Psychopathological Implications of Behavioral Patterns in Obsessive–Compulsive Rituals: A Hierarchical Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.2.1. OCD Patients
2.2.2. Healthy Controls
2.3. Treatment
2.4. Sociodemographic and Clinical Assessment
2.5. Procedure
2.6. Behavioral Assessment
- Total Number of Acts: The overall count of different actions performed during the ritual.
- Number of Functional Acts: The count of actions carried out during the ritual that contribute to achieving its specific purpose.
- Number of Non-Functional Acts: The count of actions that do not contribute to or are unrelated to the main goal of the ritual.
- Total Duration: The overall length of the ritual from beginning to end.
- Duration of Functional Acts: The total time spent performing actions that have a functional purpose within the ritual.
- Duration of Non-Functional Acts: The time dedicated to actions that do not serve the purpose of the ritual.
- Total Repetitions: The total number of acts performed throughout the ritual.
- Repetitions of Functional Acts: The total count of repetitions of functional acts during the ritual.
- Repetitions of Non-Functional Acts: The total count of repetitions of non-functional acts during the ritual.
2.7. Quantitative Analysis
2.8. Statistical Methods
2.9. First Step
2.10. Second Step
3. Results
3.1. Participants
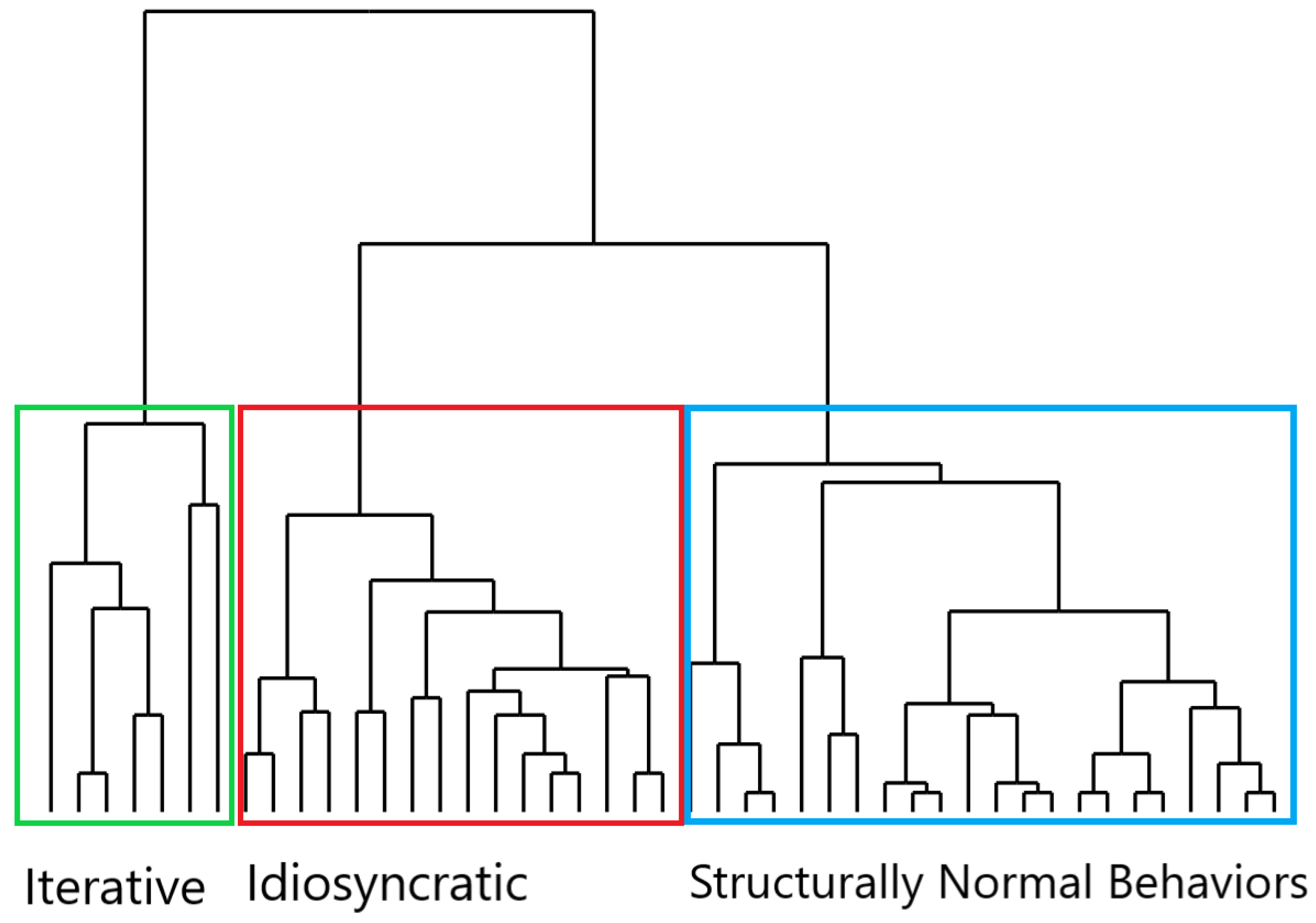
3.2. First Step: Motor Structure of Behavior and Hierarchical Analysis
3.3. Second Step: Association Between Motor Structure of Behavior and Psychopathology
4. Discussion
- -
- Behavior with High Repetitiveness and Low Non-Functionality (Cluster 1): In this cluster, the motor performance was characterized by repetitions of acts (primarily functional ones) with a limited intrusion of non-functional acts in the action flow. These behaviors, centered on repetitive actions, were mainly displayed in the OCD group, and thus, they primarily represented OCD compulsions.
- -
- Behavior with High Non-Functionality and Low Repetitiveness (Cluster 2): In this cluster, the ritual behavioral motor pattern was mainly built upon the intrusion of non-functional acts with little recourse to repetitive acts. Also, Cluster 2 behaviors were performed by OCD patients and might be identified as compulsions.
- -
- Behavior with Low Repetitiveness and Low Non-Functionality (Cluster 3): In this cluster, the motor structure was not “ritualized”, as the behavioral performance was not fragmented into parceled units through repetitive acts and/or non-functional acts [20]. The majority of the controls’ behaviors were grouped within this cluster, thus forming a composition that primarily consisted of normal behaviors, although not exclusively. Indeed, OCD patients also exhibited Cluster 3 behaviors; in this case, however, the behavior, rather than “ritualized”, was more akin to “routines”, as it was stiffened by highly rigid spatiotemporal parameters [13]. For instance, an OCD patient might engage in handwashing routinized behavior similar to the controls’ behaviors in its formal structure (no repetitive acts and few non-functional acts), yet highly rigid and inflexible in the execution, so as to respect specific spatiotemporal features.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Remmerswaal, K.C.P.; Batelaan, N.M.; Hoogendoorn, A.W.; van der Wee, N.J.A.; van Oppen, P.; van Balkom, A.J.L.M. Four-Year Course of Quality of Life and Obsessive-Compulsive Disorder. Soc. Psychiatry Psychiatr. Epidemiol. 2020, 55, 989–1000. [Google Scholar] [CrossRef]
- Macy, A.S.; Theo, J.N.; Kaufmann, S.C.V.; Ghazzaoui, R.B.; Pawlowski, P.A.; Fakhry, H.I.; Cassmassi, B.J.; IsHak, W.W. Quality of Life in Obsessive Compulsive Disorder. CNS Spectr. 2013, 18, 21–33. [Google Scholar] [CrossRef]
- Brock, H.; Hany, M. Obsessive-Compulsive Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ruscio, A.M.; Stein, D.J.; Chiu, W.T.; Kessler, R.C. The Epidemiology of Obsessive-Compulsive Disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 53–63. [Google Scholar] [CrossRef]
- American Psychiatric Association. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Hollander, E.; Kwon, J.H.; Stein, D.J.; Broatch, J.; Rowland, C.T.; Himelein, C.A. Obsessive-Compulsive and Spectrum Disorders: Overview and Quality of Life Issues. J. Clin. Psychiatry 1996, 57 (Suppl. S8), 3–6. [Google Scholar]
- Tonna, M.; Ottoni, R.; Pellegrini, C.; Mora, L.; Gambolo, L.; Di Donna, A.; Parmigiani, S.; Marchesi, C. The Motor Profile of Obsessive-Compulsive Rituals: Psychopathological and Evolutionary Implications. CNS Spectr. 2022, 28, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Eilam, D. The Cognitive Roles of Behavioral Variability: Idiosyncratic Acts as the Foundation of Identity and as Transitional, Preparatory, and Confirmatory Phases. Neurosci. Biobehav. Rev. 2015, 49, 55–70. [Google Scholar] [CrossRef]
- Keren, H.; Boyer, P.; Mort, J.; Eilam, D. Pragmatic and Idiosyncratic Acts in Human Everyday Routines: The Counterpart of Compulsive Rituals. Behav. Brain Res. 2010, 212, 90–95. [Google Scholar] [CrossRef]
- Zor, R.; Hermesh, H.; Szechtman, H.; Eilam, D. Turning Order into Chaos through Repetition and Addition of Elementary Acts in Obsessive-Compulsive Disorder (OCD). World J. Biol. Psychiatry 2009, 10, 480–487. [Google Scholar] [CrossRef]
- Eilam, D. From an Animal Model to Human Patients: An Example of a Translational Study on Obsessive Compulsive Disorder (OCD). Neurosci. Biobehav. Rev. 2017, 76, 67–76. [Google Scholar] [CrossRef]
- Zor, R.; Keren, H.; Hermesh, H.; Szechtman, H.; Mort, J.; Eilam, D. Obsessive-Compulsive Disorder: A Disorder of Pessimal (Non-Functional) Motor Behavior: OCD: A Disorder of Pessimal Behavior. Acta Psychiatr. Scand. 2009, 120, 288–298. [Google Scholar] [CrossRef]
- Eilam, D.; Zor, R.; Szechtman, H.; Hermesh, H. Rituals, Stereotypy and Compulsive Behavior in Animals and Humans. Neurosci. Biobehav. Rev. 2006, 30, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Eilam, D.; Zor, R.; Fineberg, N.; Hermesh, H. Animal Behavior as a Conceptual Framework for the Study of Obsessive-Compulsive Disorder (OCD). Behav. Brain Res. 2012, 231, 289–296. [Google Scholar] [CrossRef]
- Tonna, M.; Marchesi, C.; Parmigiani, S. The Biological Origins of Rituals: An Interdisciplinary Perspective. Neurosci. Biobehav. Rev. 2019, 98, 95–106. [Google Scholar] [CrossRef]
- Tonna, M.; Ponzi, D.; Palanza, P.; Marchesi, C.; Parmigiani, S. Proximate and Ultimate Causes of Ritual Behavior. Behav. Brain Res. 2020, 393, 112772. [Google Scholar] [CrossRef] [PubMed]
- Wolmarans, D.W.; Stein, D.J.; Harvey, B.H. A Psycho-Behavioral Perspective on Modelling Obsessive-Compulsive Disorder (OCD) in Animals: The Role of Context. Curr. Med. Chem. 2018, 25, 5662–5689. [Google Scholar] [CrossRef]
- Rappaport, R.A. Ecology, Meaning, and Religion; North Atlantic Books: Berkeley, CA, USA, 1979; ISBN 978-0-938190-27-1. [Google Scholar]
- Liénard, P.; Boyer, P. Whence Collective Rituals? A Cultural Selection Model of Ritualized Behavior. Am. Anthropol. 2006, 108, 814–827. [Google Scholar] [CrossRef]
- Boyer, P.; Liénard, P. Why Ritualized Behavior? Precaution Systems and Action Parsing in Developmental, Pathological and Cultural Rituals. Behav. Brain Sci. 2006, 29, 595–613; discussion 613–650. [Google Scholar] [CrossRef] [PubMed]
- Graybiel, A.M. Habits, Rituals, and the Evaluative Brain. Annu. Rev. Neurosci. 2008, 31, 359–387. [Google Scholar] [CrossRef]
- Blanchard, D.C.; Blanchard, R.J.; Rodgers, R.J. Risk Assessment and Animal Models of Anxiety. In Animal Models in Psychopharmacology; Olivier, B., Mos, J., Slangen, J.L., Eds.; Birkhäuser: Basel, Switzerland, 1991; pp. 117–134. ISBN 978-3-0348-6421-3. [Google Scholar]
- Schleyer, M.; Diegelmann, S.; Michels, B.; Saumweber, T.; Gerber, B. ‘Decision Making’ in Larval Drosophila. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2013; Volume 22, pp. 41–55. ISBN 978-0-12-415823-8. [Google Scholar]
- Stürzl, W.; Zeil, J.; Boeddeker, N.; Hemmi, J.M. How Wasps Acquire and Use Views for Homing. Curr. Biol. 2016, 26, 470–482. [Google Scholar] [CrossRef]
- Szechtman, H.; Woody, E. Obsessive-Compulsive Disorder as a Disturbance of Security Motivation. Psychol. Rev. 2004, 111, 111–127. [Google Scholar] [CrossRef]
- Hirsh, J.B.; Mar, R.A.; Peterson, J.B. Psychological Entropy: A Framework for Understanding Uncertainty-Related Anxiety. Psychol. Rev. 2012, 119, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Barzilay, R.; Patrick, A.; Calkins, M.E.; Moore, T.M.; Gur, R.C.; Gur, R.E. Association Between Early-life Trauma and Obsessive Compulsive Symptoms in Community Youth. Depress. Anxiety 2019, 36, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, D.F.; Cervin, M.; Ottoni, R.; Marchesi, C.; Tonna, M. Psychotic Vulnerability and Its Associations with Clinical Characteristics in Adolescents with Obsessive-Compulsive Disorder. Res. Child Adolesc. Psychopathol. 2023, 51, 1535–1548. [Google Scholar] [CrossRef]
- Borrelli, D.F.; Ottoni, R.; Provettini, A.; Morabito, C.; Dell’Uva, L.; Marchesi, C.; Tonna, M. A Clinical Investigation of Psychotic Vulnerability in Early-Onset Obsessive-Compulsive Disorder through Cognitive-Perceptive Basic Symptoms. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 195–205. [Google Scholar] [CrossRef]
- Destrée, L.; Brierley, M.-E.E.; Albertella, L.; Jobson, L.; Fontenelle, L.F. The Effect of Childhood Trauma on the Severity of Obsessive-Compulsive Symptoms: A Systematic Review. J. Psychiatr. Res. 2021, 142, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Fontenelle, L.F.; Domingues, A.M.; Souza, W.F.; Mendlowicz, M.V.; De Menezes, G.B.; Figueira, I.L.; Versiani, M. History of Trauma and Dissociative Symptoms among Patients with Obsessive-Compulsive Disorder and Social Anxiety Disorder. Psychiatr. Q. 2007, 78, 241–250. [Google Scholar] [CrossRef]
- Gershuny, B.S.; Baer, L.; Radomsky, A.S.; Wilson, K.A.; Jenike, M.A. Connections Among Symptoms of Obsessive–Compulsive Disorder and Posttraumatic Stress Disorder: A Case Series. Behav. Res. Ther. 2003, 41, 1029–1041. [Google Scholar] [CrossRef]
- Eilam, D.; Izhar, R.; Mort, J. Threat Detection: Behavioral Practices in Animals and Humans. Neurosci. Biobehav. Rev. 2011, 35, 999–1006. [Google Scholar] [CrossRef]
- Briggs, E.S.; Price, I.R. The Relationship Between Adverse Childhood Experience and Obsessive-Compulsive Symptoms and Beliefs: The Role of Anxiety, Depression, and Experiential Avoidance. J. Anxiety Disord. 2009, 23, 1037–1046. [Google Scholar] [CrossRef]
- Bottas, A.; Cooke, R.G.; Richter, M.A. Comorbidity and Pathophysiology of Obsessive-Compulsive Disorder in Schizophrenia: Is There Evidence for a Schizo-Obsessive Subtype of Schizophrenia? J. Psychiatry Neurosci. JPN 2005, 30, 187–193. [Google Scholar]
- Pallanti, S.; Quercioli, L.; Hollander, E.; Kaplan, A.; Nechmad, A.; Ratzoni, G.; Poyurovsky, M.; Meged, S.; Avidan, G.; Fuchs, C.; et al. Obsessive-Compulsive Disorder in Patients with Schizophrenia or Schizoaffective Disorder. Am. J. Psychiatry 1997, 154, 271–273. [Google Scholar] [CrossRef]
- Tibbo, P.; Warneke, L. Obsessive-Compulsive Disorder in Schizophrenia: Epidemiologic and Biologic Overlap. J. Psychiatry Neurosci. JPN 1999, 24, 15–24. [Google Scholar]
- Swets, M.; Dekker, J.; Van Emmerik-van Oortmerssen, K.; Smid, G.E.; Smit, F.; De Haan, L.; Schoevers, R.A. The Obsessive Compulsive Spectrum in Schizophrenia, a Meta-Analysis and Meta-Regression Exploring Prevalence Rates. Schizophr. Res. 2014, 152, 458–468. [Google Scholar] [CrossRef]
- Cheng, Y.-F.; Chen, V.C.-H.; Yang, Y.-H.; Chen, K.-J.; Lee, Y.-C.; Lu, M.-L. Risk of Schizophrenia Among People with Obsessive-Compulsive Disorder: A Nationwide Population-Based Cohort Study. Schizophr. Res. 2019, 209, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Sterk, B.; Lankreijer, K.; Linszen, D.H.; De Haan, L. Obsessive–Compulsive Symptoms in First Episode Psychosis and in Subjects at Ultra High Risk for Developing Psychosis; Onset and Relationship to Psychotic Symptoms. Aust. N. Z. J. Psychiatry 2011, 45, 400–406. [Google Scholar] [CrossRef]
- Tonna, M.; Ottoni, R.; Paglia, F.; Ossola, P.; De Panfilis, C.; Marchesi, C. Obsessive–Compulsive Symptom Severity in Schizophrenia: A Janus Bifrons Effect on Functioning. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 63–69. [Google Scholar] [CrossRef]
- Tonna, M.; Ottoni, R.; Paglia, F.; Ossola, P.; De Panfilis, C.; Marchesi, C. Obsessive–Compulsive Symptoms Interact with Disorganization in Influencing Social Functioning in Schizophrenia. Schizophr. Res. 2016, 171, 35–41. [Google Scholar] [CrossRef]
- Tonna, M.; Borrelli, D.F.; Aguglia, E.; Bucci, P.; Carpiniello, B.; Dell’Osso, L.; Fagiolini, A.; Meneguzzo, P.; Monteleone, P.; Pompili, M.; et al. The Relationship Between Obsessive–Compulsive Symptoms and Real-Life Functioning in Schizophrenia: New Insights from the Multicenter Study of the Italian Network for Research on Psychoses. Eur. Psychiatry 2024, 67, e37. [Google Scholar] [CrossRef]
- Tonna, M.; Ottoni, R.; Borrelli, D.F.; Gambolò, L.; Dell’Uva, L.; Di Donna, A.; Parmigiani, S.; Marchesi, C. The Association between Childhood Trauma and Motor Structure in Obsessive-Compulsive Rituals: An Ethological and Evolutionary Approach. Psychol. Trauma Theory Res. Pract. Policy 2024, 16, S204–S214. [Google Scholar] [CrossRef]
- Zor, R.; Fineberg, N.; Eilam, D.; Hermesh, H. Video Telemetry and Behavioral Analysis Discriminate Between Compulsive Cleaning and Compulsive Checking in Obsessive-Compulsive Disorder. Eur. Neuropsychopharmacol. 2011, 21, 814–824. [Google Scholar] [CrossRef]
- Hunt, A.D.; St-John Smith, P.; Abed, R. Evobiopsychosocial Medicine. Evol. Med. Public Health 2023, 11, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Cap, H.; Deleporte, P.; Joachim, J.; Reby, D. Male Vocal Behavior and Phylogeny in Deer. Cladistics 2008, 24, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Japyassú, H.F.; Machado, F. de A. Coding Behavioural Data for Cladistic Analysis: Using Dynamic Homology Without Parsimony. Cladistics 2010, 26, 625–642. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Gambolò, L.; Ottoni, R.; Di Donna, A.; Marchesi, C.; Tonna, M. Uncovering the Motor Dynamics of Obsessive-Compulsive Rituals Through Cluster Analysis. J. Nerv. Ment. Dis. 2024, 212, 557–562. [Google Scholar] [CrossRef]
- Dalmaijer, E.S.; Nord, C.L.; Astle, D.E. Statistical Power for Cluster Analysis. BMC Bioinform. 2022, 23, 205. [Google Scholar] [CrossRef]
- Kang, H. Sample Size Determination and Power Analysis Using the G*Power Software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Goodman, W.K. The Yale-Brown Obsessive Compulsive Scale: I. Development, Use, and Reliability. Arch. Gen. Psychiatry 1989, 46, 1006. [Google Scholar] [CrossRef]
- Kayser, R.R.; Haney, M.; Raskin, M.; Arout, C.; Simpson, H.B. Acute Effects of Cannabinoids on Symptoms of Obsessive-Compulsive Disorder: A Human Laboratory Study. Depress. Anxiety 2020, 37, 801–811. [Google Scholar] [CrossRef]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. User’s Guide for the SCID-5-CV Structured Clinical Interview for DSM-5®® Disorders: Clinical Version; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2016; pp. xii, 158; ISBN 978-1-58562-524-6. [Google Scholar]
- Bernstein, D.P.; Ahluvalia, T.; Pogge, D.; Handelsman, L. Validity of the Childhood Trauma Questionnaire in an Adolescent Psychiatric Population. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 340–348. [Google Scholar] [CrossRef]
- Süllwold, L. Frankfurter Beschwerde-Fragebogen; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-3-642-61647-1. [Google Scholar]
- Fusar-Poli, P.; Bechdolf, A.; Taylor, M.J.; Bonoldi, I.; Carpenter, W.T.; Yung, A.R.; McGuire, P. At Risk for Schizophrenic or Affective Psychoses? A Meta-Analysis of DSM/ICD Diagnostic Outcomes in Individuals at High Clinical Risk. Schizophr. Bull. 2013, 39, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Morosini, P.L.; Magliano, L.; Brambilla, L.; Ugolini, S.; Pioli, R. Development, Reliability and Acceptability of a New Version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to Assess Routine Social Funtioning. Acta Psychiatr. Scand. 2000, 101, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Friard, O.; Gamba, M. BORIS: A Free, Versatile Open-source Event-logging Software for Video/Audio Coding and Live Observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Blanchette, G.; O’Keefe, R.; Benuskova, L. Inference of a Phylogenetic Tree: Hierarchical Clustering versus Genetic Algorithm. In AI 2012: Advances in Artificial Intelligence; Thielscher, M., Zhang, D., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7691, pp. 300–312. ISBN 978-3-642-35100-6. [Google Scholar]
- Chakerian, J.; Holmes, S. Computational Tools for Evaluating Phylogenetic and Hierarchical Clustering Trees. J. Comput. Graph. Stat. 2012, 21, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Guttula, S.; Rao, A.; Sridhar, G.; Chakravarthy, M.; Nageshwararo, K.; Rao, P. Cluster Analysis and Phylogenetic Relationship in Biomarker Identification of Type 2 Diabetes and Nephropathy. Int. J. Diabetes Dev. Ctries. 2010, 30, 52. [Google Scholar] [CrossRef]
- Li, R. Resizable, Rescalable and Free-Style Visualization of Hierarchical Clustering and Bioinformatics Analysis. J. Data Anal. Inf. Process. 2020, 8, 229–240. [Google Scholar] [CrossRef]
- Gao, C.X.; Dwyer, D.; Zhu, Y.; Smith, C.L.; Du, L.; Filia, K.M.; Bayer, J.; Menssink, J.M.; Wang, T.; Bergmeir, C.; et al. An Overview of Clustering Methods with Guidelines for Application in Mental Health Research. Psychiatry Res. 2023, 327, 115265. [Google Scholar] [CrossRef]
- Palmucci, M.; Giordano, M. Is Cell Composition Related to the Phylogenesis of Microalgae? An Investigation Using Hierarchical Cluster Analysis of Fourier Transform Infrared Spectra of Whole Cells. Environ. Exp. Bot. 2012, 75, 220–224. [Google Scholar] [CrossRef]
- Morrison, D.A. Phylogenetic Tree-Building. Int. J. Parasitol. 1996, 26, 589–617. [Google Scholar] [CrossRef]
- Brower, A.V.Z. What Is a Cladogram and What Is Not? Cladistics 2016, 32, 573–576. [Google Scholar] [CrossRef]
- Mickevich, M.F.; Weller, S.J. Evolutionary Character Analysis: Tracing Character Change on A Cladogram. Cladistics 1990, 6, 137–170. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Gotelli, N.J. Evolutionary Patterns of Altered Behavior and Susceptibility in Parasitized Hosts. Evolution 1996, 50, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.M. Fixed Action Patterns. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T.K., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 2746–2750. ISBN 978-3-319-55065-7. [Google Scholar]
- Stokes, A.W.; Williams, H.W. Courtship Feeding in Gallinaceous Birds. Auk 1971, 88, 543–559. [Google Scholar] [CrossRef]
- Klaus Immelmann, C.B. A Dictionary of Ethology; Harvard University Press: Cambridge, MA, USA, 1989; Volume 9. [Google Scholar]
- Sosis, R.; Handwerker, W.P. Psalms and Coping with Uncertainty: Religious Israeli Women’s Responses to the 2006 Lebanon War. Am. Anthropol. 2011, 113, 40–55. [Google Scholar] [CrossRef]
- Lang, M.; Krátký, J.; Shaver, J.H.; Jerotijević, D.; Xygalatas, D. Effects of Anxiety on Spontaneous Ritualized Behavior. Curr. Biol. CB 2015, 25, 1892–1897. [Google Scholar] [CrossRef]
- Uccheddu, S.; Pierantoni, L.; Ventura, L.; Gambolo, L.; Tonna, M. Obsessive Compulsive/Compulsive Disorder in Companion Animals: An Ethological Approach. J. Vet. Behav. 2024, 71, 57–62. [Google Scholar] [CrossRef]
- Belloch, A.; Fornés, G.; Carrasco, A.; López-Solá, C.; Alonso, P.; Menchón, J.M. Incompleteness and Not Just Right Experiences in the Explanation of Obsessive–Compulsive Disorder. Psychiatry Res. 2016, 236, 1–8. [Google Scholar] [CrossRef]
- Mancini, F. The Obsessive Mind: Understanding and Treating Obsessive-Compulsive Disorder, 1st ed.; Routledge: New York, NY, USA, 2018; ISBN 978-1-138-32107-6. [Google Scholar]
- Dar, R.; Kahn, D.T.; Carmeli, R. The Relationship Between Sensory Processing, Childhood Rituals and Obsessive-Compulsive Symptoms. J. Behav. Ther. Exp. Psychiatry 2012, 43, 679–684. [Google Scholar] [CrossRef]
- Bart, O.; Bar-Shalita, T.; Mansour, H.; Dar, R. Relationships among Sensory Responsiveness, Anxiety, and Ritual Behaviors in Children with and Without Atypical Sensory Responsiveness. Phys. Occup. Ther. Pediatr. 2017, 37, 322–331. [Google Scholar] [CrossRef]
- Tonna, M.; Borrelli, D.F.; Marchesi, C.; Gerra, M.C.; Dallabona, C. Childhood obsessive-compulsive disorder, epigenetics, and heterochrony: An evolutionary and developmental approach. Dev. Psychopathol. 2025, 18, 1–15. [Google Scholar] [CrossRef]


| Patients N = 31 | Controls N = 31 | p | |
|---|---|---|---|
| Female N (%) | 11 (35) | 11 (35) | 1 |
| Age, years M (SD) | 44.9 (20.1) | 38.5 (15.8) | 0.173 |
| SOFAS M (SD) | 65.6 (16.4) | 86.0 (4.0) | <0.001 |
| YBOCS M (SD) | 21.4 (7.6) | 1.1 (1.7) | <0.001 |
| FCQ M (SD) | 25.0 (23.6) | 4.1 (2.8) | <0.001 |
| CTQ M (SD) | 34.9 (13.7) | 27.7 (1.8) | 0.005 |
| Patients (N = 31) | Controls (N = 31) | p | |
|---|---|---|---|
| Duration (s) M (SD) | 48.9 (55.9) | 9.8 (10.0) | <0.001 |
| Total Number of Acts M (SD) | 10.5 (6.9) | 5.3 (4.3) | <0.001 |
| Number of Functional Acts M (SD) | 7.8 (6.8) | 5.2 (4.2) | 0.08 |
| Number of Non-Functional Acts M (SD) | 2.8 (2.6) | 0.1 (0.3) | <0.001 |
| Total Repetitions M (SD) | 32.6 (34.9) | 9.3 (10.7) | <0.001 |
| Repetitions of Functional Acts M (SD) | 24.5 (31.5) | 9.3 (10.6) | <0.015 |
| Repetitions of Non-Functional Acts M (SD) | 8.1 (9.7) | 0.1 (0.3) | <0.001 |
| Repetitiveness M (SD) | 2.7 (1.6) | 1.4 (0.6) | <0.001 |
| Non-Functionality M (SD) | 0.4 (0.3) | 0.2 (0.2) | <0.001 |
| Cluster 1 (n = 7) | Cluster 2 (n = 25) | Cluster 3 (n = 30) | One-Way ANOVA with Bonferroni Post Hoc Analysis | |
|---|---|---|---|---|
| OCD N (%) | 7 (100) | 18 (72) | 6 (20) | p |
| Repetitiveness M (SD) | 5.5 (0.7) | 1.6 (0.6) | 1.7 (0.7) | <0.001 a,b |
| Non-Functionality M (SD) | 0.2 (1.07) | 0.6 (0.2) | 0.1 (0.1) | <0.001 c,d |
| Iterative (n = 13) | Idiosyncratic (n = 14) | p | |
|---|---|---|---|
| Age (y) M (SD) | 50.0 (19.4) | 40.9 (21.2) | 0.277 |
| Onset Age(y) M (SD) | 24.0 (12.2) | 29.6 (12.3) | 0.266 |
| Female N (%) | 7 (53.8) | 4 (28.6) | 0.182 |
| YBOCS M (SD) | 21.4 (9.3) | 18.1 (8.9) | 0.363 |
| SOFAS M (SD) | 63.5 (15.9) | 65.0 (16.4) | 0.807 |
| FCQM (SD) | 35.4 (27.7) | 17.0 (15.2) | 0.048 |
| CTQ | 42.3 (13.1) | 33.4 (6.1) | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambolò, L.; Di Donna, A.; Ottoni, R.; Parmigiani, S.; Marchesi, C.; Tonna, M. Psychopathological Implications of Behavioral Patterns in Obsessive–Compulsive Rituals: A Hierarchical Analysis. Brain Sci. 2025, 15, 552. https://doi.org/10.3390/brainsci15060552
Gambolò L, Di Donna A, Ottoni R, Parmigiani S, Marchesi C, Tonna M. Psychopathological Implications of Behavioral Patterns in Obsessive–Compulsive Rituals: A Hierarchical Analysis. Brain Sciences. 2025; 15(6):552. https://doi.org/10.3390/brainsci15060552
Chicago/Turabian StyleGambolò, Luca, Anna Di Donna, Rebecca Ottoni, Stefano Parmigiani, Carlo Marchesi, and Matteo Tonna. 2025. "Psychopathological Implications of Behavioral Patterns in Obsessive–Compulsive Rituals: A Hierarchical Analysis" Brain Sciences 15, no. 6: 552. https://doi.org/10.3390/brainsci15060552
APA StyleGambolò, L., Di Donna, A., Ottoni, R., Parmigiani, S., Marchesi, C., & Tonna, M. (2025). Psychopathological Implications of Behavioral Patterns in Obsessive–Compulsive Rituals: A Hierarchical Analysis. Brain Sciences, 15(6), 552. https://doi.org/10.3390/brainsci15060552







