A Lumped Parameter Modelling Study of Idiopathic Intracranial Hypertension Suggests the CSF Formation Rate Varies with the Capillary Transmural Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Equations
2.2. Model Input Parameters
2.3. Vessel Responses to Transmural Pressure Variations
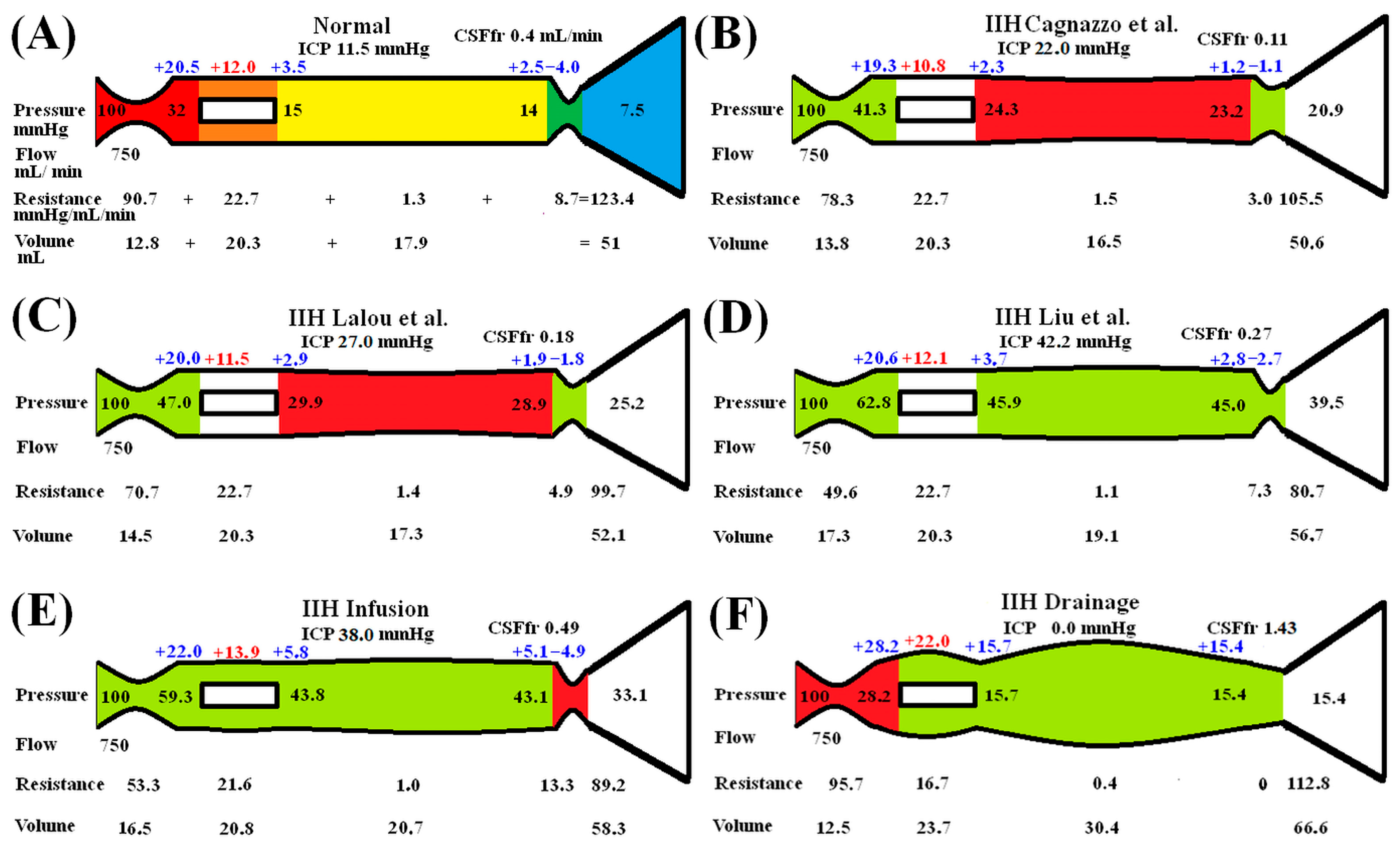
3. Results
Idiopathic Intracranial Hypertension
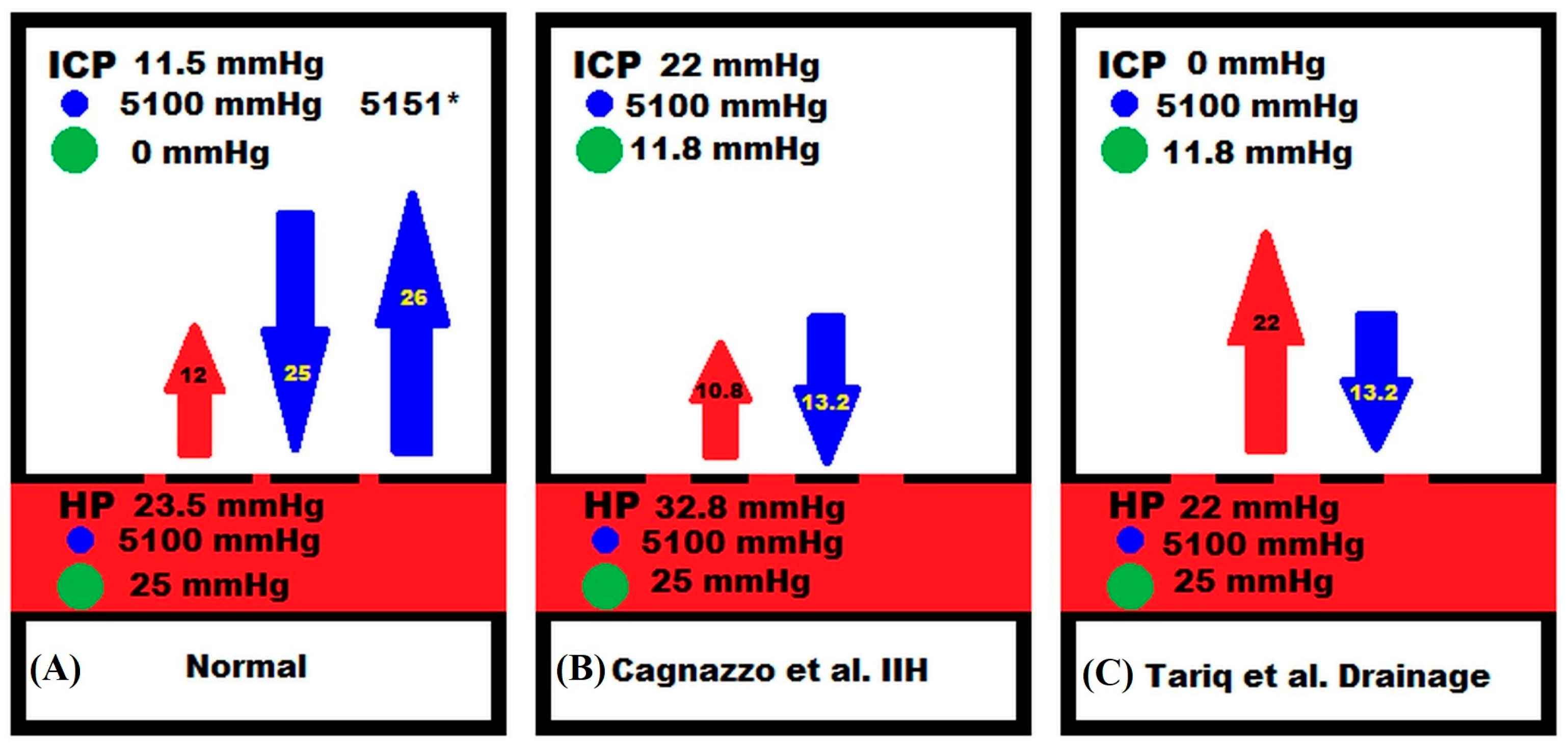
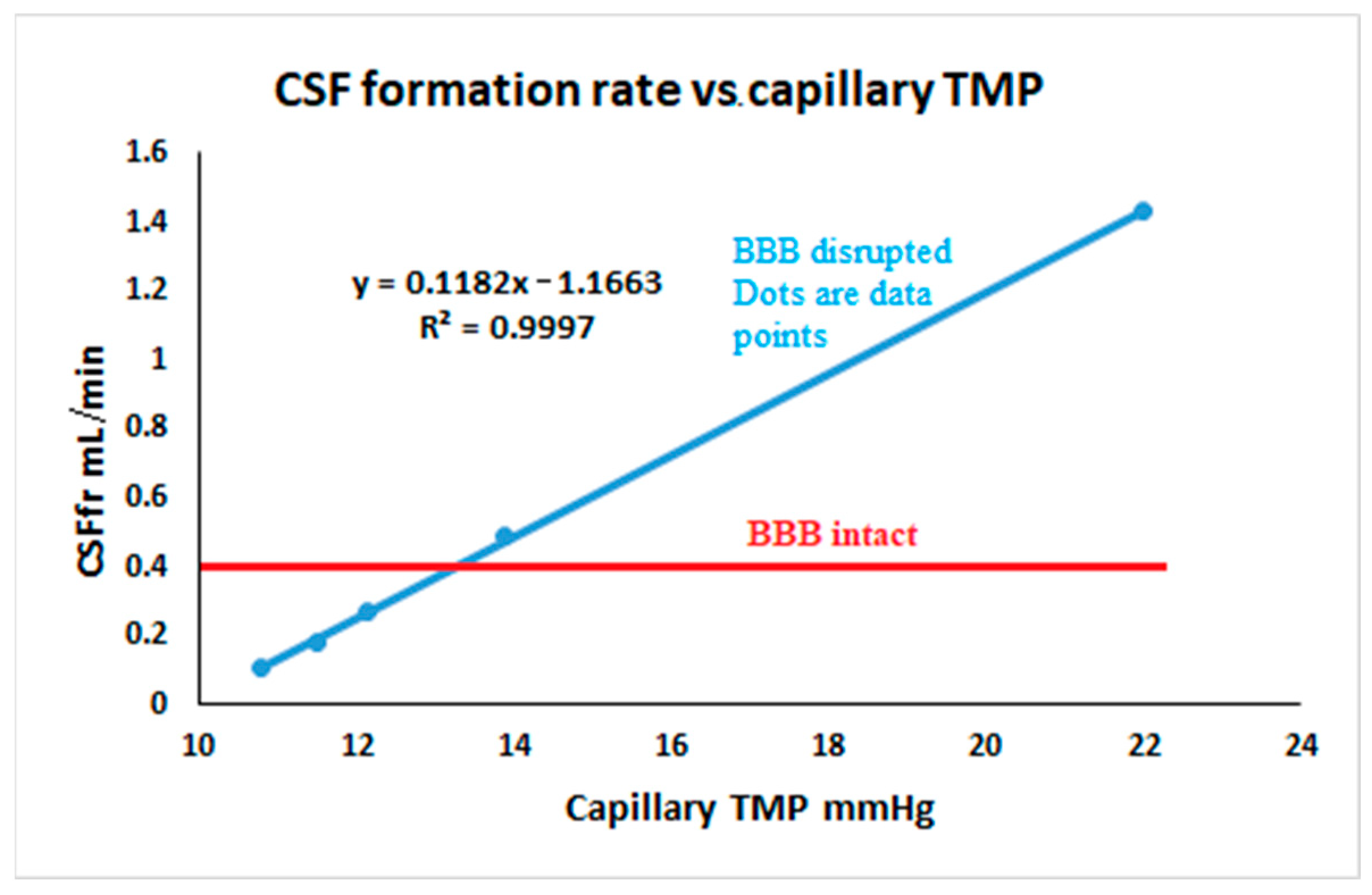
4. Discussion
4.1. Variation in CSFfr with ICP
4.2. Clinical Utility and an Explanation of Pharmacological Effects
4.3. Variations in Cerebral Blood Flow in IIH
4.4. Justification of Assumptions and Limitations
4.5. Opportunities for Further Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, I.; Hawke, S.; Halmagyi, M.; Teo, C. The pseudotumor syndrome. Disorders of cerebrospinal fluid circulation causing intracranial hypertension without ventriculomegaly. Arch. Neurol. 1991, 48, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.I.; Liu, G.T.; Digre, K.B. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013, 81, 1159–1165. [Google Scholar] [CrossRef]
- Markey, K.A.; Mollan, S.P.; Jensen, R.H.; Sinclair, A.J. Understanding idiopathic intracranial hypertension: Mechanisms, management, and future directions. Lancet Neurol. 2016, 15, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A.; Subramanian, G.M.; Yap, S.L.; Bateman, A.R. The incidence of obesity, venous sinus stenosis and cerebral hyperaemia in children referred for MRI to rule out idiopathic intracranial hypertension at a tertiary referral hospital: A 10 year review. Fluids Barriers CNS 2020, 17, 59. [Google Scholar] [CrossRef]
- Liu, K.C.; Starke, R.M.; Durst, C.R.; Wang, T.R.; Ding, D.; Crowley, R.W.; Newman, S.A. Venous sinus stenting for reduction of intracranial pressure in IIH: A prospective pilot study. J. Neurosurg. 2017, 127, 1126–1133. [Google Scholar] [CrossRef]
- Lalou, A.D.; Czosnyka, M.; Czosnyka, Z.H.; Krishnakumar, D.; Pickard, J.D.; Higgins, N.J. Coupling of CSF and sagittal sinus pressure in adult patients with pseudotumour cerebri. Acta Neurochir. 2020, 162, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Cagnazzo, F.; Villain, M.; van Dokkum, L.E.; Radu, R.A.; Morganti, R.; Gascou, G.; Dargazanli, C.; Lefevre, P.H.; Le Bars, E.; Risi, G.; et al. Concordance between venous sinus pressure and intracranial pressure in patients investigated for idiopathic intracranial hypertension. J. Headache Pain 2024, 25, 153. [Google Scholar] [CrossRef]
- Benabid, A.L.; De Rougemont, J.; Barge, M. Cerebral venous pressure, sinus pressure and intracranial pressure. Neurochirurgie 1974, 20, 623–632. [Google Scholar]
- Pollay, M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid. Res. 2010, 7, 9. [Google Scholar] [CrossRef]
- Tariq, K.; Toma, A.; Khawari, S.; Amarouche, M.; Elborady, M.A.; Thorne, L.; Watkins, L. Cerebrospinal fluid production rate in various pathological conditions: A preliminary study. Acta Neurochir. 2023, 165, 2309–2319. [Google Scholar] [CrossRef]
- Bateman, G.A.; Bateman, A.R. A lumped parameter modelling study of cerebral autoregulation in normal pressure hydrocephalus suggests the brain chooses to be ischemic. Sci. Rep. 2024, 14, 24373. [Google Scholar] [CrossRef] [PubMed]
- Davson, H.; Welch, K.; Segal, M.B. Physiology and Pathophysiology of the Cerebrospinal Fluid; Churchill Livingstone: London, UK, 1987. [Google Scholar]
- Zislin, V.; Rosenfeld, M. Impedance Pumping and Resonance in a Multi-Vessel System. Bioengineering 2018, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Ursino, M. A mathematical study of human intracranial hydrodynamics. Part 1—The cerebrospinal fluid pulse pressure. Ann. Biomed. Eng. 1988, 16, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.H.; Timperman, A.L. Effect of intracranial hypotension on cerebral blood flow. J. Neurol. Neurosurg. Psychiatry 1971, 34, 687–692. [Google Scholar] [CrossRef]
- Cirovic, S.; Walsh, C.; Fraser, W.D. Mathematical study of the role of non-linear venous compliance in the cranial volume-pressure test. Med. Biol. Eng. Comput. 2003, 41, 579–588. [Google Scholar] [CrossRef]
- Fleischman, D.; Berdahl, J.P.; Zaydlarova, J.; Stinnett, S.; Fautsch, M.P.; Allingham, R.R. Cerebrospinal fluid pressure decreases with older age. PLoS ONE 2012, 7, e52664. [Google Scholar] [CrossRef]
- Bateman, G.A.; Siddique, S.H. Cerebrospinal fluid absorption block at the vertex in chronic hydrocephalus: Obstructed arachnoid granulations or elevated venous pressure? Fluids Barriers CNS 2014, 11, 11. [Google Scholar] [CrossRef]
- Johnston, I.H.; Rowan, J.O. Raised intracranial pressure and cerebral blood flow. 3. Venous outflow tract pressures and vascular resistances in experimental intracranial hypertension. J. Neurol. Neurosurg. Psychiatry 1974, 37, 392–402. [Google Scholar] [CrossRef]
- Hua, J.; Liu, P.; Kim, T.; Donahue, M.; Rane, S.; Chen, J.J.; Qin, Q.; Kim, S.G. MRI techniques to measure arterial and venous cerebral blood volume. Neuroimage 2019, 187, 17–31. [Google Scholar] [CrossRef]
- Menéndez González, M. CNS Compartments: The Anatomy and Physiology of the Cerebrospinal Fluid. In Liquorpheresis: Cerebrospinal Fluid Filtration to Treat CNS Conditions; Menéndez González, M., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 1–19. [Google Scholar]
- Qvarlander, S.; Sundstrom, N.; Malm, J.; Eklund, A. CSF formation rate-a potential glymphatic flow parameter in hydrocephalus? Fluids Barriers CNS 2024, 21, 55. [Google Scholar] [CrossRef]
- Claassen, J.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef] [PubMed]
- Duelli, R.; Kuschinsky, W. Changes in brain capillary diameter during hypocapnia and hypercapnia. J. Cereb. Blood Flow. Metab. 1993, 13, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Roberto, D.S.; Ranieri, A.; Bonavita, V. Starling resistors, autoregulation of cerebral perfusion and the pathogenesis of idiopathic intracranial hypertension. Panminerva Med. 2017, 59, 76–89. [Google Scholar] [CrossRef]
- Bateman, G.A. A scoping review of the discrepancies in the measurement of cerebral blood flow in idiopathic intracranial hypertension: Oligemia, euvolemia or hyperemia? Fluids Barriers CNS 2023, 20, 63. [Google Scholar] [CrossRef]
- Lublinsky, S.; Kesler, A.; Friedman, A.; Horev, A.; Shelef, I. Quantifying response to intracranial pressure normalization in idiopathic intracranial hypertension via dynamic neuroimaging. J. Magn. Reson. Imaging 2018, 47, 913–927. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Wilkinson, M.; Parker, G.D.; Thurtell, M.J.; Macdonald, J.; McCluskey, P.J.; Allan, R.; Dunne, V.; Hanlon, M.; Owler, B.K.; et al. Transverse sinus stenting for idiopathic intracranial hypertension: A review of 52 patients and of model predictions. Am. J. Neuroradiol. 2011, 32, 1408–1414. [Google Scholar] [CrossRef]
- Welch, K.; Friedman, V. The cerebrospinal fluid valves. Brain 1960, 83, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Schievelkamp, A.H.; Wagele, P.; Hattingen, E. Arachnoid granulations in idiopathic intracranial hypertension: Do they have an influence? Headache 2024, 64, 722–728. [Google Scholar] [CrossRef]
- Weiss, M.H.; Wertman, N. Modulation of CSF production by alterations in cerebral perfusion pressure. Arch. Neurol. 1978, 35, 527–529. [Google Scholar] [CrossRef]
- Cserr, H.F. Physiology of the choroid plexus. Physiol. Rev. 1971, 51, 273–311. [Google Scholar] [CrossRef]
- Kimelberg, H.K. Water homeostasis in the brain: Basic concepts. Neuroscience 2004, 129, 851–860. [Google Scholar] [CrossRef]
- Brean, A.; Eide, P.K.; Stubhaug, A. Comparison of intracranial pressure measured simultaneously within the brain parenchyma and cerebral ventricles. J. Clin. Monit. Comput. 2006, 20, 411–414. [Google Scholar] [CrossRef]
- Koskinen, L.O.; Olivecrona, M. Clinical experience with the intraparenchymal intracranial pressure monitoring Codman MicroSensor system. Neurosurgery 2005, 56, 693–698; discussion 693–698. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Hua, Y.; Xi, G.; Keep, R.F. Mechanisms of cerebrospinal fluid and brain interstitial fluid production. Neurobiol. Dis. 2023, 183, 106159. [Google Scholar] [CrossRef]
- Roterman, I.; Stapor, K.; Dulak, D.; Szoniec, G.; Konieczny, L. Aquaporins as Membrane Proteins: The Current Status. Front Biosci. 2025, 17, 27967. [Google Scholar] [CrossRef] [PubMed]
- Janigro, D. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia 2012, 53 (Suppl. S1), 26–34. [Google Scholar] [CrossRef] [PubMed]
- Eide, P.K.; Hansson, H.A. A New Perspective on the Pathophysiology of Idiopathic Intracranial Hypertension: Role of the Glia-Neuro-Vascular Interface. Front. Mol. Neurosci. 2022, 15, 900057. [Google Scholar] [CrossRef]
- Fulop, G.A.; Ahire, C.; Csipo, T.; Tarantini, S.; Kiss, T.; Balasubramanian, P.; Yabluchanskiy, A.; Farkas, E.; Toth, A.; Nyul-Toth, A.; et al. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience 2019, 41, 575–589. [Google Scholar] [CrossRef]
- Bateman, G.A.; Bateman, A.R. Brain Ischemia in Alzheimer’s Disease May Partly Counteract the Disruption of the Blood–Brain Barrier. Brain Sci. 2025, 15, 269. [Google Scholar] [CrossRef]
- Bateman, G.A.; Bateman, A.R. A Lumped Parameter Model Suggests That Infusion Studies Overestimate the Cerebrospinal Fluid Outflow Resistance in Normal Pressure Hydrocephalus. Brain Sci. 2024, 14, 1242. [Google Scholar] [CrossRef]
- Taylor, A.E. Capillary fluid filtration. Starling forces and lymph flow. Circ. Res. 1981, 49, 557–575. [Google Scholar] [CrossRef]
- Borgesen, S.E.; Gjerris, F. Relationships between intracranial pressure, ventricular size, and resistance to CSF outflow. J. Neurosurg. 1987, 67, 535–539. [Google Scholar] [CrossRef]
- Parker, J.C.; Perry, M.A.; Taylor, A.E. Permeability of the Microvascular Barrier; Raven Press: New York, NY, USA, 1984. [Google Scholar]
- Boulton, M.; Young, A.; Hay, J.; Armstrong, D.; Flessner, M.; Schwartz, M.; Johnston, M. Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: Measurement of 125I-albumin clearance. Neuropathol. Appl. Neurobiol. 1996, 22, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Flessner, M.; Armstrong, D.; Hay, J.; Johnston, M. Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am. J. Physiol. 1998, 274, R88–R96. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Mase, M. Cerebrospinal Fluid Production and Absorption and Ventricular Enlargement Mechanisms in Hydrocephalus. Neurol. Med. Chir. 2023, 63, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Acetazolamide for the treatment of idiopathic intracranial hypertension. Expert. Rev. Neurother. 2015, 15, 851–856. [Google Scholar] [CrossRef]
- Piper, R.J.; Kalyvas, A.V.; Young, A.M.; Hughes, M.A.; Jamjoom, A.A.; Fouyas, I.P. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst. Rev. 2015, 2015, CD003434. [Google Scholar] [CrossRef]
- Domercq, M.; Matute, C. Neuroprotection by tetracyclines. Trends Pharmacol. Sci. 2004, 25, 609–612. [Google Scholar] [CrossRef]
- Robinson, B.D.; Isbell, C.L.; Melge, A.R.; Lomas, A.M.; Shaji, C.A.; Mohan, C.G.; Huang, J.H.; Tharakan, B. Doxycycline prevents blood-brain barrier dysfunction and microvascular hyperpermeability after traumatic brain injury. Sci. Rep. 2022, 12, 5415. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Y.; Wang, X.J.; Wang, X.T.; Zhao, Y.; Wang, L.M.; Chen, Z.Y. Retinoic acid ameliorates blood-brain barrier disruption following ischemic stroke in rats. Pharmacol. Res. 2015, 99, 125–136. [Google Scholar] [CrossRef]
- Digre, K.B.; Corbett, J.J. Idiopathic Intracranial Hypertension (Pseudotumor Cerebri): A Reappraisal. Neurologist 2001, 7, 2–68. [Google Scholar] [CrossRef]
- Friedman, D.I.; Gordon, L.K.; Egan, R.A.; Jacobson, D.M.; Pomeranz, H.; Harrison, A.R.; Goldhammer, Y. Doxycycline and intracranial hypertension. Neurology 2004, 62, 2297–2299. [Google Scholar] [CrossRef] [PubMed]
- Thurtell, M.J.; Wall, M. Idiopathic intracranial hypertension (pseudotumor cerebri): Recognition, treatment, and ongoing management. Curr. Treat. Options Neurol. 2013, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.; Shityakov, S.; Forster, C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014, 355, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Lindvall-Axelsson, M.; Hedner, P.; Owman, C. Corticosteroid action on choroid plexus: Reduction in Na+—K+-ATPase activity, choline transport capacity, and rate of CSF formation. Exp. Brain Res. 1989, 77, 605–610. [Google Scholar] [CrossRef]
- Johnston, I.; Gilday, D.L.; Hendrick, E.B. Experimental effects of steroids and steroid withdrawal on cerebrospinal fluid absorption. J. Neurosurg. 1975, 42, 690–695. [Google Scholar] [CrossRef]
- Peng, S.L.; Chen, C.M. The influence of obesity on cerebral blood flow in young adults using arterial spin labeling MRI. NMR Biomed. 2020, 33, e4375. [Google Scholar] [CrossRef]
- Yu, C.W.; Waisberg, E.; Kwok, J.M.; Micieli, J.A. Anemia and Idiopathic Intracranial Hypertension: A Systematic Review and Meta-analysis. J. Neuroophthalmol. 2022, 42, e78–e86. [Google Scholar] [CrossRef]
- Kaipainen, A.L.; Martoma, E.; Puustinen, T.; Tervonen, J.; Jyrkkanen, H.K.; Paterno, J.J.; Kotkansalo, A.; Rantala, S.; Vanhanen, U.; Leinonen, V.; et al. Cerebrospinal fluid dynamics in idiopathic intracranial hypertension: A literature review and validation of contemporary findings. Acta Neurochir. 2021, 163, 3353–3368. [Google Scholar] [CrossRef]
- Brooks, D.J.; Beaney, R.P.; Leenders, K.L.; Marshall, J.; Thomas, D.J.; Jones, T. Regional cerebral oxygen utilization, blood flow, and blood volume in benign intracranial hypertension studied by positron emission tomography. Neurology 1985, 35, 1030–1034. [Google Scholar] [CrossRef]
- Bicakci, K.; Bicakci, S.; Aksungur, E. Perfusion and diffusion magnetic resonance imaging in idiopathic intracranial hypertension. Acta Neurol. Scand. 2006, 114, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; Grubb, R.L., Jr.; Phelps, M.E.; Gado, M.H.; Caronna, J.J. Cerebral hemodynamics and metabolism in pseudotumor cerebri. Ann. Neurol. 1978, 4, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Grubb, R.L., Jr.; Raichle, M.E.; Higgins, C.S.; Eichling, J.O. Measurement of regional cerebral blood volume by emission tomography. Ann. Neurol. 1978, 4, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lan, L.; Abrigo, J.; Ip, H.L.; Soo, Y.; Zheng, D.; Wong, K.S.; Wang, D.; Shi, L.; Leung, T.W.; et al. Comparison of Newtonian and Non-newtonian Fluid Models in Blood Flow Simulation in Patients with Intracranial Arterial Stenosis. Front. Physiol. 2021, 12, 718540. [Google Scholar] [CrossRef]
- Yonan, K.A.; Greene, E.R.; Sharrar, J.M.; Caprihan, A.; Qualls, C.; Roldan, C.A. Middle cerebral artery blood flows by combining TCD velocities and MRA diameters: In vitro and in vivo validations. Ultrasound Med. Biol. 2014, 40, 2692–2699. [Google Scholar] [CrossRef]
- Iyer, A.M.; Midtlien, J.P.; Kittel, C.; Klever, L.A.; Wiater, A.; Chang, E.; Margraf, C.; Fargen, K.M. The correlation between intracranial pressure and venous sinus pressures changes after venous sinus stenting. J. NeuroInterventional Surg. 2025. [Google Scholar] [CrossRef]
- Shah, V.A.; Kardon, R.H.; Lee, A.G.; Corbett, J.J.; Wall, M. Long-term follow-up of idiopathic intracranial hypertension: The Iowa experience. Neurology 2008, 70, 634–640. [Google Scholar] [CrossRef]
- Galvin, J.A.; Van Stavern, G.P. Clinical characterization of idiopathic intracranial hypertension at the Detroit Medical Center. J. Neurol. Sci. 2004, 223, 157–160. [Google Scholar] [CrossRef]
- Kety, S.S.; Shenkin, H.A.; Schmidt, C.F. The Effects of Increased Intracranial Pressure on Cerebral Circulatory Functions in Man. J. Clin. Invest. 1948, 27, 493–499. [Google Scholar] [CrossRef]
- Bateman, G.A.; Bateman, A.R. Is the ischemia found in normal pressure hydrocephalus secondary to venous compression or arterial constriction? A comment on Ohmura et al. Fluids Barriers CNS 2025, 22, 29. [Google Scholar] [CrossRef]
- Grubb, R.L., Jr.; Raichle, M.E.; Phelps, M.E.; Ratcheson, R.A. Effects of increased intracranial pressure on cerebral blood volume, blood flow, and oxygen utilization in monkeys. J. Neurosurg. 1975, 43, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Ashoor, M.; Khorshidi, A.; Sarkhosh, L. Estimation of microvascular capillary physical parameters using MRI assuming a pseudo liquid drop as model of fluid exchange on the cellular level. Rep. Pract. Oncol. Radiother. 2019, 24, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Saiga, R.; Uesugi, M.; Takeuchi, A.; Uesugi, K.; Suzuki, Y.; Takekoshi, S.; Inomoto, C.; Nakamura, N.; Torii, Y.; Kushima, I.; et al. Brain capillary structures of schizophrenia cases and controls show a correlation with their neuron structures. Sci. Rep. 2021, 11, 11768. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bateman, G.A.; Bateman, A.R. A Lumped Parameter Modelling Study of Idiopathic Intracranial Hypertension Suggests the CSF Formation Rate Varies with the Capillary Transmural Pressure. Brain Sci. 2025, 15, 527. https://doi.org/10.3390/brainsci15050527
Bateman GA, Bateman AR. A Lumped Parameter Modelling Study of Idiopathic Intracranial Hypertension Suggests the CSF Formation Rate Varies with the Capillary Transmural Pressure. Brain Sciences. 2025; 15(5):527. https://doi.org/10.3390/brainsci15050527
Chicago/Turabian StyleBateman, Grant A., and Alexander R. Bateman. 2025. "A Lumped Parameter Modelling Study of Idiopathic Intracranial Hypertension Suggests the CSF Formation Rate Varies with the Capillary Transmural Pressure" Brain Sciences 15, no. 5: 527. https://doi.org/10.3390/brainsci15050527
APA StyleBateman, G. A., & Bateman, A. R. (2025). A Lumped Parameter Modelling Study of Idiopathic Intracranial Hypertension Suggests the CSF Formation Rate Varies with the Capillary Transmural Pressure. Brain Sciences, 15(5), 527. https://doi.org/10.3390/brainsci15050527






