The Neuroanatomical Correlates of Visceral Pain: An Activation Likelihood Estimation Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Quality Assessment
2.4. Data Extraction and Statistical Analysis
3. Results
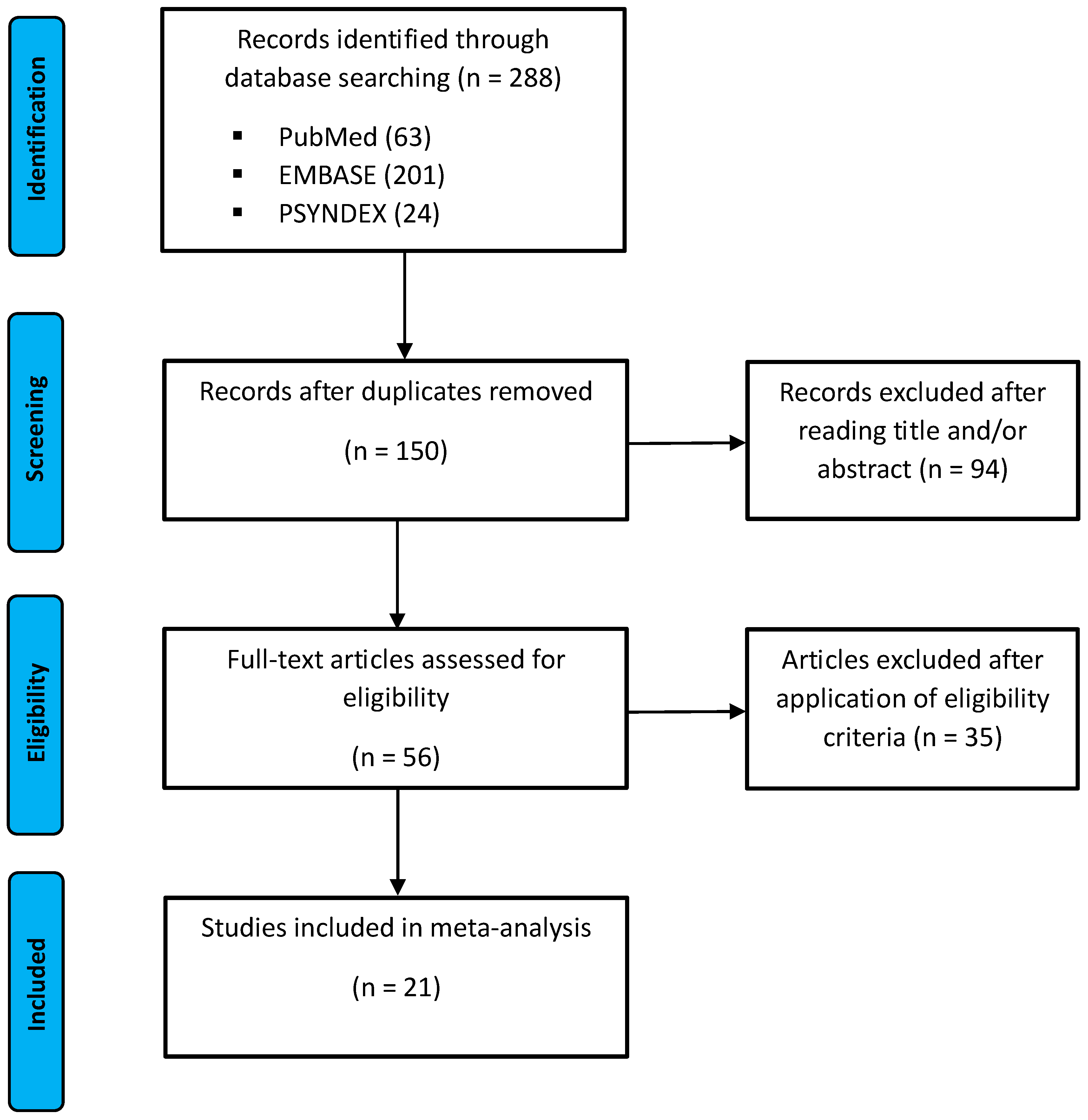
3.1. Study Selection
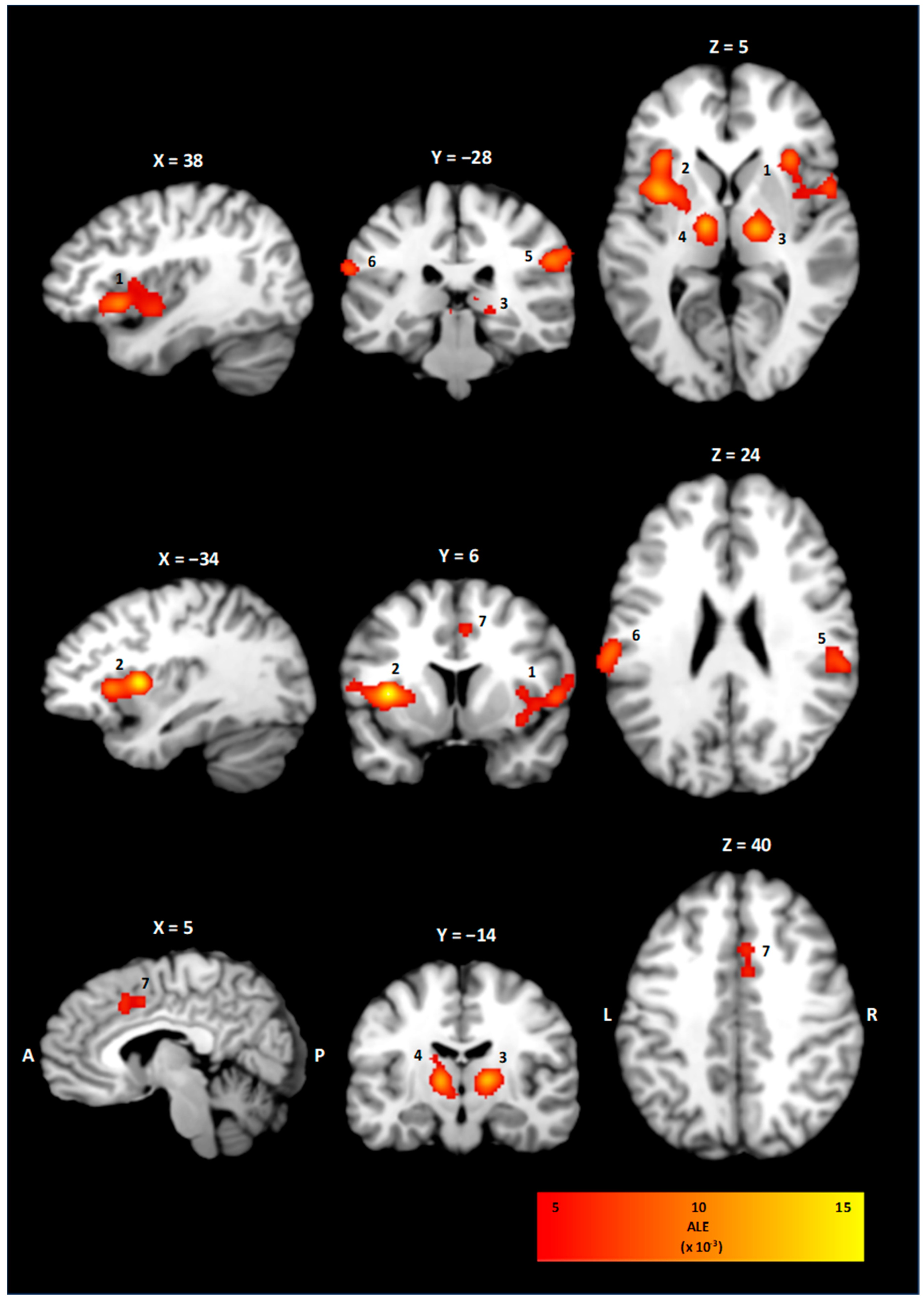
3.2. ALE Cluster Analysis
| Scheme | Population | Intervention | ||||
|---|---|---|---|---|---|---|
| Author (Year) | Study Design | Imaging Modality | Sample Size (% Female) | Mean Age ± SD (Years) | Conditions | Foci |
| Strigo et al. (2003) [20] | Prospective experimental study | 1.5 T fMRI | 7 (43%) | 25.8 range 19–34 | Esophageal balloon distension with the balloon catheter positioned 5 cm above the lower esophageal sphincter; cutaneous heat stimulation onto the upper midline chest; two stimulus intensities low/high; stimuli were presented three times in a counter-balanced and quasi-randomized order. | 24 |
| Verne et al. (2003) [21] | Prospective experimental study | 1.5 T fMRI | 9 (67%) | 29 ± 9 | Phasic rectal balloon distension to pressures of 35 mmHg or 55 mmHg for 20 s with 20 s interstimulus control period for cycles; cutaneous pain tested with a heated water bath at three temperatures (35, 45, 47 °C); pain rating on a VAS; psychological tests with BDI, Somatic Focus (PILL), STAI, State-Trait Anger Expression Inventory, Coping Strategies Questionnaire, and the NEO-FFI. | 5 |
| Lu et al. (2004) [22] | Prospective experimental study | 3 T fMRI | 10 (20%) | 23.6 | Gastric fundus distension with an air inflated balloon; conditions of either non-painful or painful gastric distension (60–70% on a VAS rating scale); psychological test with STAI, BDI, NEO-FFI. | 48 |
| Andresen et al. (2005) [23] | Prospective experimental study | 1.5 T fMRI | 8 (63%) | 41.3 range 27–64 | Rectal balloon distensions of subliminal, liminal, and supraliminal stimulation intensities adapted to the individual perception threshold; perception rating from 1 to 6, each distension lasted for 20 s followed by a rest of 10 s. | 12 |
| Ladabaum et al. (2006) [24] | Prospective experimental study | 1.5 T fMRI | 18 (78%) | 32 ± 6.5 | Gastric balloon distension sequences of ten 45 s isobaric inflations to gastric sensation of ≥6 and <9 on a VAS scale; sequences of 45 s deflations to minimal distending pressure. | 39 |
| Hui-Song et al. (2006) [25] | Prospective experimental study | 3 T fMRI | 12 (100%) | 23.0 | Rectal balloon distension with a pressure of 20% above the pain detection threshold; foot cold pressor test with an ice water bath at 4 °C, rating on a 5-points scale. | 15 |
| Berman et al. (2008) [26] | Prospective experimental study | 1.5 T fMRI | 15 (100%) | 36.3 ± 7.3 | Rectal balloon distension, four to six stimulus sets containing 16 trials with pressures of 5, 25 or 45 mmHg. | 22 |
| Study ID | Population | Intervention | ||||
|---|---|---|---|---|---|---|
| Author (Year) | Study Design | Imaging Modality | Sample Size (% Female) | Mean Age ± SD (Years) | Conditions | Foci |
| Coen et al. (2009) [27] | Prospective experimental study | 1.5 T fMRI | 12 (0%) | 26.0 21–31 | Esophageal balloon catheter inflated to produce 40 trials of either painful or non-painful sensations; stimulation was performed under neutral or negative emotions. | 11 |
| Elsenbruch et al. (2009) [28] | Prospective experimental study | 1.5 T fMRI | 12 (100%) | 31.4 ± 2.3 | Rectal balloon distension, staircase increments of 2–10 mmHg until a subjective threshold of 5 on a 6-point scale was reached; ratings on HADS, STAI-S, and SCL-90-R. | 6 |
| Moisset et al. (2010) [29] | Prospective experimental study | 1.5 T fMRI | 11 (100%) | 38.4 ± 3.1 | Rectal balloon distension until a subjective threshold of either 2 (non-painful stimulus) or 5 (painful stimulus) on a 6-point scale; psychological test with PAS and short-form McGill questionnaire. | 21 |
| Lu et al. (2010) [30] | Prospective experimental study | 3 T fMRI | 14 (64%) | 23.9 ± 3.9 | Esophageal balloon distension until 60–70% of subjective pain intensity, administration of normal saline during control condition; during placebo conditions, participants were told to receive an opioid intravenously; ratings on PCS, VAS, and McGill pain questionnaire. | 27 |
| Benson et al. (2011) [31] | Prospective experimental study | 1.5 T fMRI | 30 (50%) | 25.75 ± 6.1 | Rectal balloon distensions until an individual pain threshold of 5 on a 6 points scale; eight phases of distension alternated with eight phases without distension and a duration of 31 s for each condition; auditory cue before distension to assess anticipation; rating with HADS and VAS. | 17 |
| Geeraerts et al. (2011) [39] | Prospective experimental study | PET | 14 (29%) | 26.3 ± 1.8 | Gastric fundus balloon distension and continuous and stepwise infusion until individualized abdominal discomfort threshold; comparison between intragastric volumes of balloon distension and continuous or stepwise meal infusion; rating on satiation or upper abdominal sensation scale. | 6 |
| Smith et al. (2011) [32] | Prospective experimental study | 3 T fMRI | 14 (100%) | n.a. | Rectal balloon distension; five repetitions of four conditions consisting of no stimulus, subliminal stimulus, liminal stimulus and painful stimulus for 40 s each; rating with HAD, PHQ-15, and VAS. | 34 |
| Study ID | Population | Intervention | ||||
|---|---|---|---|---|---|---|
| Author (Year) | Study Design | Imaging Modality | Sample Size (% Female) | Mean Age ± SD (Years) | Conditions | Foci |
| Schmid et al. (2013) [33] | Prospective experimental study | 3 T fMRI | 36 (50%) | 29.7 ± 1.8 | Rectal balloon distention until a subjective pain threshold between 5 and 6 on 6-point scale; two groups of intravenous infusion of saline/spasmolytic drug (placebo) or saline/opioid antagonist (nocebo); investigation of anticipated effect; rating on HADS, BDI and baseline cortisol levels. | 15 |
| Theysohn et al. (2014) [34] | Prospective experimental study | 3 T fMRI | 30 (50%) | 34.7 ± 3.2 | Rectal balloon distension until an individualized pain threshold following a visual stimulus, counterbalanced by visually cued resting periods; participants were told that they would receive an analgesic drug or an inert substance; rating on a VAS scale. | 10 |
| Gramsch et al. (2014) [35] | Prospective experimental study | 3 T fMRI | 24 (54%) | 28.8 ± 8.5 | Rectal balloon distension, conditioning paradigm with visual cues to measure distension and anticipation-related neural activation; pain threshold set at subjective pain rating between 5 and 6 on a 6-point scale; ratings on VAS and HADS. | 11 |
| Icenhour et al. (2016) [36] | Prospective experimental study | 3 T fMRI | 40 (53%) | 26.00 ± 3.27 range 20–32 | Rectal balloon distensions of high or low intensity paired with visual cues to assess pain and anticipation-related neural activation; pain threshold defined as low intensity for a rating of 4 and high intensity between 5 and 6 on a 6-point scale; rating on VAS, HADS, and STAI. | 6 |
| Tanaka et al. (2016) [40] | Prospective experimental study | PET | 16 (0%) | 22.8 ± 2.5 | Rectal balloon distensions at mild (20 mmHg), intense (40 mmHg) compared to baseline and no distension (0 mmHg); effect of CRH or saline intravenously on brain activation was assessed; plasma ACTH, serum cortisol and plasma noradrenaline levels at each stimulation. | 12 |
| Guleria et al. (2017) [37] | Prospective experimental study | 3 T fMRI | 10 (0%) | 28.5 range 26.5–31.5 | Rectal balloon distensions to an individualized pain threshold; comparison between healthy controls and patients with IBS. | 5 |
| Icenhour et al. (2021) [38] | Prospective experimental study | 3 T fMRI | 27 (44%) | 25.7 ± 1.0 | Rectal balloon distensions in different visual contexts; interoceptive cues were followed by visceral pain as conditioned stimulus; ratings on VAS, HADS, TICS, and STAI. | 22 |
| Cluster | Brain Region | BA | x | y | z | Volume (mm3) | ALE (×10−3) |
|---|---|---|---|---|---|---|---|
| 1 | Right Insula | 13 | 41 | 11 | 3 | 7024 | 27.0 |
| 2 | Left Insula | 13 | −36 | 9 | 7 | 6080 | 37.5 |
| 3 | Right Thalamus | - | 16 | −16 | 6 | 3592 | 30.1 |
| 4 | Left Thalamus | - | −11 | −14 | 6 | 2944 | 30.1 |
| 5 | Right Inferior Parietal Lobe | 40 | 57 | −28 | 29 | 2648 | 26.2 |
| 6 | Left Postcentral Gyrus | 40 | −62 | −24 | 23 | 1592 | 23.1 |
| 7 | Right Cingulate Cortex | 32 | 4 | 14 | 41 | 1344 | 16.4 |
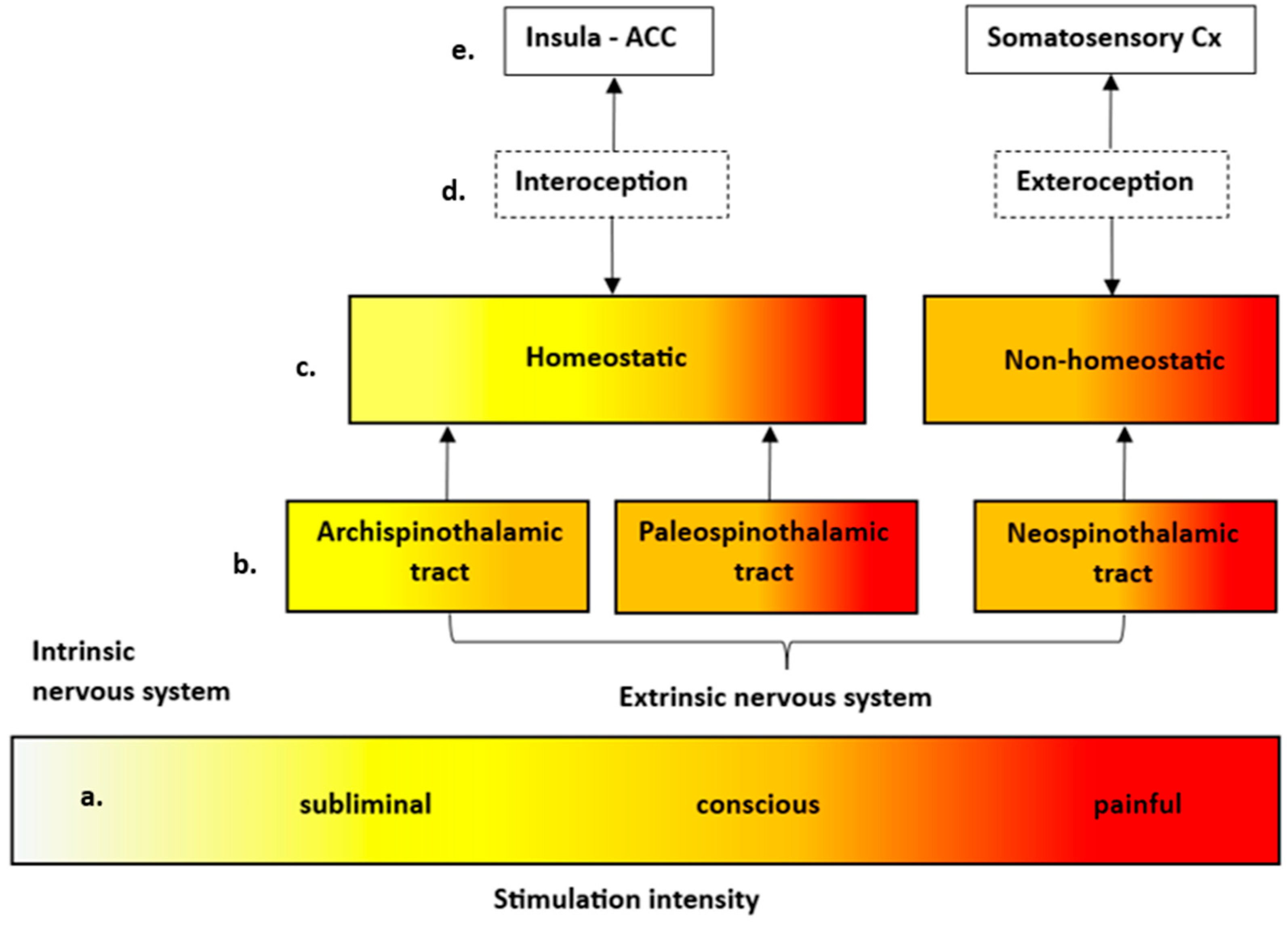
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Anterior cingulate cortex |
| ALE | Activation likelihood estimation |
| BA | Brodmann area |
| fMRI | Functional magnetic resonance imaging |
| FWHM | Full width half maximum |
| IBS | Irritable bowel syndrome |
| MNI | Montreal Neurological Imaging |
| PCC | Posterior cingulate cortex |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| VMA | Visceromotor areas |
References
- Grundy, L.; Erickson, A.; Brierley, S.M. Visceral pain. Annu. Rev. Physiol. 2019, 81, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Caporale, N.; Morselli-Labate, A.M.; Nardi, E.; Cogliandro, R.; Cavazza, M.; Stanghellini, V. Acute abdominal pain in the emergency department of a university hospital in Italy. United Eur. Gastroenterol. J. 2016, 4, 297–304. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Casey, K.L. The imaging of pain: Background and rationale. In Pain Imaging; Casey, K.L., Bushnell, M.C., Eds.; IASP Press: Washington, DC, USA, 2000; pp. 1–29. [Google Scholar]
- Cervero, F. Visceral versus Somatic Pain: Similarities and Differences. Dig. Dis. 2009, 27 (Suppl. 1), 3–10. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R.; Gebhart, G.F. Inside information-The unique features of visceral sensation. Mol. Interv. 2008, 8, 242–253. [Google Scholar] [CrossRef]
- Lelic, D.; Nissen, T.D.; Brock, C.; Aziz, Q.; Drewes, A.M. Rapid balloon distension as a tool to study cortical processing of visceral sensations and pain. Neurogastroenterol. Motil. 2015, 27, 832–840. [Google Scholar] [CrossRef]
- Treede, R.-D.; Kenshalo, D.R.; Gracely, R.H.; Jones, A.K. The cortical representation of pain. Pain 1999, 79, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Fenton, B.W.; Shih, E.; Zolton, J. The neurobiology of pain perception in normal and persistent pain. Pain Manag. 2015, 5, 297–317. [Google Scholar] [CrossRef]
- Barrett, L.F.; Simmons, W.K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015, 16, 419–429. [Google Scholar] [CrossRef]
- Ceunen, E.; Vlaeyen, J.W.S.; Van Diest, I. On the origin of interoception. Front. Psychol. 2016, 7, 743. [Google Scholar] [CrossRef]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The emerging science of interoception: Sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Daunizeau, J.; Kilner, J.; Kiebel, S.J. Action and behavior: A free-energy formulation. Biol. Cybern. 2010, 102, 227–260. [Google Scholar] [CrossRef] [PubMed]
- Pezzulo, G.; Rigoli, F.; Friston, K. Active inference, homeostatic regulation and adaptive behavioural control. Prog. Neurobiol. 2015, 134, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-based activation likelihood estimation meta analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation meta-analysis revisited. NeuroImage 2012, 59, 2349–2361. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. he PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Sterne, J.A. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Strigo, I.A.; Duncan, G.H.; Boivin, M.; Bushnell, M.C. Differentiation of visceral and cutaneous pain in the human brain. J. Neurophysiol. 2003, 89, 3294–3303. [Google Scholar] [CrossRef]
- Verne, N.G.; Himes, N.C.; Robinson, M.E.; Gopinath, K.S.; Briggs, R.W.; Crosson, B.; Price, D.D. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain 2003, 103, 99–110. [Google Scholar] [CrossRef]
- Lu, C.L.; Wu, Y.T.; Yeh, T.C.; Chen, L.F.; Chang, F.Y.; Lee, S.D.; Ho, L.T.; Hsieh, J.C. Neuronal correlates of gastric pain induced by fundus distension: A 3T-fMRI study. Neurogastroenterol. Motil. 2004, 16, 575–587. [Google Scholar] [CrossRef]
- Andresen, V.; Bach, D.R.; Poellinger, A.; Tsrouya, C.; Stroh, A.; Foerschler, A.; Georgiewa, P.; Zimmer, C.; Mönnikes, H. Brain activation responses to subliminal or supraliminal rectal stimuli and to auditory stimuli in irritable bowel syndrome. Neurogastroenterol. Motil. 2005, 17, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Roberts, T.P.; McGonigle, D.J. Gastric fundic distension activates fronto-limbic structures but not primary so-matosensory cortex: A functional magnetic resonance imaging study. NeuroImage 2007, 34, 724–732. [Google Scholar] [CrossRef]
- Song, G.H.; Venkatraman, V.; Ho, K.Y.; Chee, M.W.; Yeoh, K.G.; Wilder-Smith, C.H. Cortical effects of anticipation and en-dogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain 2006, 126, 79–90. [Google Scholar] [CrossRef]
- Berman, S.M.; Naliboff, B.D.; Suyenobu, B.; Labus, J.S.; Stains, J.; Ohning, G.; Kilpatrick, L.; Bueller, J.A.; Ruby, K.; Jarcho, J.; et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J. Neurosci. 2008, 28, 349–359. [Google Scholar] [CrossRef]
- Coen, S.J.; Yágüez, L.; Aziz, Q.; Mitterschiffthaler, M.T.; Brammer, M.; Williams, S.C.; Gregory, L.J. Negative mood affects brain processing of visceral sensation. Gastroenterology 2009, 137, 253–261. [Google Scholar] [CrossRef]
- Elsenbruch, S.; Rosenberger, C.; Enck, P.; Forsting, M.; Schedlowski, M.; Gizewski, E.R. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: An fMRI study. Gut 2010, 59, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Bouhassira, D.; Ducreux, D.; Glutron, D.; Coffin, B.; Sabaté, J. Anatomical connections between brain areas ac-tivated during rectal distension in healthy volunteers: A visceral pain network. Eur. J. Pain 2010, 14, 142–148. [Google Scholar] [CrossRef]
- Lu, H.-C.; Hsieh, J.-C.; Lu, C.-L.; Niddam, D.M.; Wu, Y.-T.; Yeh, T.-C.; Cheng, C.-M.; Chang, F.-Y.; Lee, S.-D. Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: A 3T-fMRI study. Pain 2010, 148, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.; Kotsis, V.; Rosenberger, C.; Bingel, U.; Forsting, M.; Schedlowski, M.; Gizewski, E.R.; Elsenbruch, S. Behavioural and neural correlates of visceral pain sensitivity in healthy men and women: Does sex matter? Eur. J. Pain 2011, 16, 349–358. [Google Scholar] [CrossRef]
- Smith, J.K.; Humes, D.J.; Head, K.E.; Bush, D.; White, T.P.; Stevenson, C.M.; Brookes, M.J.; Marciani, L.; Spiller, R.C.; Gowland, P.A.; et al. fMRI and MEG analysis of visceral pain in healthy volunteers. Neurogastroenterol. Motil. 2011, 23, 648-e260. [Google Scholar] [CrossRef]
- Schmid, J.; Theysohn, N.; Gaß, F.; Benson, S.; Gramsch, C.; Forsting, M.; Gizewski, E.R.; Elsenbruch, S. Neural mechanisms mediating positive and negative treatment expectations in visceral pain: A functional magnetic resonance imaging study on placebo and nocebo effects in healthy volunteers. Pain 2013, 154, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Theysohn, N.; Schmid, J.; Icenhour, A.; Mewes, C.; Forsting, M.; Gizewski, E.R.; Schedlowski, M.; Elsenbruch, S.; Benson, S. Are there sex differences in placebo analgesia during visceral pain processing? A fMRI study in healthy subjects. Neurogastroenterol. Motil. 2014, 126, 1743–1753. [Google Scholar] [CrossRef]
- Gramsch, C.; Kattoor, J.; Icenhour, A.; Forsting, M.; Schedlowski, M.; Gizewski, E.R.; Elsenbruch, S. Learning pain-related fear: Neural mechanisms mediating rapid differential conditioning, extinction and reinstatement processes in human visceral pain. Neurobiol. Learn. Mem. 2014, 116, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Icenhour, A.; Labrenz, F.; Ritter, C.; Theysohn, N.; Forsting, M.; Bingel, U.; Elsenbruch, S. Learning by experience? Visceral pain-related neural and behavioral responses in a classical conditioning paradigm. Neurogastroenterol. Motil. 2017, 29, e13026. [Google Scholar] [CrossRef]
- Guleria, A.; Karyampudi, A.; Singh, R.; Khetrapal, C.L.; Verma, A.; Ghoshal, U.C.; Kumar, D. Mapping of brain activations to rectal balloon distension stimuli in male patients with irritable bowel syndrome using functional magnetic resonance imaging. J. Neurogastroenterol. Motil. 2017, 23, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Icenhour, A.; Petrakova, L.; Hazzan, N.; Theysohn, N.; Merz, C.J.; Elsenbruch, S. When gut feelings teach the brain to fear pain: Context-dependent activation of the central fear network in a novel interoceptive conditioning paradigm. NeuroImage 2021, 238, 118229. [Google Scholar] [CrossRef]
- Geeraerts, B.; Van Oudenhove, L.; Dupont, P.; Vanderghinste, D.; Bormans, G.; Van Laere, K.; Tack, J. Different regional brain activity during physiological gastric distension compared to balloon distension: A H215O-PET study. Neurogastroenterol. Motil. 2010, 23, 533-e203. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kanazawa, M.; Kano, M.; Morishita, J.; Hamaguchi, T.; Van Oudenhove, L.; Ly, H.G.; Dupont, P.; Tack, J.; Yamaguchi, T.; et al. Differential activation in amygdala and plasma noradrenaline during colorectal distention by administration of corticotropin-releasing hormone between healthy individuals and patients with irritable bowel syndrome. PLoS ONE 2016, 11, e0157347. [Google Scholar] [CrossRef]
- Blackshaw, L.A.; Brookes, S.J.H.; Grundy, D.; Schemann, M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol. Motil. 2007, 19, 1–19. [Google Scholar] [CrossRef]
- Schemann, M.; Neunlist, M. The human enteric nervous system. Neurogastroenterol. Motil. 2004, 16, 55–59. [Google Scholar] [CrossRef]
- Critchley, H.D.; Harrison, N.A. Visceral influences on brain and behavior. Neuron 2013, 77, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.F. Pathobiology of visceral pain: Molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G834–G838. [Google Scholar] [CrossRef]
- Almeida, T.F.; Roizenblatt, S.; Tufik, S. Afferent pain pathways: A neuroanatomical review. Brain Res. 2004, 1000, 40–56. [Google Scholar] [CrossRef]
- Kendroud, S.; Fitzgerald, L.A.; Murray, I.V.; Hanna, A. Physiology, Nociceptive Pathways. In StatPearls; Ineligible Companies: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470255/ (accessed on 10 April 2025).
- Millan, M.J. The induction of pain: An integrative review. Prog. Neurobiol. 1999, 57, 1–164. [Google Scholar] [CrossRef]
- Norton, J.A. (Ed.) Essential Practice of Surgery: Basic Science and Clinical Evidence; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Ferrari, P.F.; Rozzi, S.; Fogassi, L. The inferior parietal lobule: Where action becomes perception. In Percept, Decision, Action: Bridging the Gaps: Novartis Foundation Symposium 270; John Wiley & Sons, Ltd.: Chichester, UK, 2006; Volume 13, pp. 129–145. [Google Scholar] [CrossRef]
- Saper, C.B. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 2002, 25, 433–469. [Google Scholar] [CrossRef]
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Damasio, A.R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. B Biol. Sci. 1996, 351, 1413–1420. [Google Scholar] [CrossRef]
- Stephani, C.; Fernandez-Baca, V.; Maciunas, R.; Koubeissi, M.; Lüders, H.O. Functional neuroanatomy of the insular lobe. Brain Struct. Funct. 2011, 216, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and function of the human insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef]
- Ungerleider, L.G.; Haxby, J.V. ‘What’ and ‘where’ in the human brain. Curr. Opin. Neurobiol. 1994, 4, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129 Pt 3, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R.; Voogd, J.; van Huijzen, C. Telencephalon: Hippocampus and related structures. In The Human Central Nervous System; Springer: Berlin/Heidelberg, Germany, 2008; pp. 361–400. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Morrell, M.J.; Vogt, B.A. Contributions of anterior cingulate cortex to behaviour. Brain 1995, 118, 279–306. [Google Scholar] [CrossRef]
- Friston, K.; Kiebel, S. Predictive coding under the free-energy principle. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1211–1221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, C.; Maxeiner, H. The Neuroanatomical Correlates of Visceral Pain: An Activation Likelihood Estimation Meta-Analysis. Brain Sci. 2025, 15, 651. https://doi.org/10.3390/brainsci15060651
Müller C, Maxeiner H. The Neuroanatomical Correlates of Visceral Pain: An Activation Likelihood Estimation Meta-Analysis. Brain Sciences. 2025; 15(6):651. https://doi.org/10.3390/brainsci15060651
Chicago/Turabian StyleMüller, Christoph, and Hagen Maxeiner. 2025. "The Neuroanatomical Correlates of Visceral Pain: An Activation Likelihood Estimation Meta-Analysis" Brain Sciences 15, no. 6: 651. https://doi.org/10.3390/brainsci15060651
APA StyleMüller, C., & Maxeiner, H. (2025). The Neuroanatomical Correlates of Visceral Pain: An Activation Likelihood Estimation Meta-Analysis. Brain Sciences, 15(6), 651. https://doi.org/10.3390/brainsci15060651





