Exploring the Neural Correlates of Metal Exposure in Motor Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Experimental Design
2.2. Inclusion Criteria
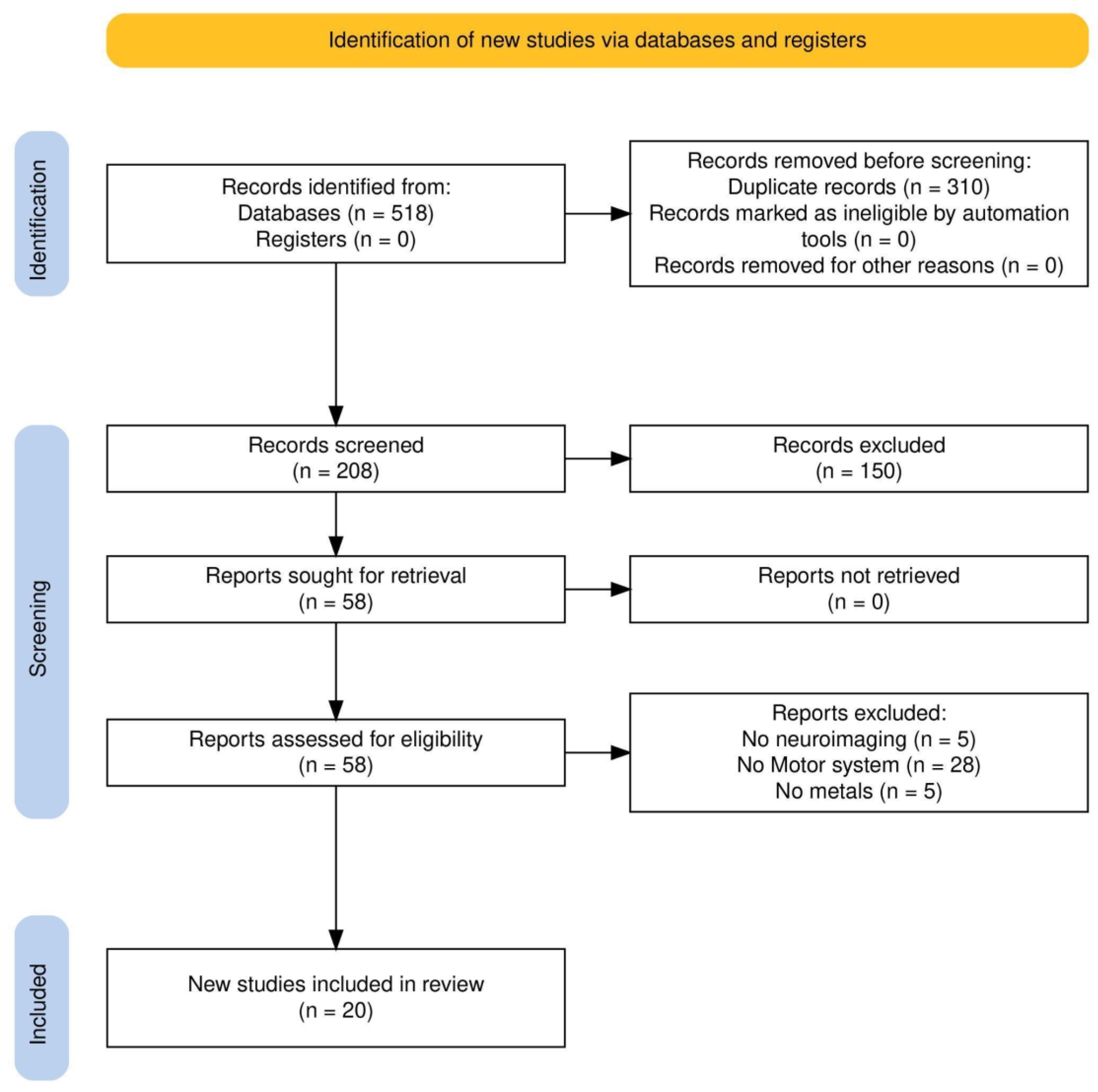
2.3. Study Selection
3. Results
3.1. Manganese
3.1.1. Adolescents
3.1.2. Welders
3.1.3. Single Case
| Authors | Year | Functional Outcomes | Sample Size | Entry Route |
|---|---|---|---|---|
| Sen et al. [19] | 2011 | OB and other brain regions | 14 | Inhalation |
| Kim et al. [18] | 2011 | Brain microstructural abnormalities | 49 | Inhalation |
| Chang et al. [14] | 2013 | Changes in the GP and cerebellum | 66 | Inhalation |
| Long et al. [12] | 2014 | Alterations in GABA | 32 | Inhalation |
| Lao et al. [17] | 2017 | Altered BG neurodevelopment | 23 | Water |
| Ma et al. [13] | 2018 | Thalamic GABA | 39 | Inhalation |
| Lee et al. [25] | 2018 | Brain and functional changes | Review | Inhalation |
| de Water et al. [16] | 2019 | iFC | 14 | All |
| Lotz et al. [21] | 2021 | Fine motor functions and relaxation rates R1 in GP and substantia nigra | 78 | Inhalation |
| Wu et al. [20] | 2022 | Grey matter volume and structural covariance patterns | 75 | Inhalation |
| Alikunju et al. [24] | 2023 | Upper motor neuron type weakness on patient’s face left side and spastic dysarthria | 1 | Inhalation |
| Thunberg et al. [23] | 2024 | R1 measured using whole-brain quantitative MRI in an encompassing pre- and post-central gyri region | 51 | Inhalation |
| Monsivais et al. [22] | 2024 | High-resolution 3D MRI and R1 relaxation maps to identify Mn accumulation | 59 | Inhalation |
3.1.4. Review
3.2. Lead
3.3. Mercury and Copper
| Authors | Year | Functional Outcomes | Sample Size | Entry Route |
|---|---|---|---|---|
| Bleecker et al. [26] | 2007 | Cerebral WM changes | 61 | All |
| Cecil et al. [27] | 2008 | Gray matter volume | 157 | All |
| Brubaker et al. [28] | 2009 | Myelination and axonal integrity | 91 | All |
| Seo et al. [29] | 2014 | Functional abnormalities in the frontoparietal working memory network | 65 | All |
| Takeuchi et al. [30] | 2021 | Microstructural properties of gray matter areas, and brain activity | 920 | All |
| Migneron-Foisy et al. [32] | 2022 | Alterations of the CC | 89 | All |
| Authors | Year | Functional Outcomes | Sample Size | Entry Route |
|---|---|---|---|---|
| Pujol et al. [31] | 2016 | Alterations in BG structure and function | 263 | Inhalation |
| Migneron-Foisy et al. [32] | 2022 | Alterations in the CC | 89 | Food |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Antonioni, A.; Govoni, V.; Brancaleoni, L.; Donà, A.; Granieri, E.; Bergamini, M.; Gerdol, R.; Pugliatti, M. Amyotrophic Lateral Sclerosis and Air Pollutants in the Province of Ferrara, Northern Italy: An Ecological Study. Int. J. Environ. Res. Public Health 2023, 20, 5591. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Kim, J.; Prado-Rico, J.M.; Du, G.; Lewis, M.M.; Kong, L.; Yanosky, J.D.; Eslinger, P.; Kim, B.-G.; Hong, Y.-S.; et al. Effects of mixed metal exposures on MRI diffusion features in the medial temporal lobe. NeuroToxicology 2024, 105, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, T.J.; Dietrich, K.N.; Wright, J.P.; Altaye, M.; Cecil, K.M. Criminal arrests associated with reduced regional brain volumes in an adult population with documented childhood lead exposure. Environ. Res. 2021, 201, 111559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greger, J.L. Nutrition versus toxicology of manganese in humans: Evaluation of potential biomarkers. Neurotoxicology 1998, 20, 205–212. [Google Scholar]
- Crossgrove, J.; Zheng, W. Manganese toxicity upon overexposure. NMR Biomed. 2004, 17, 544–553. [Google Scholar] [CrossRef]
- Seo, J.; Chang, Y.; Jang, K.E.; Park, J.W.; Kim, Y.-T.; Park, S.-J.; Jeong, K.S.; Kim, A.; Kim, S.H. Altered executive function in the welders: A functional magnetic resonance imaging study. Neurotoxicology Teratol. 2016, 56, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.M.; Kornblith, E.S.; Gocheva, V.V.; Colledge, M.A.; Bollweg, G.; Kim, Y.; Beseler, C.L.; Wright, C.W.; Adams, S.W.; Lobdell, D.T. Environmental exposure to manganese in air: Associations with cognitive functions. NeuroToxicology 2015, 49, 139–148. [Google Scholar] [CrossRef]
- Invernizzi, A.; Rechtman, E.; Oluyemi, K.; Renzetti, S.; Curtin, P.; Colicino, E.; Ambrosi, C.; Mascaro, L.; Patrono, A.; Corbo, D.; et al. Topological network properties of resting-state functional connectivity patterns are associated with metal mixture exposure in adolescents. Front. Neurosci. 2023, 17, 1098441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corbo, D.; Placidi, D.; Gasparotti, R.; Wright, R.; Smith, D.R.; Lucchini, R.G.; Horton, M.K.; Colicino, E. The Luria-Nebraska Neuropsychological Battery Neuromotor Tasks: From Conventional to Image-Derived Measures. Brain Sci. 2022, 12, 757. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Environmental Protection Agency (EPA) Manganese (CASRN 7439-96-5). Environmental Protection Agency. 2010. Available online: http://www.epa.gov/iris/subst/0373.htm (accessed on 1 January 2010).
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, R.; Maynard, A.; Ito, Y.; Finkelstein, J.; et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef]
- Long, Z.; Li, X.-R.; Xu, J.; Edden, R.A.E.; Qin, W.-P.; Long, L.-L.; Murdoch, J.B.; Zheng, W.; Jiang, Y.-M.; Dydak, U.; et al. Thalamic GABA predicts fine motor performance in manganese-exposed smelter workers. PLoS ONE 2014, 9, e88220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, R.E.; Ward, E.J.; Yeh, C.-L.; Snyder, S.; Long, Z.; Yavuz, F.G.; Zauber, S.E.; Dydak, U. Thalamic GABA levels and occupational manganese neurotoxicity: Association with exposure levels and brain MRI. NeuroToxicology 2018, 64, 30–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, Y.; Jin, S.-U.; Kim, Y.; Shin, K.M.; Lee, H.J.; Kim, S.H.; Ahn, J.-H.; Park, S.-J.; Jeong, K.S.; Weon, Y.C.; et al. Decreased brain volumes in manganese-exposed welders. NeuroToxicology 2013, 37, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- De Water, E.; Papazaharias, D.M.; Ambrosi, C.; Mascaro, L.; Iannilli, E.; Gasparotti, R.; Lucchini, R.G.; Austin, C.; Arora, M.; Tang, C.Y.; et al. Early-life dentine manganese concentrations and intrinsic functional brain connectivity in adolescents: A pilot study. PLoS ONE 2019, 14, e0220790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lao, Y.; Dion, L.-A.; Gilbert, G.; Bouchard, M.F.; Rocha, G.; Wang, Y.; Leporé, N.; Saint-Amour, D. Mapping the basal ganglia alterations in children chronically exposed to manganese. Sci. Rep. 2017, 7, 41804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.; Jeong, K.S.; Song, H.-J.; Lee, J.-J.; Seo, J.-H.; Kim, G.-C.; Lee, H.J.; Kim, H.J.; Ahn, J.-H.; Park, S.-J.; et al. Altered white matter microstructural integrity revealed by voxel-wise analysis of diffusion tensor imaging in welders with manganese exposure. NeuroToxicology 2011, 32, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Flynn, M.R.; Du, G.; Tröster, A.I.; An, H.; Huang, X. Manganese accumulation in the olfactory bulbs and other brain regions of “asymptomatic” welders. Toxicol. Sci. 2011, 121, 160–167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.; Zhang, Q.; Sun, P.; Zhang, H.; Gao, M.; Ma, M.; Dong, Y.; Liu, P.; Wu, X. Gray matter microstructural alterations in manganese-exposed welders: A preliminary neuroimaging study. Eur. Radiol. 2022, 32, 8649–8658. [Google Scholar] [CrossRef]
- Lotz, A.; Pesch, B.; Casjens, S.; Lehnert, M.; Zschiesche, W.; Taeger, D.; Yeh, C.-L.; Weiss, T.; Schmidt-Wilcke, T.; Quetscher, C.; et al. Association of exposure to manganese and fine motor skills in welders—Results from the WELDOX II study. NeuroToxicology 2021, 82, 137–145. [Google Scholar] [CrossRef]
- Monsivais, H.; Yeh, C.-L.; Edmondson, A.; Harold, R.; Snyder, S.; Wells, E.M.; Schmidt-Wilcke, T.; Foti, D.; Zauber, S.E.; Dydak, U. Whole-brain mapping of increased manganese levels in welders and its association with exposure and motor function. NeuroImage 2024, 288, 120523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thunberg, P.; Wastensson, G.; Lidén, G.; Adjeiwaah, M.; Tellman, J.; Bergström, B.; Fornander, L.; Lundberg, P. Welding techniques and manganese concentrations in blood and brain: Results from the WELDFUMES study. NeuroToxicology 2024, 105, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Alikunju, M.; Misiriyyah, N.; Iqbal, S.S.; Khan, M. Manganese Neurotoxicity as a Stroke Mimic: A Case Report. Cureus 2023, 15, e37247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, E.-Y.; Flynn, M.R.; Lewis, M.M.; Mailman, R.B.; Huang, X. Welding-related brain and functional changes in welders with chronic and low-level exposure. NeuroToxicology 2018, 64, 50–59. [Google Scholar] [CrossRef]
- Bleecker, M.L.; Ford, D.P.; Vaughan, C.G.; Walsh, K.S.; Lindgren, K.N. The association of lead exposure and motor performance mediated by cerebral white matter change. NeuroToxicology 2007, 28, 318–323. [Google Scholar] [CrossRef]
- Cecil, K.M.; Brubaker, C.J.; Adler, C.M.; Dietrich, K.N.; Altaye, M.; Egelhoff, J.C.; Wessel, S.; Elangovan, I.; Hornung, R.; Jarvis, K.; et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008, 5, e112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brubaker, C.J.; Schmithorst, V.J.; Haynes, E.N.; Dietrich, K.N.; Egelhoff, J.C.; Lindquist, D.M.; Lanphear, B.P.; Cecil, K.M. Altered myelination and axonal integrity in adults with childhood lead exposure: A diffusion tensor imaging study. NeuroToxicology 2009, 30, 867–875. [Google Scholar] [CrossRef]
- Seo, J.; Lee, B.-K.; Jin, S.-U.; Park, J.W.; Kim, Y.-T.; Ryeom, H.-K.; Lee, J.; Suh, K.J.; Kim, S.H.; Park, S.-J.; et al. Lead-induced impairments in the neural processes related to working memory function. PLoS ONE 2014, 9, e105308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takeuchi, H.; Taki, Y.; Nouchi, R.; Yokoyama, R.; Kotozaki, Y.; Nakagawa, S.; Sekiguchi, A.; Iizuka, K.; Hanawa, S.; Araki, T.; et al. Lead exposure is associated with functional and microstructural changes in the healthy human brain. Commun. Biol. 2021, 4, 912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pujol, J.; Fenoll, R.; Macià, D.; Martínez-Vilavella, G.; Alvarez-Pedrerol, M.; Rivas, I.; Forns, J.; Deus, J.; Blanco-Hinojo, L.; Querol, X.; et al. Airborne copper exposure in school environments associated with poorer motor performance and altered basal ganglia. Brain Behav. 2016, 6, e00467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Migneron-Foisy, V.; Muckle, G.; Jacobson, J.L.; Ayotte, P.; Jacobson, S.W.; Saint-Amour, D. Impact of chronic exposure to legacy environmental contaminants on the corpus callosum microstructure: A diffusion MRI study of Inuit adolescents. NeuroToxicology 2022, 92, 200–211. [Google Scholar] [CrossRef] [PubMed]
- McLeod, K.R.; Langevin, L.M.; Goodyear, B.G.; Dewey, D. Functional connectivity of neural motor networks is disrupted in children with developmental coordination disorder and attention-deficit/hyperactivity disorder. NeuroImage Clin. 2014, 4, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.I.; Miall, R.C.; McNab, F.; Galea, J.M. Individual differences in explicit and implicit visuomotor learning and working memory capacity. Sci. Rep. 2016, 6, 36633. [Google Scholar] [CrossRef] [PubMed]
- Suk, W.A.; Olden, K.; Yang, R.S.H. Chemical mixtures research: Significance and future perspectives. Environ. Health Perspect. 2002, 110 (Suppl. S6), 891–892. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbo, D.; Gasparotti, R.; Renzetti, S. Exploring the Neural Correlates of Metal Exposure in Motor Areas. Brain Sci. 2025, 15, 679. https://doi.org/10.3390/brainsci15070679
Corbo D, Gasparotti R, Renzetti S. Exploring the Neural Correlates of Metal Exposure in Motor Areas. Brain Sciences. 2025; 15(7):679. https://doi.org/10.3390/brainsci15070679
Chicago/Turabian StyleCorbo, Daniele, Roberto Gasparotti, and Stefano Renzetti. 2025. "Exploring the Neural Correlates of Metal Exposure in Motor Areas" Brain Sciences 15, no. 7: 679. https://doi.org/10.3390/brainsci15070679
APA StyleCorbo, D., Gasparotti, R., & Renzetti, S. (2025). Exploring the Neural Correlates of Metal Exposure in Motor Areas. Brain Sciences, 15(7), 679. https://doi.org/10.3390/brainsci15070679






