A New Method for Inducing Mental Fatigue: A High Mental Workload Task Paradigm Based on Complex Cognitive Abilities and Time Pressure
Abstract
1. Introduction
1.1. MF and Theoretical Models
1.2. Methods for Inducing MF Through Cognitive Tasks
2. Methods
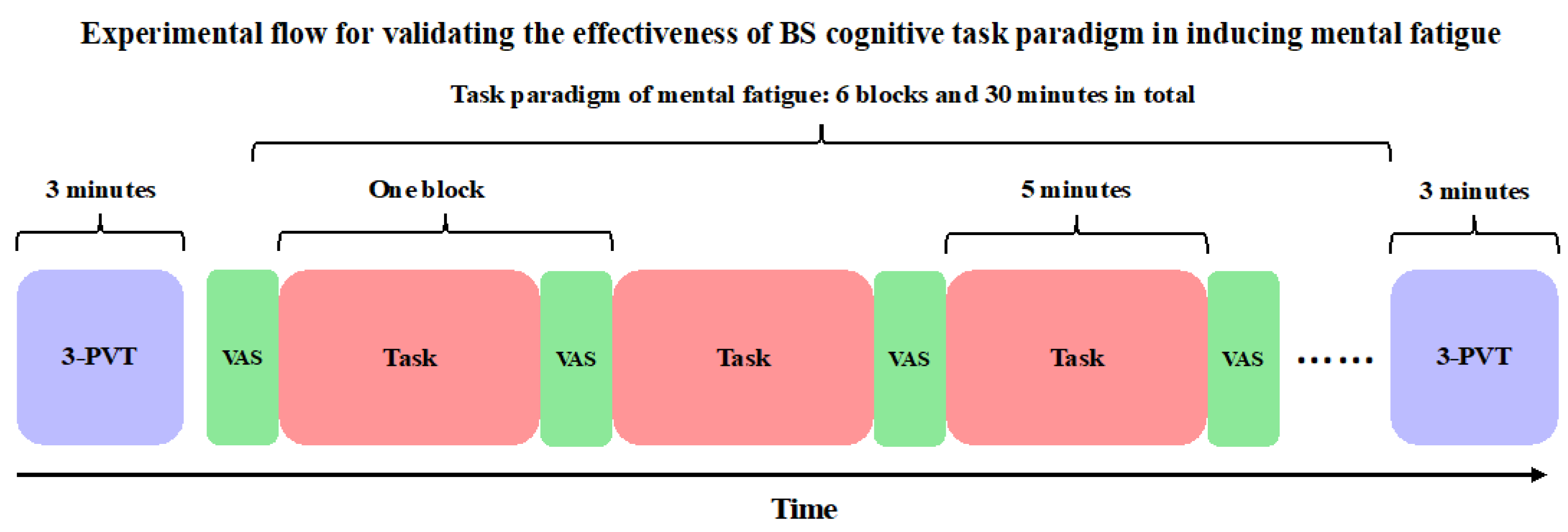
2.1. Research Design
2.2. Participants
2.3. Materials
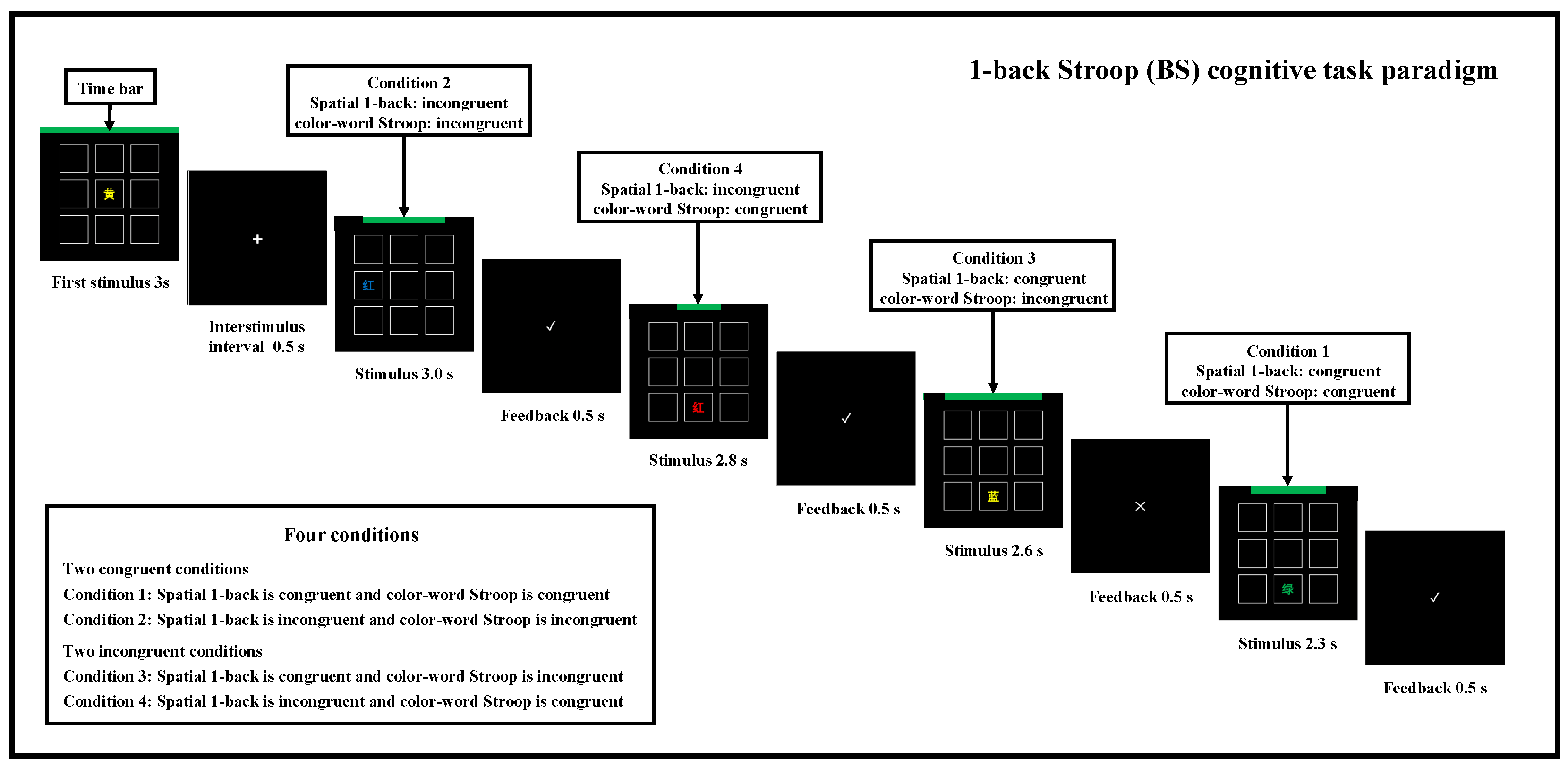
2.3.1. BS Task Paradigm
2.3.2. 2-Back Task
2.3.3. NASA-TLX
2.3.4. PVT
2.3.5. VAS
2.4. Statistical Analysis
2.4.1. Statistical Analysis for Construction of Task Complexity and Time Pressure for BS Cognitive Task Paradigm
2.4.2. Statistical Analysis for HMW Induction Validity
2.4.3. Statistical Analysis for MF Induction Validity
3. Results
3.1. Construction of Task Complexity and Time Pressure for BS Cognitive Task Paradigm
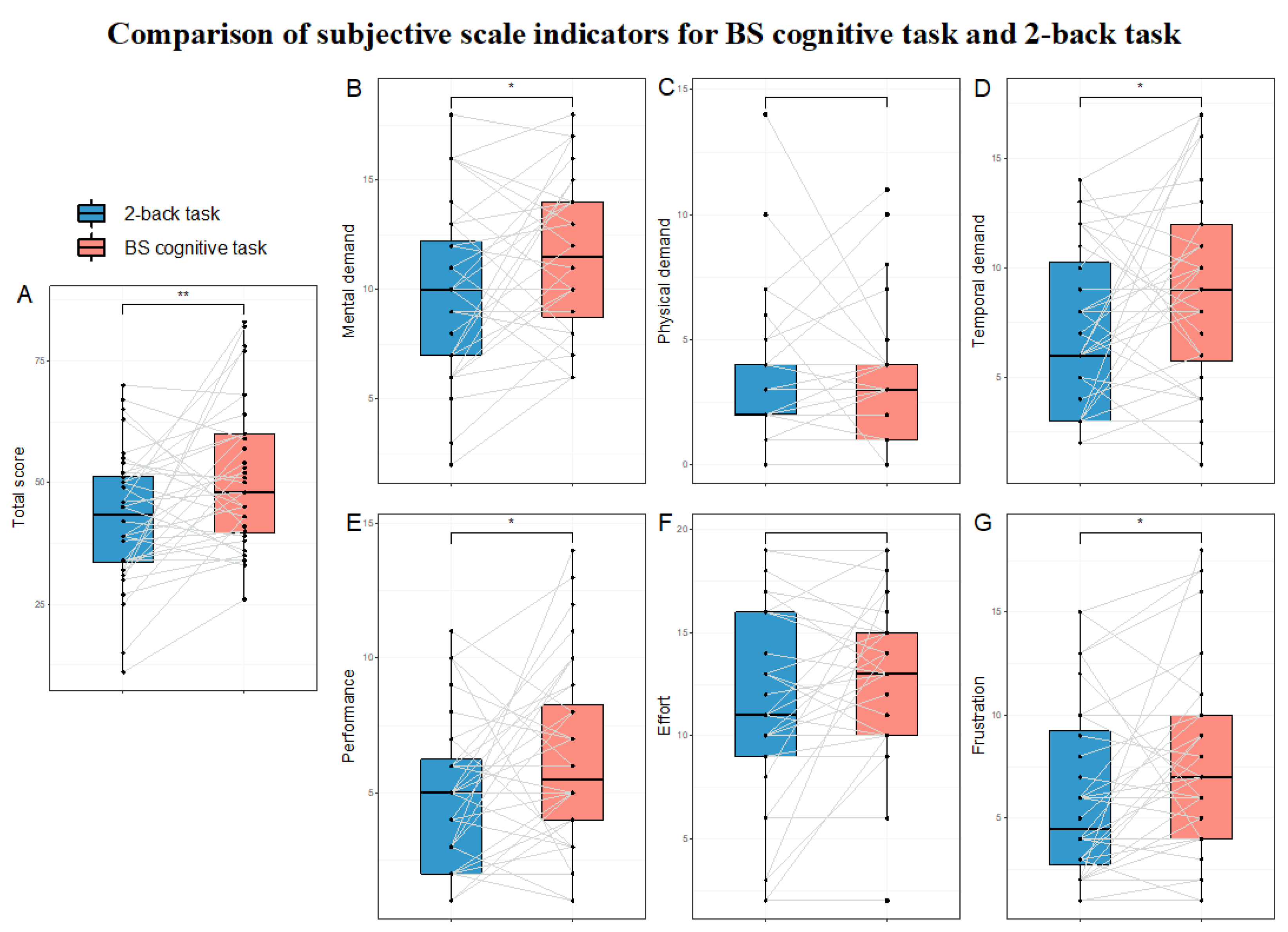
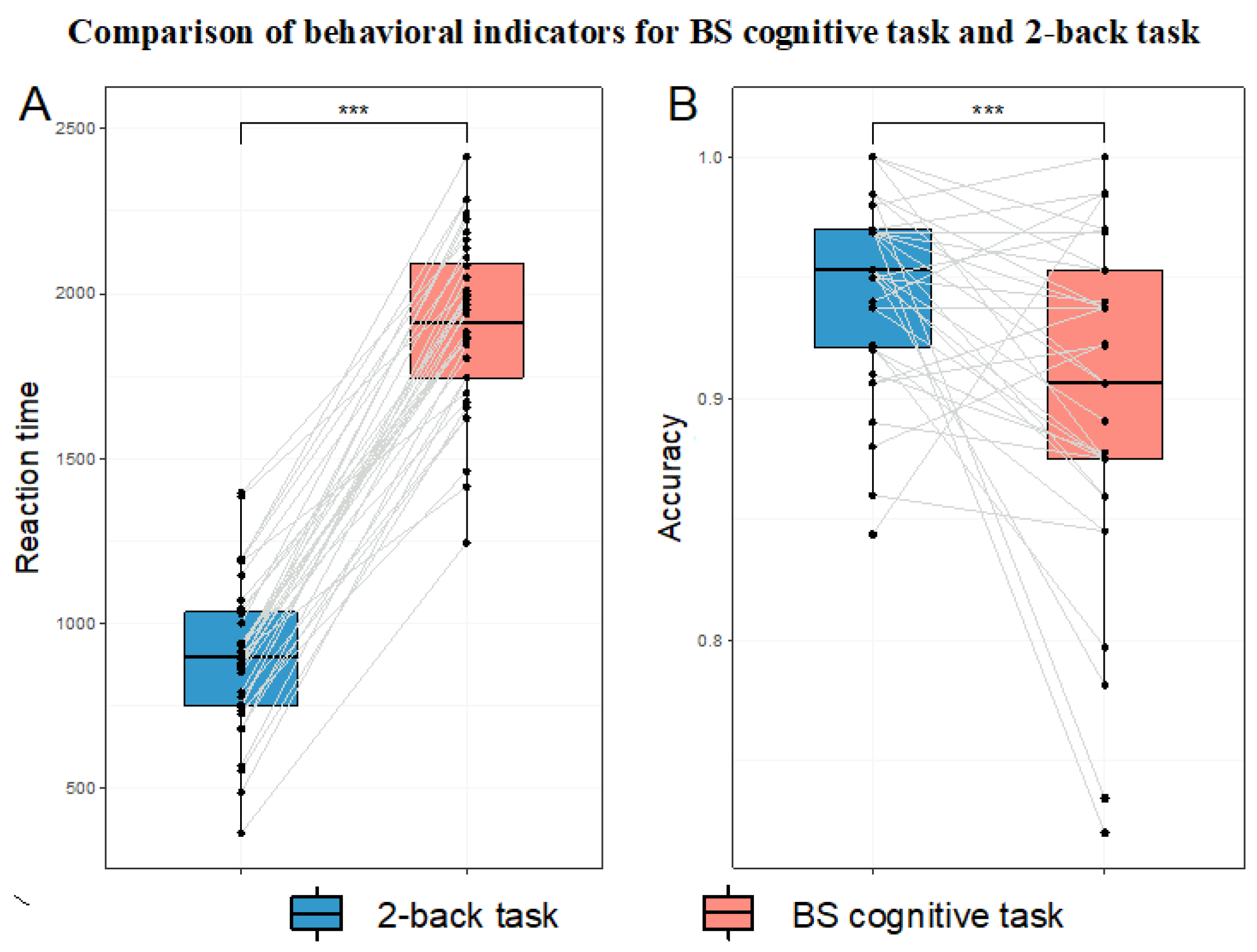
3.2. Validation of HMW Induction Effectiveness
3.2.1. Comparison of Subjective Scale Indicators
3.2.2. Comparison of Behavioral Indicators
3.3. Validation of MF Induction Effectiveness
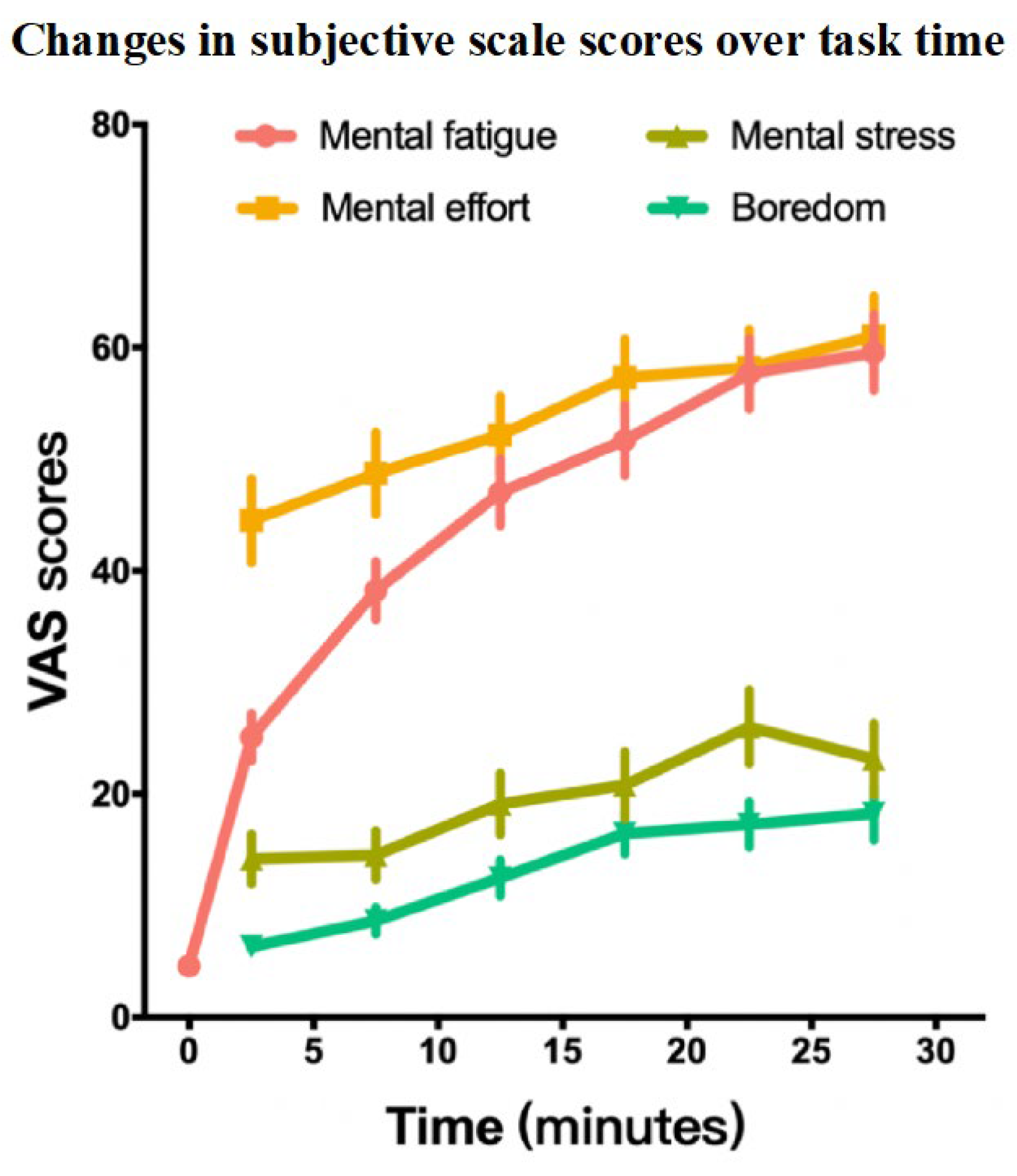
3.3.1. Comparison of VAS Indicators
3.3.2. Comparison of BS Cognitive Task Behavioral Indicators
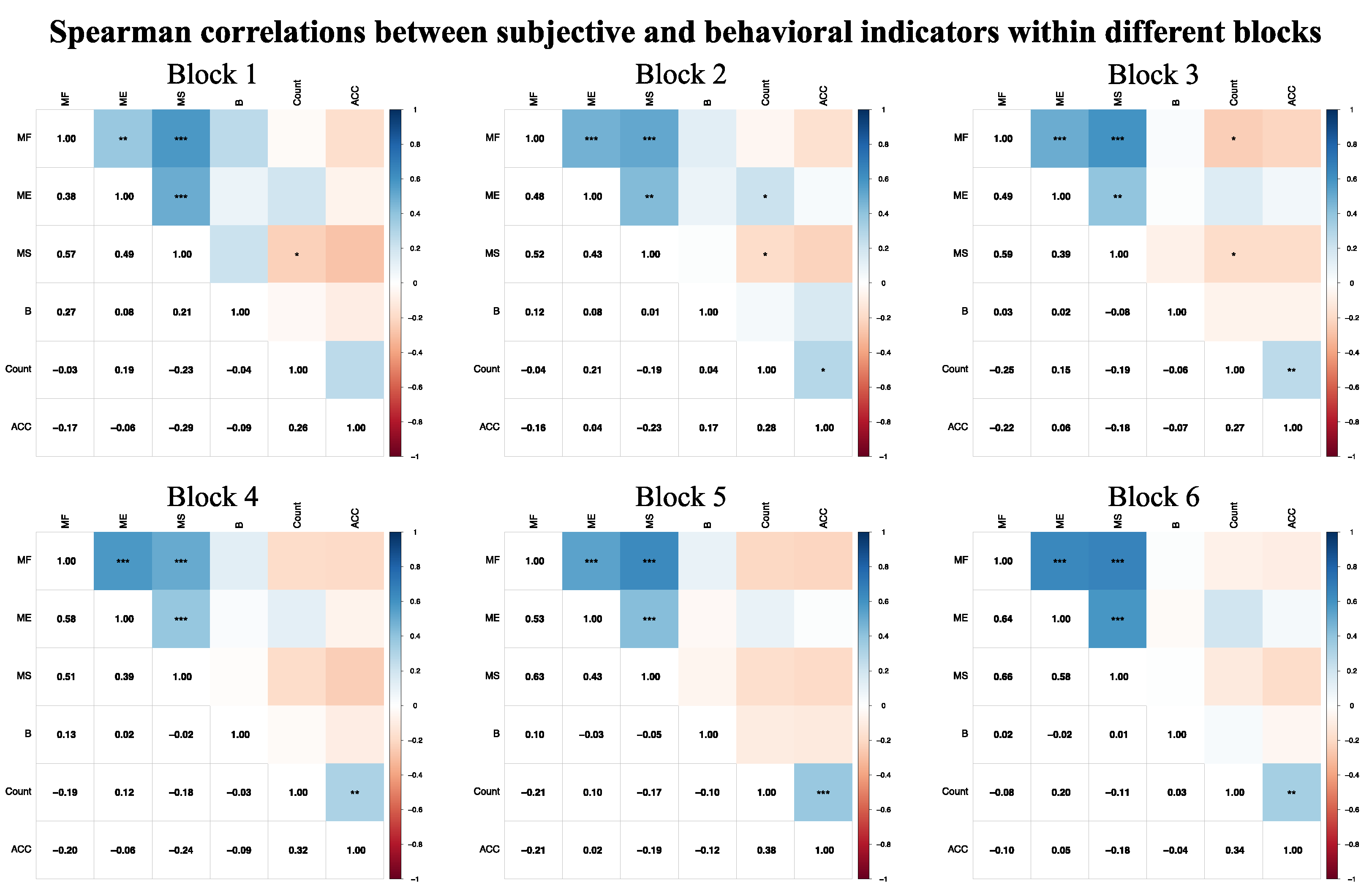
3.3.3. Spearman Correlations Between Subjective and Behavioral Indicators
3.3.4. Comparison of PVT Task Behavioral Indicators
4. Discussion
4.1. Validation of HMW Induction Effectiveness
4.2. Validation of MF Induction Effectiveness
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habay, J.; Van Cutsem, J.; Verschueren, J.; De Bock, S.; Proost, M.; De Wachter, J.; Tassignon, B.; Meeusen, R.; Roelands, B. Mental fatigue and sport-specific psychomotor performance: A systematic review. Sports Med. 2021, 51, 1527–1548. [Google Scholar] [CrossRef] [PubMed]
- Salihu, A.T.; Hill, K.D.; Jaberzadeh, S. Neural mechanisms underlying state mental fatigue: A systematic review and activation likelihood estimation meta-analysis. Prog. Neurobiol. 2022, 33, 889–917. [Google Scholar] [CrossRef]
- Van Cutsem, J.; Marcora, S.; De Pauw, K.; Bailey, S.; Meeusen, R.; Roelands, B. The effects of mental fatigue on physical performance: A systematic review. Sports Med. 2017, 47, 1569–1588. [Google Scholar] [CrossRef]
- Behrens, M.; Gube, M.; Chaabene, H.; Prieske, O.; Zenon, A.; Broscheid, K.-C.; Schega, L.; Husmann, F.; Weippert, M. Fatigue and human performance: An updated framework. Sports Med. 2022, 53, 7–31. [Google Scholar] [CrossRef]
- Wiehler, A.; Branzoli, F.; Adanyeguh, I.; Mochel, F.; Pessiglione, M. A neuro-metabolic account of why daylong cognitive work alters the control of economic decisions. Curr. Biol. 2022, 32, 3564–3575. [Google Scholar] [CrossRef]
- Holtzer, R.; Shuman, M.; Mahoney, J.R.; Lipton, R.; Verghese, J. Cognitive fatigue defined in the context of attention networks. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2011, 18, 108–128. [Google Scholar] [CrossRef]
- Qi, P.; Ru, H.; Gao, L.; Zhang, X.; Zhou, T.; Tian, Y.; Thakor, N.; Bezerianos, A.; Li, J.; Sun, Y. Neural mechanisms of mental fatigue revisited: New insights from the brain connectome. Engineering 2019, 5, 276–286. [Google Scholar] [CrossRef]
- Herlambang, M.B.; Taatgen, N.A.; Cnossen, F. The role of motivation as a factor in mental fatigue. Hum. Factors 2019, 61, 1171–1185. [Google Scholar] [CrossRef]
- Kurzban, R.; Duckworth, A.; Kable, J.W.; Myers, J. An opportunity cost model of subjective effort and task performance. Behav. Brain Sci. 2013, 36, 661–679. [Google Scholar] [CrossRef]
- Boksem, M.A.; Tops, M. Mental fatigue: Costs and benefits. Brain Res. Rev. 2008, 59, 125–139. [Google Scholar] [CrossRef]
- Pattyn, N.; Neyt, X.; Henderickx, D.; Soetens, E. Psychophysiological investigation of vigilance decrement: Boredom or cognitive fatigue? Physiol. Behav. 2008, 93, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Langner, R.; Eickhoff, S.B. Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull. 2013, 139, 870–900. [Google Scholar] [CrossRef] [PubMed]
- Grier, R.A.; Warm, J.S.; Dember, W.N.; Matthews, G.; Galinsky, T.L.; Szalma, J.L.; Parasuraman, R. The vigilance decrement reflects limitations in effortful attention, not mindlessness. Hum. Factors 2003, 45, 349–359. [Google Scholar] [CrossRef]
- Helton, W.S.; Russell, P.N. Feature absence–presence and two theories of lapses of sustained attention. Psychol. Res. 2010, 75, 384–392. [Google Scholar] [CrossRef]
- Holroyd, C.B. The Waste Disposal Problem of Effortful Control; Psychology Press: London, UK, 2016. [Google Scholar]
- Bafna, T.; Hansen, J.P. Mental fatigue measurement using eye metrics: A systematic literature review. Psychophysiology 2021, 58, e13828. [Google Scholar] [CrossRef]
- Tran, Y.; Craig, A.; Craig, R.; Chai, R.; Nguyen, H. The influence of mental fatigue on brain activity: Evidence from a systematic review with meta-analyses. Psychophysiology 2020, 57, e13554. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, M.; Yang, Y.; Gao, J.; Rao, N.; Lin, P. The reorganization of human brain networks modulated by driving mental fatigue. IEEE J. Biomed. Health Inform. 2017, 21, 743–755. [Google Scholar] [CrossRef]
- Dimitrakopoulos, G.N.; Kakkos, I.; Thakor, N.V.; Bezerianos, A.; Sun, Y. A mental fatigue index based on regression using mulitband EEG features with application in simulated driving. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju-do, Republic of Korea, 11–15 July 2017; pp. 3220–3223. [Google Scholar]
- Zhai, Y.; Li, Y.; Zhou, S.; Zhang, C.; Luo, E.; Tang, C.; Xie, K. Mental fatigue decreases complexity: Evidence from multiscale entropy analysis of instantaneous frequency variation in alpha rhythm. Front. Hum. Neurosci. 2022, 16, 906735. [Google Scholar] [CrossRef]
- Li, W.; Cheng, S.; Wang, H.; Chang, Y. EEG microstate changes according to mental fatigue induced by aircraft piloting simulation: An exploratory study. Behav. Brain Res. 2022, 438, 114203. [Google Scholar] [CrossRef]
- Quiquempoix, M.; Sauvet, F.; Erblang, M.; Van Beers, P.; Guillard, M.; Drogou, C.; Trignol, A.; Vergez, A.; Léger, D.; Chennaoui, M.; et al. Effects of caffeine intake on cognitive performance related to total sleep deprivation and time on task: A randomized cross-over double-blind study. Nat. Sci. Sleep 2022, 14, 457–473. [Google Scholar] [CrossRef]
- Heaton, K.J.; Maule, A.L.; Maruta, J.; Kryskow, E.M.; Ghajar, J. Attention and visual tracking degradation during acute sleep deprivation in a military sample. Aviat. Space Environ. Med. 2014, 85, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Kayser, K.C.; Puig, V.A.; Estepp, J.R. Predicting and mitigating fatigue effects due to sleep deprivation: A review. Front. Neurosci. 2022, 16, 930280. [Google Scholar] [CrossRef]
- Ikegami, K.; Ogyu, S.; Arakomo, Y.; Suzuki, K.; Mafune, K.; Hiro, H.; Nagata, S. Recovery of cognitive performance and fatigue after one night of sleep deprivation. J. Occup. Health 2009, 51, 412–422. [Google Scholar] [CrossRef]
- Dallaway, N.; Lucas, S.J.E.; Ring, C. Cognitive tasks elicit mental fatigue and impair subsequent physical task endurance: Effects of task duration and type. Psychophysiology 2022, 59, e14126. [Google Scholar] [CrossRef]
- Watanabe, K.; Sasaki, A.T.; Tajima, K.; Mizuseki, K.; Mizuno, K.; Watanabe, Y. Mental fatigue is linked with attentional bias for sad stimuli. Sci. Rep. 2019, 9, 8797. [Google Scholar] [CrossRef]
- Angius, L.; Merlini, M.; Hopker, J.; Bianchi, M.; Fois, F.; Piras, F.; Cugia, P.; Russell, J.; Marcora, S.M. Physical and mental fatigue reduce psychomotor vigilance in professional football players. Int. J. Sports Physiol. Perform. 2022, 17, 1391–1398. [Google Scholar] [CrossRef]
- Magnuson, J.R.; Doesburg, S.M.; McNeil, C.J. Development and recovery time of mental fatigue and its impact on motor function. Biol. Psychol. 2021, 161, 108076. [Google Scholar] [CrossRef]
- Xu, X.; Tang, J.; Xu, T.; Lin, M. Mental fatigue degree recognition based on relative band power and fuzzy entropy of EEG. Int. J. Environ. Res. Public Health 2023, 20, 1447. [Google Scholar] [CrossRef]
- Bruijel, J.; Quaedflieg, C.; Otto, T.; van de Ven, V.; Stapert, S.; van Heugten, C.; Vermeeren, A. Task-induced subjective fatigue and resting-state striatal connectivity following traumatic brain injury. NeuroImage Clin. 2022, 33, 102936. [Google Scholar] [CrossRef]
- Kowalski, K.L.; Tierney, B.C.; Christie, A.D. Mental fatigue does not substantially alter neuromuscular function in young, healthy males and females. Physiol. Behav. 2022, 253, 113855. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Bhavsar, R.; Mao, T.; Mamikonyan, E.; Raizen, D.; Detre, J.A.; Weintraub, D.; Rao, H. Perfusion imaging of fatigue and time-on-task effects in patients with Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 901203. [Google Scholar] [CrossRef] [PubMed]
- Gui, D.; Xu, S.; Zhu, S.; Fang, Z.; Spaeth, A.M.; Xin, Y.; Feng, T.; Rao, H. Resting spontaneous activity in the default mode network predicts performance decline during prolonged attention workload. NeuroImage 2015, 120, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Borragán, G.; Slama, H.; Bartolomei, M.; Peigneux, P. Cognitive fatigue: A Time-based Resource-sharing account. Cortex 2017, 89, 71–84. [Google Scholar] [CrossRef]
- Salomone, M.; Burle, B.; Fabre, L.; Berberian, B. An electromyographic analysis of the effects of cognitive fatigue on online and anticipatory action control. Front. Hum. Neurosci. 2021, 14, 615046. [Google Scholar] [CrossRef]
- Borragán, G.; Slama, H.; Destrebecqz, A.; Peigneux, P. Cognitive fatigue facilitates procedural sequence learning. Front. Hum. Neurosci. 2016, 10, 86. [Google Scholar] [CrossRef]
- Han, M.; Wang, S.; Wang, Y.; Niu, H.; Liu, Z.; Liu, T. Research Progress of Pilots’ Mental Fatigue Evaluation Based on EEG. Manned Spaceflight 2021, 27, 639–645. [Google Scholar]
- Mandrick, K.; Peysakhovich, V.; Rémy, F.; Lepron, E.; Causse, M. Neural and psychophysiological correlates of human performance under stress and high mental workload. Biol. Psychol. 2016, 121, 62–73. [Google Scholar] [CrossRef]
- Dasari, D.; Shou, G.; Ding, L. ICA-Derived EEG Correlates to mental fatigue, effort, and workload in a realistically simulated air traffic control task. Front. Neurosci. 2017, 11, 297. [Google Scholar] [CrossRef]
- Ackerman, P.L.; Kanfer, R. Test length and cognitive fatigue: An empirical examination of effects on performance and test-taker reactions. J. Exp. Psychol. Appl. 2009, 15, 163–181. [Google Scholar] [CrossRef]
- Lim, J.; Wu, W.-C.; Wang, J.; Detre, J.A.; Dinges, D.F.; Rao, H. Imaging brain fatigue from sustained mental workload: An ASL perfusion study of the time-on-task effect. NeuroImage 2010, 49, 3426–3435. [Google Scholar] [CrossRef]
- Dehais, F.; Lafont, A.; Roy, R.; Fairclough, S. A neuroergonomics approach to mental workload, engagement and human performance. Front. Neurosci. 2020, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, K.; Hodder, S.; Lloyd, A. A comparison of methods used for inducing mental fatigue in performance research: Indi-vidualised, dual-task and short duration cognitive tests are most effective. Ergonomics 2020, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D. Attention and Effort; Prentice-Hall: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Borghini, G.; Astolfi, L.; Vecchiato, G.; Mattia, D.; Babiloni, F. Measuring neurophysiological signals in aircraft pilots and car drivers for the assessment of mental workload, fatigue and drowsiness. Neurosci. Biobehav. Rev. 2012, 44, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Young, M.S.; Brookhuis, K.A.; Wickens, C.D.; Hancock, P.A. State of science: Mental workload in ergonomics. Ergonomics 2015, 58, 1–17. [Google Scholar] [CrossRef]
- Longo, L.; Wickens, C.D.; Hancock, G.M.; Hancock, P.A. Human mental workload: A survey and a novel inclusive definition. Front. Psychol. 2022, 13, 883321. [Google Scholar] [CrossRef]
- Diaz-Piedra, C.; Rieiro, H.; Suárez, J.; Rios-Tejada, F.; Catena, A.; Di Stasi, L.L. Fatigue in the military: Towards a fatigue detection test based on the saccadic velocity. Physiol. Meas. 2016, 37, 62–75. [Google Scholar] [CrossRef]
- Martin, K.; Périard, J.; Rattray, B.; Pyne, D.B. Physiological factors which influence cognitive performance in military personnel. Hum. Factors 2019, 62, 93–123. [Google Scholar] [CrossRef]
- Flood, A.; Keegan, R.J. Cognitive resilience to psychological stress in military personnel. Front. Psychol. 2022, 13, 809003. [Google Scholar] [CrossRef]
- Milyavskaya, M.; Inzlicht, M.; Johnson, T.; Larson, M.J. Reward sensitivity following boredom and cognitive effort: A high-powered neurophysiological investigation. Neuropsychologia 2019, 123, 159–168. [Google Scholar] [CrossRef]
- Siegel, A.I.; Wolf, J.J. Man-Machine Simulation Models: Psychosocial and Performance Interaction; Wiley-Interscience: Hoboken, NJ, USA, 1969. [Google Scholar]
- Wang, L.; He, X.; Chen, Y. Quantitative relationship model between workload and time pressure under different flight operation tasks. Int. J. Ind. Ergon. 2016, 54, 93–102. [Google Scholar] [CrossRef]
- Rendon-Velez, E.; van Leeuwen, P.M.; Happee, R.; Horváth, I.; van der Vegte, W.; de Winter, J. The effects of time pressure on driver performance and physiological activity: A driving simulator study. Transp. Res. Part F Traffic Psychol. Behav. 2016, 41, 150–169. [Google Scholar] [CrossRef]
- Jung, W.; Park, J.; Kim, J. A simulator study and its application to an HRA in NPPs. In Proceedings of the 2007 IEEE 8th Human Factors and Power Plants and HPRCT 13th Annual Meeting, Monterey, CA, USA, 26–31 August 2007. [Google Scholar]
- Park, J.; Jung, W. OPERA—A human performance database under simulated emergencies of nuclear power plants. Reliab. Eng. Syst. Saf. 2007, 92, 503–519. [Google Scholar] [CrossRef]
- Hogervorst, M.A.; Brouwer, A.-M.; van Erp, J.B.F. Combining and comparing EEG, peripheral physiology and eye-related measures for the assessment of mental workload. Front. Neurosci. 2014, 8, 322. [Google Scholar] [CrossRef]
- Liu, Y.; Ayaz, H.; Shewokis, P.A. Mental workload classification with concurrent electroencephalography and functional near-infrared spectroscopy. Brain-Comput. Interfaces 2017, 4, 175–185. [Google Scholar] [CrossRef]
- Lim, L.G.; Ung, W.C.; Chan, Y.L.; Lu, C.-K.; Sutoko, S.; Funane, T.; Kiguchi, M.; Tang, T.B. A unified analytical framework with multiple fNIRS features for mental workload assessment in the prefrontal cortex. IEEE Trans. Neural Syst. Rehabilit. Eng. 2020, 28, 2367–2376. [Google Scholar] [CrossRef]
- Baldwin, C.L.; Penaranda, B. Adaptive training using an artificial neural network and EEG metrics for within- and cross-task workload classification. NeuroImage 2012, 59, 48–56. [Google Scholar] [CrossRef]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (task load index): Results of empirical and theoretical research. Adv. Psychol. 1988, 52, 139–183. [Google Scholar]
- Hart, S.G. Nasa-task load index (NASA-TLX); 20 years later. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, Sydney, Australia, 20–22 November 2006; Volume 50, pp. 904–908. [Google Scholar]
- Kabilmiharbi, N.; Khamis, N.K.; Noh, N.A. Commonly used assessment method to evaluate mental workload for multiple driving distractions: A systematic review. Iran. J. Public Health 2022, 51, 482–494. [Google Scholar]
- Galy, E.; Paxion, J.; Berthelon, C. Measuring mental workload with the NASA-TLX needs to examine each dimension rather than relying on the global score: An example with driving. Ergonomics 2018, 61, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, J.; Rogers, N.L.; Dinges, D.F. Psychomotor vigilance performance: A neurocognitive assay sensitive to sleep loss. In Sleep Deprivation: Clinical Issues, Pharmacology and Sleep Loss Effects; Marcel Dekker, Inc.: New York, NY, USA, 2010; pp. 39–70. [Google Scholar]
- Lim, J.; Dinges, D.F. Sleep deprivation and vigilant attention. Ann. N. Y. Acad. Sci. 2008, 1129, 305–322. [Google Scholar] [CrossRef]
- Basner, M.; Dinges, D.F. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep 2011, 34, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Basner, M.; Mollicone, D.; Dinges, D.F. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 2011, 69, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Lamond, N.; Dawson, D.; Roach, G.D. Fatigue assessment in the field: Validation of a hand-held electronic psychomotor vigilance task. Aviat. Space Environ. Med. 2005, 76, 486–489. [Google Scholar]
- Lee, I.S.; Bardwell, W.A.; Ancoli-Israel, S.; Dimsdale, J.E. Number of lapses during the psychomotor vigilance task as an objective measure of fatigue. J. Clin. Sleep Med. 2010, 6, 163–168. [Google Scholar] [CrossRef]
- Linnhoff, S.; Fiene, M.; Heinze, H.-J.; Zaehle, T. Cognitive fatigue in multiple sclerosis: An objective approach to diagnosis and treatment by transcranial electrical stimulation. Brain Sci. 2019, 9, 100. [Google Scholar] [CrossRef]
- Smith, M.R.; Chai, R.; Nguyen, H.T.; Marcora, S.M.; Coutts, A.J. Comparing the effects of three cognitive tasks on indicators of mental fatigue. J. Psychol. 2019, 153, 759–783. [Google Scholar] [CrossRef]
- Xiao, F.; Sun, T.; Cai, X.-L.; Chen, Q.-F. Task relevance effect on number/shape conflict detection in the number-matching task: An ERP study. Acta Psychol. 2020, 208, 103126. [Google Scholar] [CrossRef]
- Maule, A.; Hockey, G.J.; Bdzola, L. Effects of time-pressure on decision-making under uncertainty: Changes in affective state and information processing strategy. Acta Psychol. 2000, 104, 283–301. [Google Scholar] [CrossRef]
- Sussman, R.F.; Sekuler, R. Feeling rushed? Perceived time pressure impacts executive function and stress. Acta Psychol. 2022, 229, 103702. [Google Scholar] [CrossRef]
- Saunders, B.; Inzlicht, M. Vigour and fatigue: How variation in affect underlies effective self-control. In Motivation and Cognitive Control; Braver, T.S., Ed.; Routledge/Taylor & Francis Group: Oxfordshire, UK, 2015; pp. 211–234. [Google Scholar]
- Shigihara, Y.; Tanaka, M.; Ishii, A.; Kanai, E.; Funakura, M.; Watanabe, Y. Two types of mental fatigue affect spontaneous oscillatory brain activities in different ways. Behav. Brain Funct. 2013, 9, 2. [Google Scholar] [CrossRef]
- Shenhav, A.; Musslick, S.; Lieder, F.; Kool, W.; Griffiths, T.L.; Cohen, J.D.; Botvinick, M.M. Toward a rational and mechanistic account of mental effort. Annu. Rev. Neurosci. 2017, 40, 99–124. [Google Scholar] [CrossRef] [PubMed]
- Szalma, J.L.; Warm, J.S.; Matthews, G.; Dember, W.N.; Weiler, E.M.; Meier, A.; Eggemeier, F.T. Effects of sensory modality and task duration on performance, workload, and stress in sustained attention. Hum. Factors 2004, 46, 219–233. [Google Scholar] [CrossRef]
- Goldberg, Y.K.; Eastwood, J.D.; LaGuardia, J.; Danckert, J. Boredom: An emotional experience distinct from apathy, anhedonia, or depression. J. Soc. Clin. Psychol. 2011, 30, 647–666. [Google Scholar] [CrossRef]
- Raffaelli, Q.; Mills, C.; Christoff, K. The knowns and unknowns of boredom: A review of the literature. Exp. Brain Res. 2017, 236, 2451–2462. [Google Scholar] [CrossRef]
- Hagger, M.S.; Chatzisarantis, N.L.D.; Alberts, H.; Anggono, C.O.; Batailler, C.; Birt, A.R.; Brand, R.; Brandt, M.J.; Brewer, G.; Bruyneel, S.; et al. A multilab preregistered replication of the ego-depletion effect. Perspect. Psychol. Sci. 2016, 11, 546–573. [Google Scholar] [CrossRef]
- Hockey, G. A Motivational Control Theory of Cognitive Fatigue; American Psychological Association: Worcester, MA, USA, 2011. [Google Scholar]
- Noé, F.; Hachard, B.; Ceyte, H.; Bru, N.; Paillard, T. Relationship between the level of mental fatigue induced by a prolonged cognitive task and the degree of balance disturbance. Exp. Brain Res. 2021, 239, 2273–2283. [Google Scholar] [CrossRef]
- Verschueren, J.O.; Tassignon, B.; Proost, M.; Teugels, A.; Van Cutsem, J.; Roelands, B.; Verhagen, E.; Meeusen, R. Does Mental Fatigue Negatively Affect Outcomes of Functional Performance Tests? Med. Sci. Sports Exerc. 2020, 52, 2002–2010. [Google Scholar] [CrossRef]
- Blanken, T.F.; Bathelt, J.; Deserno, M.K.; Voge, L.; Borsboom, D.; Douw, L. Connecting brain and behavior in clinical neuroscience: A network approach. Neurosci. Biobehav. Rev. 2021, 130, 81–90. [Google Scholar] [CrossRef]
- Hilland, E.; Landrø, N.I.; Kraft, B.; Tamnes, C.K.; Fried, E.I.; Maglanoc, L.A.; Jonassen, R. Exploring the links between specific depression symptoms and brain structure: A network study. Psychiatry Clin. Neurosci. 2019, 74, 220–221. [Google Scholar] [CrossRef]
- Bailey, A.; Channon, S.; Beaumont, J.G. The relationship between subjective fatigue and cognitive fatigue in advanced multiple sclerosis. Mult. Scler. J. 2007, 13, 73–80. [Google Scholar] [CrossRef]
- Morrow, S.; Weinstock-Guttman, B.; Munschauer, F.; Hojnacki, D.; Benedict, R. Subjective fatigue is not associated with cognitive impairment in multiple sclerosis: Cross-sectional and longitudinal analysis. Mult. Scler. J. 2009, 15, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, S.; Hassa, T.; Claros-Salinas, D.; Dettmers, C.; Schoenfeld, M.A. Neural correlates of effort-dependent and effort-independent cognitive fatigue components in patients with multiple sclerosis. Mult. Scler. J. 2017, 25, 256–266. [Google Scholar] [CrossRef]
- Dang, J.; King, K.M.; Inzlicht, M. Why are self-report and behavioral measures weakly correlated? Trends Cogn. Sci. 2020, 24, 267–269. [Google Scholar] [CrossRef]
- DeLuca, J.; Genova, H.M.; Capili, E.J.; Wylie, G.R. Functional neuroimaging of fatigue. Phys. Med. Rehabil. Clin. N. Am. 2009, 20, 325–337. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Guerdoux-Ninot, E.; Trouillet, R. Impact of perceived stress on cognitive performance: Moderating effect of mild cognitive impairment and Alzheimer’s disease. J. Clin. Exp. Neuropsychol. 2019, 41, 364–379. [Google Scholar] [CrossRef]
- Beilock, S.L.; Carr, T.H. When high-powered people fail: Working memory and “choking under pressure” in math. Psychol. Sci. 2005, 16, 101–105. [Google Scholar] [CrossRef]
- McEwen, B.S.; Sapolsky, R.M. Stress and cognitive function. Curr. Opin. Neurobiol. 1995, 5, 205–216. [Google Scholar] [CrossRef]
- Liu, P.; Li, Z. Quantitative relationship between time margin and human reliability. Int. J. Ind. Ergon. 2020, 78, 102977. [Google Scholar] [CrossRef]



| Variables | Mean | SD |
|---|---|---|
| Reaction Times (ms) | ||
| Condition 1 | 1815.63 | 503.94 |
| Condition 2 | 2394.17 | 572.98 |
| Condition 3 | 2110.47 | 558.54 |
| Condition 4 | 2251.46 | 545.22 |
| Accuracy | ||
| Condition 1 | 0.92 | 0.07 |
| Condition 2 | 0.88 | 0.09 |
| Condition 3 | 0.91 | 0.06 |
| Condition 4 | 0.91 | 0.07 |
| Indicator | BS Cognitive Task | 2-Back Task | p | Effect Size | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Subjective Scale | ||||||
| NASA-TLX | 50.67 | 14.80 | 42.56 | 13.69 | 0.008 a | 0.472 c |
| Mental demand | 11.64 | 3.51 | 9.94 | 3.83 | 0.014 a | 0.429 c |
| Physical demand | 3.08 | 2.62 | 3.22 | 2.80 | 0.781 b | 0.069 d |
| Time demand | 9.11 | 4.67 | 7.03 | 3.86 | 0.024 a | 0.394 c |
| Performance | 6.31 | 3.34 | 4.89 | 2.90 | 0.029 a | 0.381 c |
| Effort | 12.81 | 3.71 | 11.42 | 4.73 | 0.110 b | 0.340 d |
| Frustration | 7.72 | 4.56 | 6.06 | 4.23 | 0.015 b | 0.510 d |
| Behavioral Results | ||||||
| Reaction Time (ms) | 1910 | 260 | 902 | 232 | <0.001 a | 4.321 c |
| Accuracy (%) | 0.90 | 0.07 | 0.95 | 0.04 | <0.001 b | −0.671 d |
| Indicator | Pre-Test | Block 1 | Block 2 | Block 3 | Block 4 | Block 5 | Block 6 | F/χ2 | p | Effect Size |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||||
| Subjective Scale | ||||||||||
| MF | 4.60 ± 5.35 | 25.08 ± 18.13 | 38.24 ± 22.23 | 47.00 ± 25.38 | 51.71 ± 27.09 | 57.65 ± 27.07 | 59.56 ± 28.89 | 310.89 | <0.001 b | 0.720 d |
| ME | — | 44.51 ± 32.05 | 48.75 ± 31.17 | 52.17 ± 29.72 | 57.32 ± 29.38 | 58.21 ± 29.16 | 61.03 ± 30.65 | 58.45 | <0.001 b | 0.162 d |
| MS | — | 14.18 ± 18.84 | 14.51 ± 18.60 | 19.10 ± 23.55 | 20.81 ± 25.46 | 26.01 ± 28.13 | 23.11 ± 27.45 | 43.71 | <0.001 b | 0.121 d |
| Boredom | — | 6.33 ± 8.14 | 8.64 ± 10.25 | 12.50 ± 13.97 | 16.49 ± 15.78 | 17.28 ± 17.31 | 18.26 ± 20.32 | 72.97 | <0.001 b | 0.203 d |
| Behavioral Results | ||||||||||
| Correct trials | — | 150.47 ± 29.27 | 153.22 ± 28.69 | 148.14 ± 27.91 | 145.06 ± 27.00 | 142.56 ± 27.99 | 141.07 ± 28.72 | 17.43 | <0.001 a | 0.200 c |
| Accuracy (%) | — | 94.68 ± 3.50 | 93.94 ± 3.72 | 93.11 ± 4.05 | 92.75 ± 4.11 | 92.29 ± 4.42 | 91.77 ± 4.72 | 66.91 | <0.001 b | 0.186 d |
| Indicator | Pre-Test | Post-Test | p | Effect Size |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Reaction Time (ms) | 276.32 ± 27.22 | 291.29 ± 37.67 | <0.001 | −0.682 |
| Attention Lapse | 0.15 ± 0.43 | 0.54 ± 1.01 | 0.001 | −0.754 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Wu, L.; Feng, T.; Liu, X. A New Method for Inducing Mental Fatigue: A High Mental Workload Task Paradigm Based on Complex Cognitive Abilities and Time Pressure. Brain Sci. 2025, 15, 541. https://doi.org/10.3390/brainsci15060541
Ren L, Wu L, Feng T, Liu X. A New Method for Inducing Mental Fatigue: A High Mental Workload Task Paradigm Based on Complex Cognitive Abilities and Time Pressure. Brain Sciences. 2025; 15(6):541. https://doi.org/10.3390/brainsci15060541
Chicago/Turabian StyleRen, Lei, Lin Wu, Tingwei Feng, and Xufeng Liu. 2025. "A New Method for Inducing Mental Fatigue: A High Mental Workload Task Paradigm Based on Complex Cognitive Abilities and Time Pressure" Brain Sciences 15, no. 6: 541. https://doi.org/10.3390/brainsci15060541
APA StyleRen, L., Wu, L., Feng, T., & Liu, X. (2025). A New Method for Inducing Mental Fatigue: A High Mental Workload Task Paradigm Based on Complex Cognitive Abilities and Time Pressure. Brain Sciences, 15(6), 541. https://doi.org/10.3390/brainsci15060541





