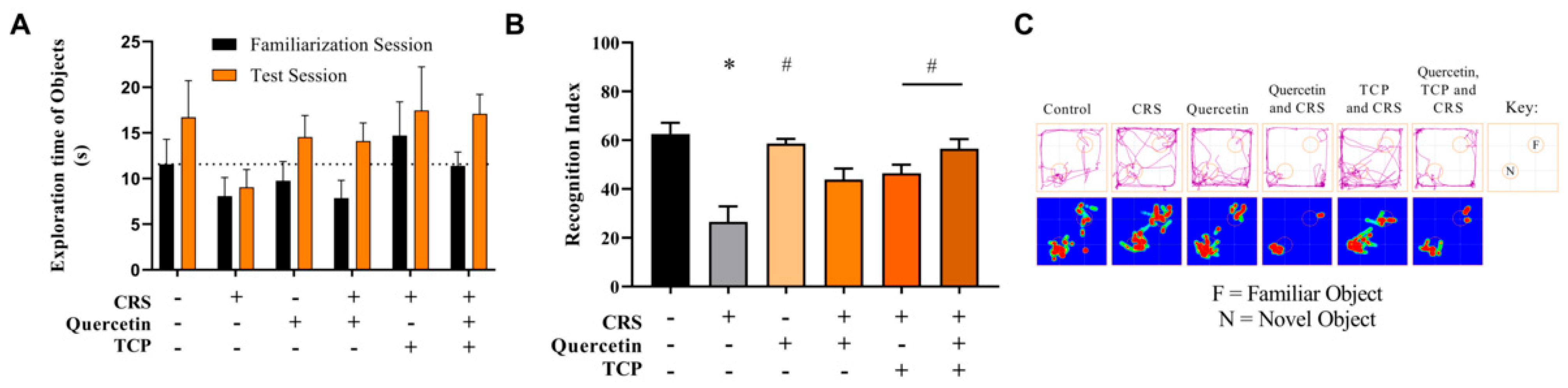
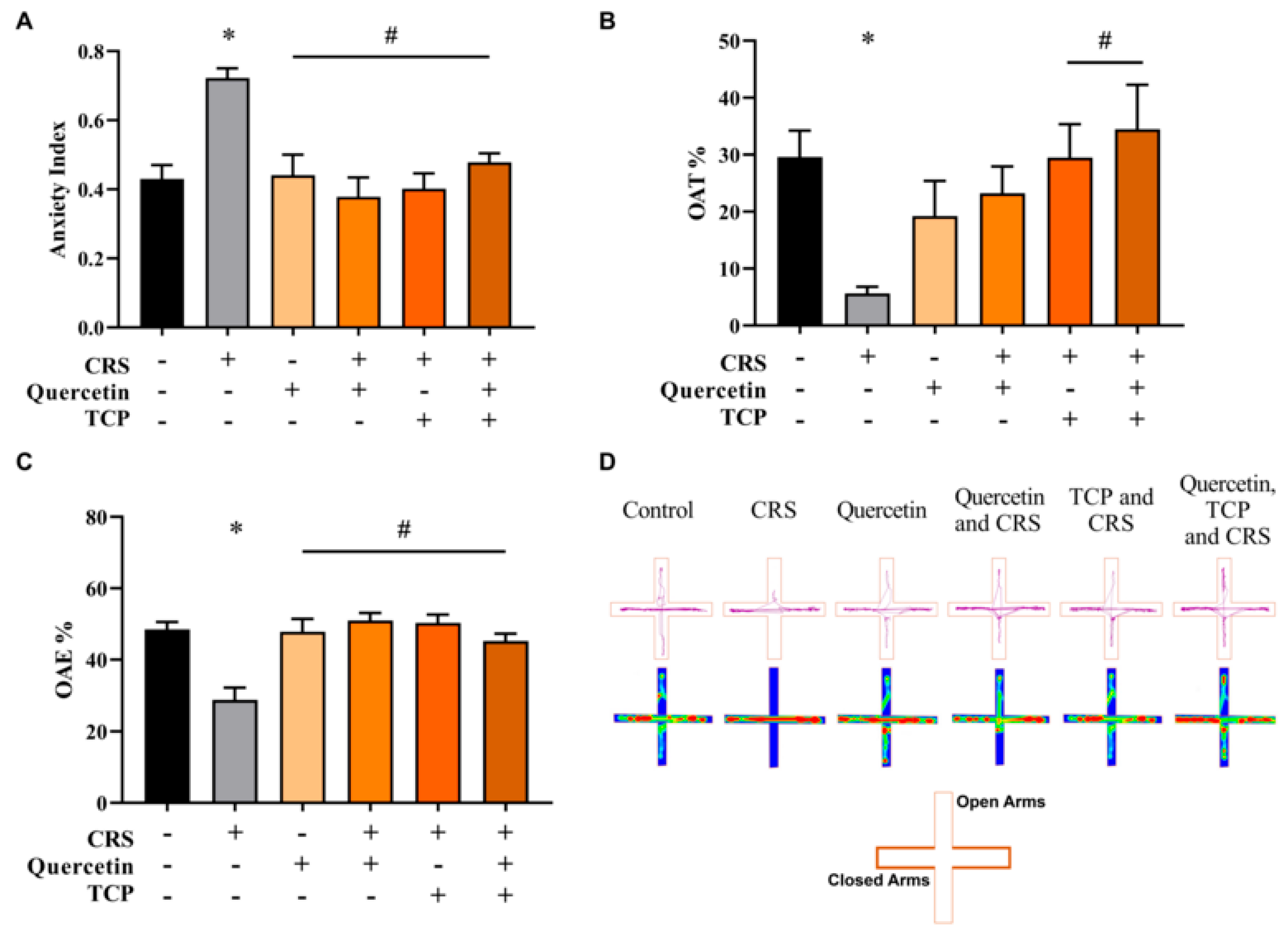
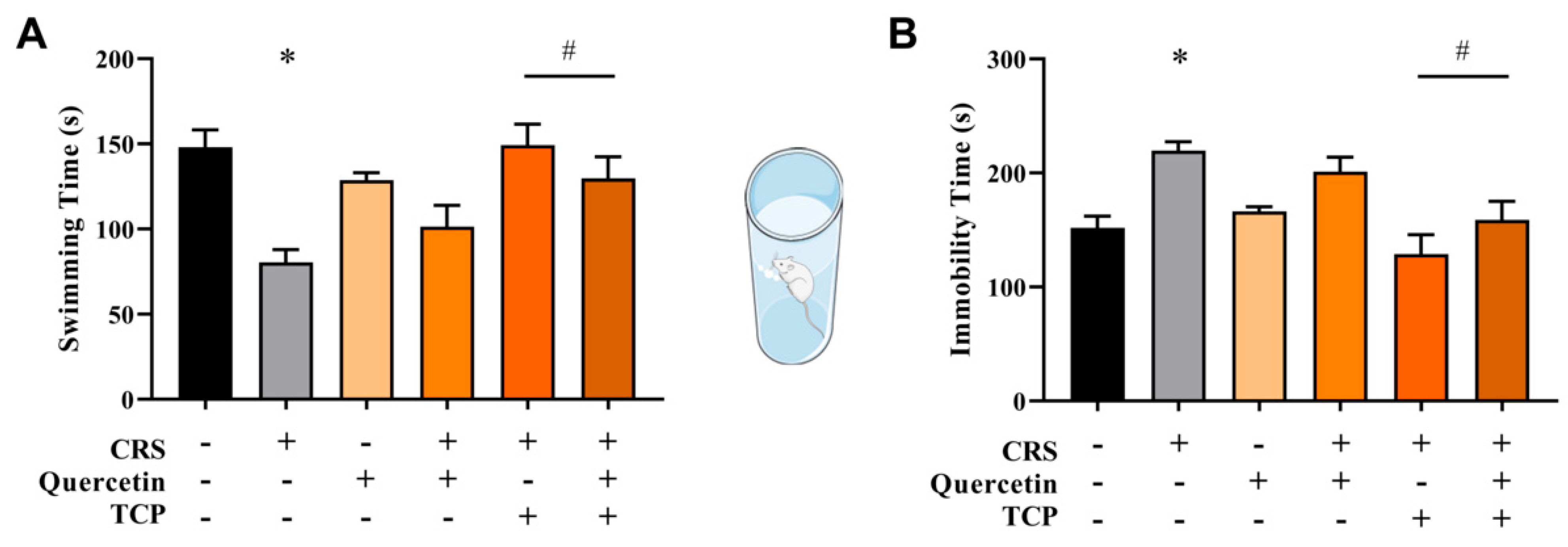
3.2. Quercetin and TCP Interventions Improve Memory, Depression-like, and Anxiety-like Behaviors in Male Rats Subjected to CRS
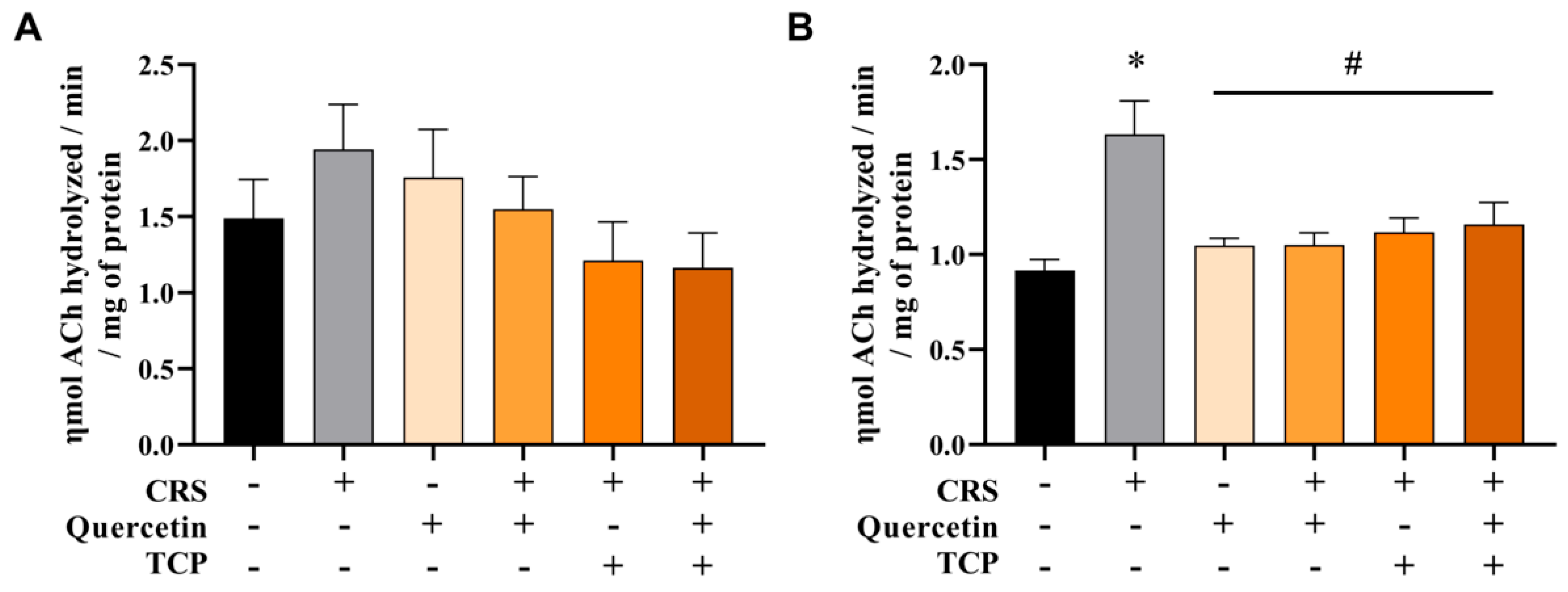
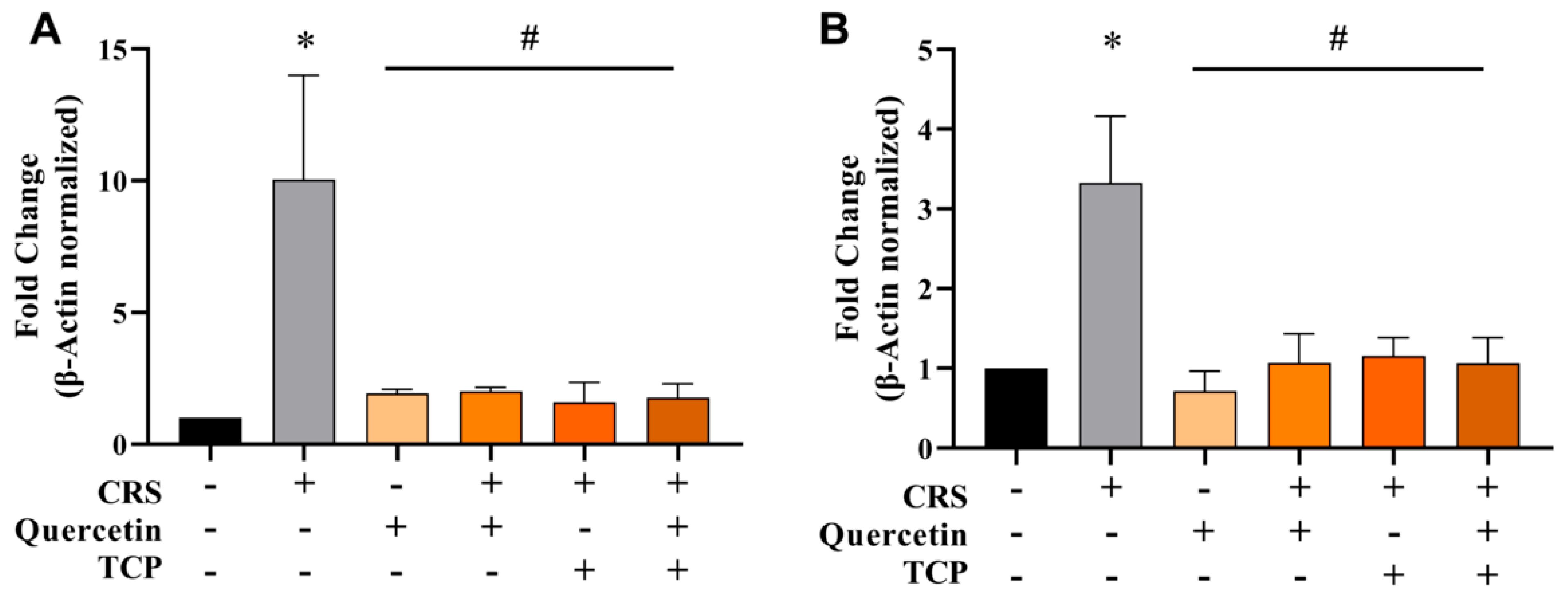
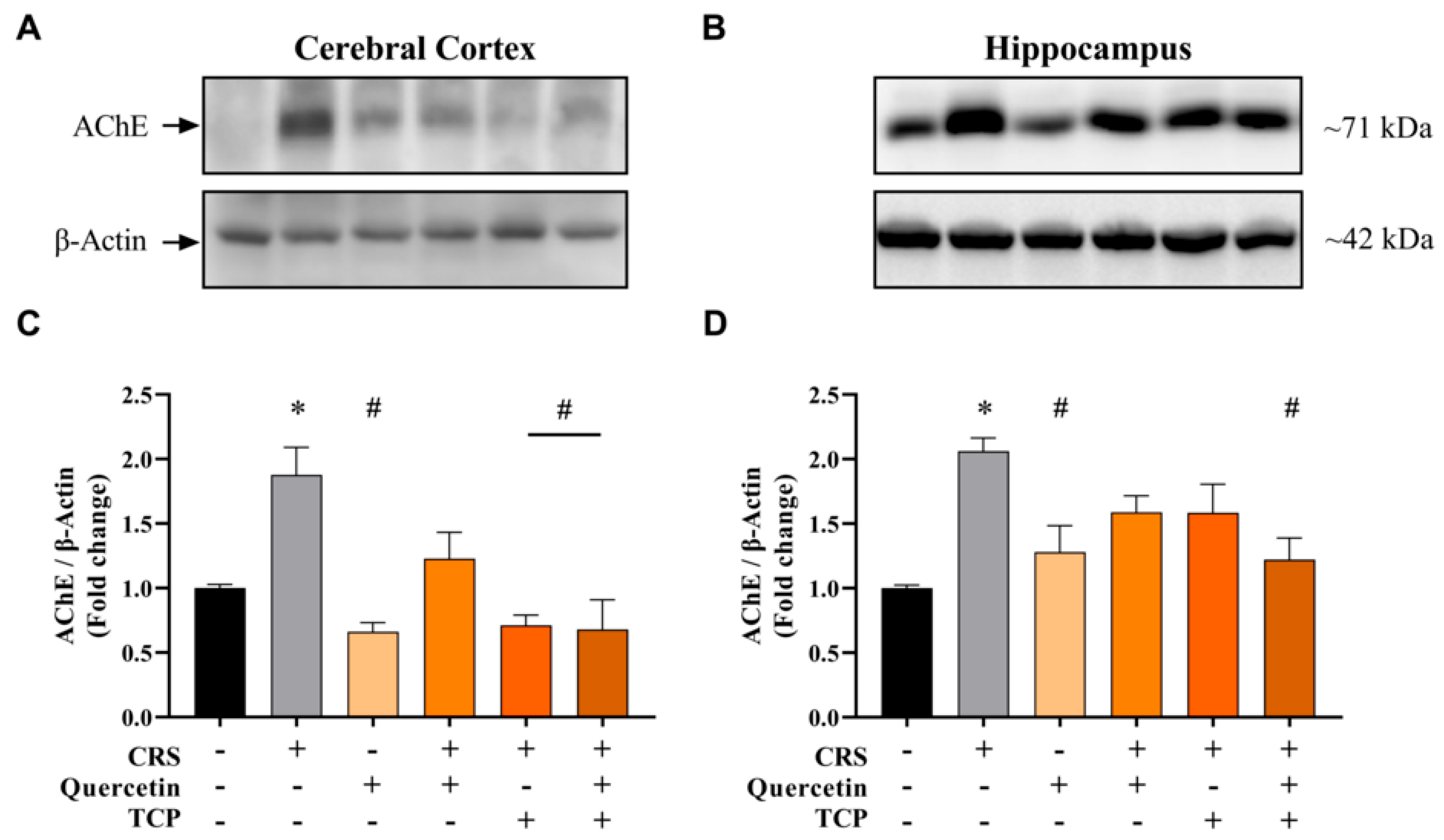
3.3. CRS-Induced Alterations in Cholinergic Parameters Are Reversed by Quercetin and TCP Treatments in the Brain of Male Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 7 March 2025).
- Kennedy, S.H. Core symptoms of major depressive disorder: Relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008, 10, 271–277. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Villas Boas, G.R.; Boerngen de Lacerda, R.; Paes, M.M.; Gubert, P.; Almeida, W.L.D.C.; Rescia, V.C.; de Carvalho, P.M.G.; de Carvalho, A.A.V.; Oesterreich, S.A. Molecular aspects of depression: A review from neurobiology to treatment. Eur. J. Pharmacol. 2019, 851, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Dagytė, G.; Den Boer, J.A.; Trentani, A. The cholinergic system and depression. Behav. Brain Res. 2011, 221, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Dulawa, S.C.; Janowsky, D.S. Cholinergic regulation of mood: From basic and clinical studies to emerging therapeutics. Mol. Psychiatry 2019, 24, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Thakare, V.N.; Dhakane, V.D.; Patel, B.M. Attenuation of acute restraint stress-induced depressive like behavior and hippocampal alterations with protocatechuic acid treatment in mice. Metab. Brain Dis. 2017, 32, 401–413. [Google Scholar] [CrossRef]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61 (Suppl. 6), 7–11. [Google Scholar]
- Ramaholimihaso, T.; Bouazzaoui, F.; Kaladjian, A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence-A Narrative Review. Front. Psychiatry 2020, 11, 572533. [Google Scholar] [CrossRef]
- Park, H.; Han, K.M.; Jeon, H.; Lee, J.S.; Lee, H.; Jeon, S.G.; Park, J.H.; Kim, Y.G.; Lin, Y.; Lee, Y.H.; et al. The MAO Inhibitor Tranylcypromine Alters LPS- and Aβ-Mediated Neuroinflammatory Responses in Wild-type Mice and a Mouse Model of AD. Cells 2020, 9, 1982. [Google Scholar] [CrossRef]
- Tomaz, V.S.; Chaves Filho, A.J.M.; Cordeiro, R.C.; Jucá, P.M.; Soares, M.V.R.; Barroso, P.N.; Cristino, L.M.F.; Jiang, W.; Teixeira, A.L.; de Lucena, D.F.; et al. Antidepressants of different classes cause distinct behavioral and brain pro- and anti-inflammatory changes in mice submitted to an inflammatory model of depression. J. Affect. Disord. 2020, 268, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Lee, S.A.; Hong, S.S.; Lee, K.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Monoamine oxidase inhibitory components from the roots of Sophora flavescens. Arch. Pharm. Res. 2005, 28, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Hong, S.S.; Hwang, J.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Monoamine oxidase inhibitory components from Cayratia japonica. Arch. Pharm. Res. 2007, 30, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Saaby, L.; Rasmussen, H.B.; Jäger, A.K. MAO-A inhibitory activity of quercetin from Calluna vulgaris (L.) Hull. J. Ethnopharmacol. 2009, 121, 178–181. [Google Scholar] [CrossRef]
- Yoshino, S.; Hara, A.; Sakakibara, H.; Kawabata, K.; Tokumura, A.; Ishisaka, A.; Kawai, Y.; Terao, J. Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria. Nutrition 2011, 27, 847–852. [Google Scholar] [CrossRef]
- Bandaruk, Y.; Mukai, R.; Kawamura, T.; Nemoto, H.; Terao, J. Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria. J. Agric. Food Chem. 2012, 60, 10270–10277. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods, 3rd ed.; U.S. Department of Agriculture: Sacramento, CA, USA, 2011; pp. 1–156. [Google Scholar]
- Silvestro, S.; Bramanti, P.; Mazzon, E. Role of Quercetin in Depressive-Like Behaviors: Findings from Animal Models. Appl. Sci. 2021, 11, 7116. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yao, L.; Gu, W.; Zhao, S.; Shen, Z.; Lin, Z.; Liu, W.; Yan, T. Pharmacological Activity of Quercetin: An Updated Review. Evid. Based Complement. Altern. Med. 2022, 2022, 3997190. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Gao, Y.; Nie, K.; Wang, H.; Su, H.; Wang, Z.; Lu, F.; Huang, W.; Dong, H. Antidepressant Potential of Quercetin and its Glycoside Derivatives: A Comprehensive Review and Update. Front. Pharmacol. 2022, 13, 865376. [Google Scholar] [CrossRef]
- Abdalla, F.H.; Schmatz, R.; Cardoso, A.M.; Carvalho, F.B.; Baldissarelli, J.; de Oliveira, J.S.; Rosa, M.M.; Gonçalves Nunes, M.A.; Rubin, M.A.; da Cruz, I.B.; et al. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na(+),K(+)-ATPase activities. Physiol. Behav. 2014, 135, 152–167. [Google Scholar] [CrossRef]
- Maciel, R.M.; Carvalho, F.B.; Olabiyi, A.A.; Schmatz, R.; Gutierres, J.M.; Stefanello, N.; Zanini, D.; Rosa, M.M.; Andrade, C.M.; Rubin, M.A.; et al. Neuroprotective effects of quercetin on memory and anxiogenic-like behavior in diabetic rats: Role of ectonucleotidases and acetylcholinesterase activities. Biomed. Pharmacother. 2016, 84, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.M.; Canas, P.M.; Oliveira, C.R.; Cunha, R.A. Increased density and synapto-protective effect of adenosine A2A receptors upon sub-chronic restraint stress. Neuroscience 2006, 141, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.; Lopes, C.R.; Gonçalves, F.Q.; Nunes, A.; Pochmann, D.; Machado, N.J.; Tomé, A.R.; Agostinho, P.; Cunha, R.A. Crosstalk Between ATP-P2X7 and Adenosine A2A Receptors Controlling Neuroinflammation in Rats Subject to Repeated Restraint Stress. Front. Cell. Neurosci. 2021, 15, 639322. [Google Scholar] [CrossRef]
- Pereira Braga, C.; Momentti, A.C.; Barbosa Peixoto, F.; de Fátima Ferreira Baptista, R.; dos Santos, F.A.; Fava, F.H.; Fernandes, A.A. Influence of treatment with quercetin on lipid parameters and oxidative stress of pregnant diabetic rats. Can. J. Physiol. Pharmacol. 2013, 91, 171–177. [Google Scholar] [CrossRef] [PubMed]
- De Mattos, B.D.S.; Soares, M.S.P.; Spohr, L.; Pedra, N.S.; Teixeira, F.C.; de Souza, A.A.; Stefanello, F.M.; Baldissarelli, J.; Gamaro, G.D.; Spanevello, R.M. Quercetin prevents alterations of behavioral parameters, delta-aminolevulinic dehydratase activity, and oxidative damage in brain of rats in a prenatal model of autism. Int. J. Dev. Neurosci. 2020, 80, 287–302. [Google Scholar] [CrossRef]
- Gorbenko, N.I.; Borikov, O.Y.; Kiprych, T.V.; Ivanova, O.V.; Taran, K.V.; Litvinova, T.S. Quercetin improves myocardial redox status in rats with type 2 diabetes. Endocr. Regul. 2021, 55, 142–152. [Google Scholar] [CrossRef]
- Greenshaw, A.J.; Nazarali, A.J.; Rao, T.S.; Baker, G.B.; Coutts, R.T. Chronic tranylcypromine treatment induces functional alpha 2-adrenoceptor down-regulation in rats. Eur. J. Pharmacol. 1988, 154, 67–72. [Google Scholar] [CrossRef]
- Malyszko, J.; Urano, T.; Takada, Y.; Takada, A. Serotonergic systems in brain and blood under stress and tranylcypromine treatment in rats. Brain Res. Bull. 1994, 35, 9–13. [Google Scholar] [CrossRef]
- Castro, M.F.V.; Assmann, C.E.; Stefanello, N.; Reichert, K.P.; Palma, T.V.; da Silva, A.D.; Miron, V.V.; Mostardeiro, V.B.; Morsch, V.M.M.; Schetinger, M.R.C. Caffeic acid attenuates neuroinflammation and cognitive impairment in streptozotocin-induced diabetic rats: Pivotal role of the cholinergic and purinergic signaling pathways. J. Nutr. Biochem. 2023, 115, 109280. [Google Scholar] [CrossRef]
- Carvalho, F.B.; Gutierres, J.M.; Bueno, A.; Agostinho, P.; Zago, A.M.; Vieira, J.; Frühauf, P.; Cechella, J.L.; Nogueira, C.W.; Oliveira, S.M.; et al. Anthocyanins control neuroinflammation and consequent memory dysfunction in mice exposed to lipopolysaccharide. Mol. Neurobiol. 2017, 54, 3350–3367. [Google Scholar] [CrossRef]
- Miron, V.V.; Assmann, C.E.; Mostardeiro, V.B.; da Silveira, M.V.; Copetti, P.M.; Bissacotti, B.F.; Schirmann, A.A.; Castro, M.F.V.; Gutierres, J.M.; da Cruz Fernandes, M.; et al. Neuroprotective effect of long-term resistance physical exercise against memory damage elicited by a lipopolysaccharide-induced neuroinflammation model in male rats. J. Neurosci. Res. 2024, 102, e25370. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.T.K.; Marques, L.S.; Zborowski, V.A.; Silva, G.L.; Nogueira, C.W.; Zeni, G. Resistance Training Modulates Hippocampal Neuroinflammation and Protects Anxiety-Depression-like Dyad Induced by an Emotional Single Prolonged Stress Model. Mol. Neurobiol. 2023, 60, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, 52587. [Google Scholar] [CrossRef]
- Ellman, G.l.; Courtney, K.d.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Assmann, C.E.; Mostardeiro, V.B.; Weis, G.C.C.; Reichert, K.P.; de Oliveira Alves, A.; Miron, V.V.; Bagatini, M.D.; Palma, T.V.; de Andrade, C.M.; Pillat, M.M.; et al. Aluminum-Induced Alterations in Purinergic System Parameters of BV-2 Brain Microglial Cells. J. Immunol. Res. 2021, 2021, 2695490. [Google Scholar] [CrossRef]
- Rebola, N.; Simões, A.P.; Canas, P.M.; Tomé, A.R.; Andrade, G.M.; Barry, C.E.; Agostinho, P.M.; Lynch, M.A.; Cunha, R.A. Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J. Neurochem. 2011, 117, 100–111. [Google Scholar] [CrossRef]
- Tian, H.; Hu, Z.; Xu, J.; Wang, C. The molecular pathophysiology of depression and the new therapeutics. MedComm 2022, 3, e156. [Google Scholar] [CrossRef]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef] [PubMed]
- Seewoo, B.J.; Hennessy, L.A.; Feindel, K.W.; Etherington, S.J.; Croarkin, P.E.; Rodger, J. Validation of Chronic Restraint Stress Model in Young Adult Rats for the Study of Depression Using Longitudinal Multimodal MR Imaging. eNeuro 2020, 7, ENEURO.0113-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Xu, Y.; Yuan, X. Validity of chronic restraint stress for modeling anhedonic-like behavior in rodents: A systematic review and meta-analysis. J. Int. Med. Res. 2022, 50, 3000605221075816. [Google Scholar] [CrossRef] [PubMed]
- Olave, F.A.; Aguayo, F.I.; Román-Albasini, L.; Corrales, W.A.; Silva, J.P.; González, P.I.; Lagos, S.; García, M.A.; Alarcón-Mardones, M.; Rojas, P.S.; et al. Chronic restraint stress produces sex-specific behavioral and molecular outcomes in the dorsal and ventral rat hippocampus. Neurobiol. Stress 2022, 17, 100440. [Google Scholar] [CrossRef]
- Pansarim, V.; Leite-Panissi, C.R.A.; Schmidt, A. Chronic restraint stress alters rat behavior depending on sex and duration of stress. Behav. Processes 2023, 207, 104856. [Google Scholar] [CrossRef]
- Assareh, N.; ElBatsh, M.M.; Marsden, C.A.; Kendall, D.A. The effects of chronic administration of tranylcypromine and rimonabant on behaviour and protein expression in brain regions of the rat. Pharmacol. Biochem. Behav. 2012, 100, 506–512. [Google Scholar] [CrossRef]
- Shemesh, A.; Abdulla, A.; Yang, F.; Chua, S.C.; Pessin, J.E.; Zong, H. The antidepressant trans-2-phenylcyclopropylamine protects mice from high-fat-diet-induced obesity. PLoS ONE 2014, 9, e89199. [Google Scholar] [CrossRef]
- Carpéné, C.; Boulet, N.; Chaplin, A.; Mercader, J. Past, Present and Future Anti-Obesity Effects of Flavin-Containing and/or Copper-Containing Amine Oxidase Inhibitors. Medicines 2019, 6, 9. [Google Scholar] [CrossRef]
- Slattery, D.A.; Cryan, J.F. Modelling depression in animals: At the interface of reward and stress pathways. Psychopharmacology 2017, 234, 1451–1465. [Google Scholar] [CrossRef]
- Markov, D.D. Sucrose Preference Test as a Measure of Anhedonic Behavior in a Chronic Unpredictable Mild Stress Model of Depression: Outstanding Issues. Brain Sci. 2022, 12, 1287. [Google Scholar] [CrossRef]
- Berger, S.; Gureczny, S.; Reisinger, S.N.; Horvath, O.; Pollak, D.D. Effect of Chronic Corticosterone Treatment on Depression-Like Behavior and Sociability in Female and Male C57BL/6N Mice. Cells 2019, 8, 1018. [Google Scholar] [CrossRef] [PubMed]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef]
- Taché, Y.; Million, M. Role of Corticotropin-releasing Factor Signaling in Stress-related Alterations of Colonic Motility and Hyperalgesia. J. Neurogastroenterol. Motil. 2015, 21, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F.; Autore, G. Effects of quercetin on the gastrointestinal tract in rats and mice. Phytother. Res. 1994, 8, 42–45. [Google Scholar] [CrossRef]
- Ausderau, K.K.; Colman, R.J.; Kabakov, S.; Schultz-Darken, N.; Emborg, M.E. Evaluating depression- and anxiety-like behaviors in non-human primates. Front. Behav. Neurosci. 2023, 16, 1006065. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Ubgade, A.; Quazi, M.; Umathe, S.; Mundhada, D. Reversal by quercetin of corticotrophin releasing factor induced anxiety- and depression-like effect in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 955–960. [Google Scholar] [CrossRef]
- Toumi, M.L.; Merzoug, S.; Baudin, B.; Tahraoui, A. Quercetin alleviates predator stress-induced anxiety-like and brain oxidative signs in pregnant rats and immune count disturbance in their offspring. Pharmacol. Biochem. Behav. 2013, 107, 1–10. [Google Scholar] [CrossRef]
- Samad, N.; Saleem, A.; Yasmin, F.; Shehzad, M.A. Quercetin protects against stress-induced anxiety- and depression-like behavior and improves memory in male mice. Physiol. Res. 2018, 67, 795–808. [Google Scholar] [CrossRef]
- Ge, C.; Wang, S.; Wu, X.; Lei, L. Quercetin mitigates depression-like behavior via the suppression of neuroinflammation and oxidative damage in corticosterone-induced mice. J. Chem. Neuroanat. 2023, 132, 102313. [Google Scholar] [CrossRef]
- Wang, M.; Wei, X.; Jia, Y.; Wang, C.; Wang, X.; Zhang, X.; Li, D.; Wang, Y.; Gao, Y. Quercetin alleviates chronic unpredictable mild stress-induced depression-like behavior by inhibiting NMDAR1 with α2δ-1 in rats. CNS Neurosci. Ther. 2024, 30, e14724. [Google Scholar] [CrossRef]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: New approaches to hippocampal neurogenesis. J. Neuroinflamm. 2023, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Pappalardo, G.; Basile, L.; Giuffrida, A.; Copani, A.; Tosto, R.; Sinopoli, A.; Giuffrida, M.L.; Pirrone, E.; Drago, F.; et al. Neuroprotective effects of the monoamine oxidase inhibitor tranylcypromine and its amide derivatives against Aβ(1-42)-induced toxicity. Eur. J. Pharmacol. 2015, 764, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.S.; Goudarzi, I.; Lashkarbolouki, T.; Abrari, K.; Elahdadi Salmani, M. Chronic administration of quercetin prevent spatial learning and memory deficits provoked by chronic stress in rats. Behav. Brain Res. 2014, 270, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J.; Hale, P.J.; Ghimire, A.; Watson, B.O. The cholinesterase inhibitor donepezil has antidepressant-like properties in the mouse forced swim test. Transl. Psychiatry 2020, 10, 255. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Hale, P.J.; Ghimire, A.; Watson, B.O. Multiple cholinesterase inhibitors have antidepressant-like properties in the mouse forced swim test. Behav. Brain Res. 2021, 409, 113323. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Hale, P.J.; Ghimire, A.; Watson, B.O. Repurposing Cholinesterase Inhibitors as Antidepressants? Dose and Stress-Sensitivity May Be Critical to Opening Possibilities. Front. Behav. Neurosci. 2021, 14, 620119. [Google Scholar] [CrossRef]
- Srikumar, B.N.; Raju, T.R.; Shankaranarayana Rao, B.S. The involvement of cholinergic and noradrenergic systems in behavioral recovery following oxotremorine treatment to chronically stressed rats. Neuroscience 2006, 143, 679–688. [Google Scholar] [CrossRef]
- Abdalla, F.H.; Cardoso, A.M.; Pereira, L.B.; Schmatz, R.; Gonçalves, J.F.; Stefanello, N.; Fiorenza, A.M.; Gutierres, J.M.; Serres, J.D.; Zanini, D.; et al. Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol. Cell. Biochem. 2013, 381, 1–8. [Google Scholar] [CrossRef]
- Baldissarelli, J.; Santi, A.; Schmatz, R.; Abdalla, F.H.; Cardoso, A.M.; Martins, C.C.; Dias, G.R.; Calgaroto, N.S.; Pelinson, L.P.; Reichert, K.P.; et al. Hypothyroidism Enhanced Ectonucleotidases and Acetylcholinesterase Activities in Rat Synaptosomes can be Prevented by the Naturally Occurring Polyphenol Quercetin. Cell Mol. Neurobiol. 2017, 37, 53–63. [Google Scholar] [CrossRef]
- Islam, M.R.; Zaman, A.; Jahan, I.; Chakravorty, R.; Chakraborty, S. In silico QSAR analysis of quercetin reveals its potential as therapeutic drug for Alzheimer’s disease. J. Young Pharm. 2013, 5, 173–179. [Google Scholar] [CrossRef]
- Liao, Y.; Mai, X.; Wu, X.; Hu, X.; Luo, X.; Zhang, G. Exploring the Inhibition of Quercetin on Acetylcholinesterase by Multispectroscopic and In Silico Approaches and Evaluation of Its Neuroprotective Effects on PC12 Cells. Molecules 2022, 27, 7971. [Google Scholar] [CrossRef]
- Fortes, Z.B.; Reis, C.C.; Scivoletto, R. Anticholinesterase activity of tranylcypromine and its isomers. Rev. Bras. Pesqui. Med. Biol. 1980, 13, 71–74. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostardeiro, V.B.; Assmann, C.E.; Schirmann, A.A.; da Silveira, M.V.; Rambo, B.V.C.; Schott, M.; Pereira, A.d.S.; Miron, V.V.; Soares, H.W.; Dambrós, L.V.; et al. Quercetin and Tranylcypromine Improve Memory, Behavioral Performance, and Cholinergic Function in Male Rats Subjected to Chronic Restraint Stress. Brain Sci. 2025, 15, 709. https://doi.org/10.3390/brainsci15070709
Mostardeiro VB, Assmann CE, Schirmann AA, da Silveira MV, Rambo BVC, Schott M, Pereira AdS, Miron VV, Soares HW, Dambrós LV, et al. Quercetin and Tranylcypromine Improve Memory, Behavioral Performance, and Cholinergic Function in Male Rats Subjected to Chronic Restraint Stress. Brain Sciences. 2025; 15(7):709. https://doi.org/10.3390/brainsci15070709
Chicago/Turabian StyleMostardeiro, Vitor Bastianello, Charles Elias Assmann, Adriel Antonio Schirmann, Marcylene Vieira da Silveira, Bianca Vedoin Copês Rambo, Mairin Schott, Aline da Silva Pereira, Vanessa Valéria Miron, Heloiza Winck Soares, Larissa Varotto Dambrós, and et al. 2025. "Quercetin and Tranylcypromine Improve Memory, Behavioral Performance, and Cholinergic Function in Male Rats Subjected to Chronic Restraint Stress" Brain Sciences 15, no. 7: 709. https://doi.org/10.3390/brainsci15070709
APA StyleMostardeiro, V. B., Assmann, C. E., Schirmann, A. A., da Silveira, M. V., Rambo, B. V. C., Schott, M., Pereira, A. d. S., Miron, V. V., Soares, H. W., Dambrós, L. V., Belinazo, S. F., Vidal, T. G., Bagatini, M. D., Schetinger, M. R. C., & Morsch, V. M. M. (2025). Quercetin and Tranylcypromine Improve Memory, Behavioral Performance, and Cholinergic Function in Male Rats Subjected to Chronic Restraint Stress. Brain Sciences, 15(7), 709. https://doi.org/10.3390/brainsci15070709





