Effectiveness and Safety of CGRP-Targeted Therapies Combined with Lifestyle Modifications for Chronic Migraine in Korean Pediatric Patients: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
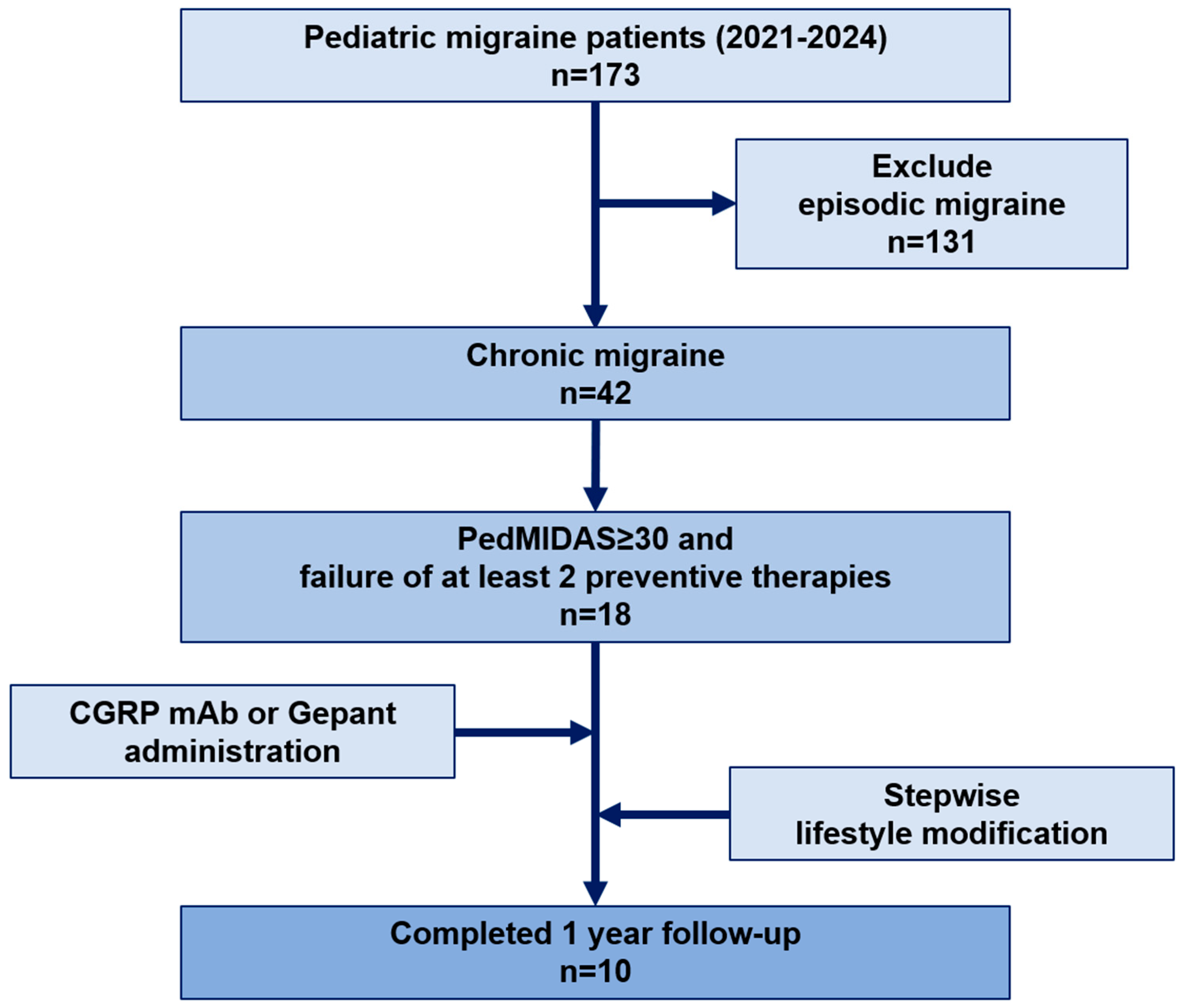
2.1. Study Design and Participants
2.2. Ethics Approval and Patient Consent
2.3. Data Collection
2.4. Headache Questionnaires and Neuropsychological Tests
2.5. Neuroimaging, Electroencephalography, and Genetic Testing
2.6. Administration of CGRP-Targeted Therapies
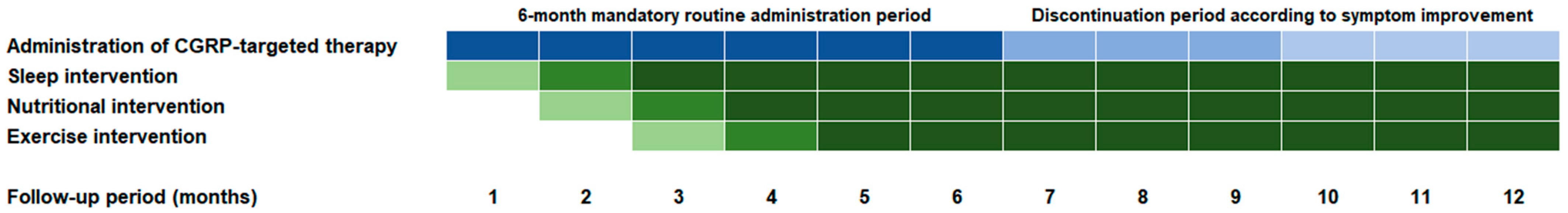
2.7. Lifestyle Modification
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
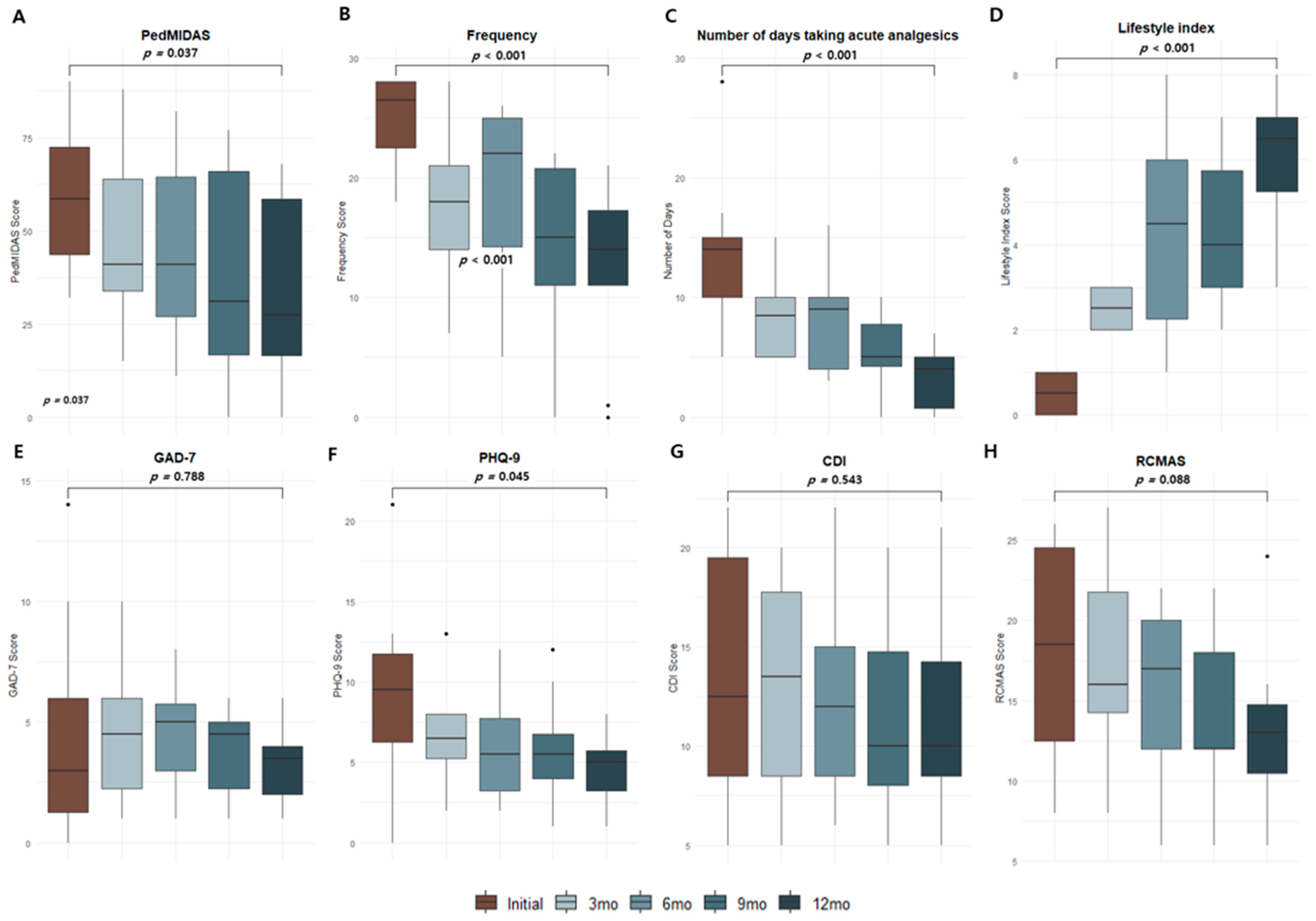
3.2. Changes in Migraine-Related Parameters over Time After CGRP-Targeted Therapies
3.3. Side Effects of CGRP-Targeted Therapies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CM | Chronic migraine |
| CGRP | Calcitonin gene-related peptide |
| ICHD-3 | International Classification of Headache Disorders, Third Edition |
| PedMIDAS | Pediatric Migraine Disability Assessment Scale |
| VAS | Visual Analogue Scale |
| CDI | Children’s Depression Inventory |
| RCMAS | Revised Children’s Manifest Anxiety Scale |
| GAD-7 | Generalized Anxiety Disorder-7 |
| PHQ-9 | Patient Health Questionnaire-9 |
| WISC-V | Wechsler Intelligence Scale for Children, Fifth Edition |
| FSIQ | Full-scale intelligence quotient |
| MQ | Memory quotient |
| SQ | Social quotient |
| MRI | Magnetic resonance imaging |
| MRA | Magnetic resonance angiography |
| EEG | Electroencephalography |
References
- Patterson-Gentile, C.; Szperka, C.L. The changing landscape of pediatric migraine therapy: A review. JAMA Neurol. 2018, 75, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Locher, C.; Kossowsky, J.; Koechlin, H.; Lam, T.L.; Barthel, J.; Berde, C.B.; Gaab, J.; Schwarzer, G.; Linde, K.; Meissner, K. Efficacy, safety, and acceptability of pharmacologic treatments for pediatric migraine prophylaxis: A systematic review and network meta-analysis. JAMA Pediatr. 2020, 174, 341–349. [Google Scholar] [CrossRef]
- Powers, S.W.; Coffey, C.S.; Hershey, A.D.; Chamberlin, L.A.; Ecklund, D.J.; Klingner, E.A.; Yankey, J.W.; Korbee, L.L.; Porter, L.L. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N. Engl. J. Med. 2017, 376, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Pringsheim, T.; Holler-Managan, Y.; Billinghurst, L.; Potrebic, S.; Gersz, E.M.; Gloss, D.; Leininger, E.; Licking, N.; Mack, K.; et al. Practice guideline update summary: Pharmacologic treatment for pediatric migraine prevention. Neurology 2019, 93, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Pringsheim, T.; Holler-Managan, Y.; Potrebic, S.; Billinghurst, L.; Gloss, D.; Hershey, A.D.; Licking, N.; Sowell, M.; Victorio, M.C.; et al. Practice guideline update summary: Acute treatment of migraine in children and adolescents. Neurology 2019, 93, 487–499. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Dodick, D.W.; Silberstein, S.D.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Effect of fremanezumab compared with placebo for prevention of episodic migraine. JAMA 2018, 319, 1999–2008. [Google Scholar] [CrossRef]
- Lamb, Y.N. Galcanezumab: First global approval. Drugs 2018, 78, 1761–1767. [Google Scholar] [CrossRef]
- VanderPluym, J.H.; Victorio, M.C.C.; Oakley, C.B.; Rastogi, R.G.; Orr, S.L. Beyond the guidelines: A narrative review of treatments on the horizon for migraine in children and adolescents. Neurology 2023, 101, 788–797. [Google Scholar] [CrossRef]
- Szperka, C.; Vander Pluym, J.; Orr, S.L.; Oakley, C.B.; Qubty, W.; Patniyot, I.; Lagman-Bartolome, A.M.; Morris, C.; Gautreaux, J.; Victorio, M.C.; et al. Recommendations on the use of anti-CGRP monoclonal antibodies in children and adolescents. Headache 2018, 58, 1658–1669. [Google Scholar] [CrossRef]
- Charles, J.A. In response to recommendations on the use of anti-CGRP monoclonal antibodies in children and adolescents. Headache 2020, 60, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Bandatmakur, A.S.M.; Dave, P.; Kerr, M.; Brunick, C.; Wen, S.; Hansen, N. Effectiveness and tolerability of anti-calcitonin gene-related peptide therapy for migraine and other chronic headaches in adolescents and young adults: A retrospective study in the USA. Brain Sci. 2024, 14, 879. [Google Scholar] [CrossRef]
- Kalika, P.; Monteith, T.S. New daily persistent headache in the pediatric and adolescent population: An updated review. Life 2024, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Greene, K.A.; Gentile, C.P.; Szperka, C.L.; Yonker, M.; Gelfand, A.A.; Grimes, B.; Irwin, S.L. Calcitonin gene-related peptide monoclonal antibody use for the preventive treatment of refractory headache disorders in adolescents. Pediatr. Neurol. 2021, 114, 62–67. [Google Scholar] [CrossRef]
- Rastogi, R.G.; Hastriter, E.V.; Evans, R.L.; Bassal, F.; Hickman, C.; Karnik, K.T.; Little, R.; Lewis, K.S. Advances in the acute and preventive treatment of pediatric migraine. Curr. Pain Headache Rep. 2023, 27, 521–529. [Google Scholar] [CrossRef]
- Marshall, A.; Lindsay, R.; Clementi, M.A.; Gelfand, A.A.; Orr, S.L. Outpatient approach to resistant and refractory migraine in children and adolescents: A narrative review. Curr. Neurol. Neurosci. Rep. 2022, 22, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed.; Cephalalgia: London, UK, 2018; Volume 38, pp. 1–211. [Google Scholar]
- Hershey, A.D.; Powers, S.W.; Vockell, A.L.; LeCates, S.; Kabbouche, M.A.; Maynard, M.K. PedMIDAS: Development of a questionnaire to assess disability of migraines in children. Neurology 2001, 57, 2034–2039. [Google Scholar] [CrossRef]
- Lee, S.M.; Yoon, J.R.; Yi, Y.Y.; Eom, S.; Lee, J.S.; Kim, H.D.; Cheon, K.-A.; Kang, H.-C. Screening for depression and anxiety disorder in children with headache. Korean J. Pediatr. 2015, 58, 64–68. [Google Scholar] [CrossRef]
- Hans, A.; Stonnington, C.M.; Zhang, N.; Butterfield, R.; Friedman, D.I. The impact of resilience on headache disability as measured by the Migraine Disability Assessment (MIDAS). Headache 2023, 63, 743–750. [Google Scholar] [CrossRef]
- Gelfand, A.A.; Allen, I.E.; Grimes, B.; Irwin, S.; Qubty, W.; Greene, K.; Waung, M.; Powers, S.W.; Szperka, C.L. Melatonin for migraine prevention in children and adolescents: A randomized, double-blind, placebo-controlled trial. Headache 2023, 63, 1314–1326. [Google Scholar] [CrossRef]
- Nguyen, K.V.; Schytz, H.W. The evidence for diet as a treatment in migraine: A review. Nutrients 2024, 16, 3415. [Google Scholar] [CrossRef]
- Hindiyeh, N.A.; Zhang, N.; Farrar, M.; Banerjee, P.; Lombard, L.; Aurora, S.K. The role of diet and nutrition in migraine triggers and treatment: A systematic literature review. Headache 2020, 60, 1300–1316. [Google Scholar] [CrossRef]
- La Touche, R.; Fierro-Marrero, J.; Sánchez-Ruíz, I.; de Rivera-Romero, B.R.; Cabrera-López, C.D.; Lerma-Lara, S.; Requejo-Salinas, N.; de Asís-Fernández, F.; Elizagaray-García, I.; Fernández-Carnero, J.; et al. Prescription of therapeutic exercise in migraine: An evidence-based clinical practice guideline. J. Headache Pain 2023, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Frattale, I.; Ferilli, M.A.N.; Ursitti, F.; Sforza, G.; Monte, G.; Checchi, M.P.; Tarantino, S.; Mazzone, L.; Valeriani, M.; Papetti, L. Unsatisfactory response to acute medications does not affect the medication overuse headache development in pediatric chronic migraine. J. Headache Pain 2024, 25, 61. [Google Scholar] [CrossRef]
- Shimomura, H. Emotional problems in pediatric headache patients. Curr. Pain Headache Rep. 2022, 26, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.C.; Kuo, P.H.; Lee, M.T.; Chang, S.H.; Chiou, L.C. Plasma calcitonin gene-related peptide: A potential biomarker for diagnosis and therapeutic responses in pediatric migraine. Front. Neurol. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, G.; Dan, Y.; Liu, X. CGRP and PACAP-38 play an important role in diagnosing pediatric migraine. J. Headache Pain 2022, 23, 68. [Google Scholar] [CrossRef]
- Moore, L.; Pakalnis, A. Calcitonin gene-related peptide inhibitors in the treatment of migraine in the pediatric and adolescent populations: A review. Pediatr. Neurol. 2024, 157, 87–95. [Google Scholar] [CrossRef]
- Seng, E.K.; Martin, P.R.; Houle, T.T. Lifestyle factors and migraine. Lancet Neurol. 2022, 21, 911–921. [Google Scholar] [CrossRef]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M. Gut-brain axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef]
- Charles, A.C.; Digre, K.B.; Goadsby, P.J.; Robbins, M.S.; Hershey, A.; Society, T.A.H. Calcitonin gene-related peptide-targeting therapies are a first-line option for the prevention of migraine: An American Headache Society position statement update. Headache 2024, 64, 333–341. [Google Scholar] [CrossRef] [PubMed]
| General Characteristics | N = 10 |
|---|---|
| Sex | M:F = 2:8 |
| Weight (kg) | 55.0 (45.0–71.4) |
| Onset of headache (year) | 10.6 (8.1–14.4) |
| Age at diagnosis of chronic migraine (year) | 13.8 (9.6–14.9) |
| Age at first dose of CGRP-targeted therapies (year) | 14 (12–16) |
| Time from diagnosis of chronic migraine to administration of CGRP-targeted therapies | 18.0 (3.9–43.0) |
| Presence of aura | 4 |
| Brain MRI + MRA findings (N = 10) | Normal (N = 10) |
| EEG findings (n = 4) | Normal (n = 4) |
| Genetic test findings (n = 4) | Normal (n = 4) |
| Baseline characteristics of migraine | |
| Average number of analgesics taken (per month) | 14 (5–28) |
| Conventional preventive treatment ever tried | 4.5 (3–7) |
| Pattern of migraine | Pulsating (N = 10) |
| PedMIDAS at diagnosis (median, range) | 58.5 (32–90) |
| Duration of migraine (median, range) | 4 (3–18) |
| Intensity of migraine (median, range) | 7.5 (6–9) |
| Frequency of migraine (median, range) | 26.5 (18–28) |
| Prior history of head trauma | none |
| Presence of sleep disturbance | 9 |
| Other neurological problems | none |
| Initial lifestyle index | 0.5 (0–1) |
| Neuropsychological test at diagnosis | |
| Full-Scale Intelligence Quotient (FSIQ) (median, range) | 103 (92–118) |
| Memory Quotient (MQ) (median, range) | 100 (49–113) |
| Social Quotient (SQ) (median, range) | 103 (98–115) |
| Patient Health Questionnaire-9 (PHQ-9) (median, range) | 18.5 (8–26) |
| Generalized Anxiety Disorder-7 (GAD-7) (median, range) | 3 (0–14) |
| Children’s Depression Inventory (CDI) (median, range) | 12.5 (5–22) |
| Revised Children’s Manifest Anxiety Scale (RCMAS) (median, range) | 18.5 (8–26) |
| Initial choice of CGRP-targeted therapies | |
| Galcanezumab | 6 |
| Fremanezumab | 4 |
| Change of Medication | Number of Patients | Reason for Changing Medication |
| Galcanezumab -> Fremanezumab | 2 | Less effective over time |
| Fremanezumab -> Galcanezumab | 1 | Less effective over time |
| Galcanezumab -> Atogepant | 2 | Pain and redness at injection site, myalgia |
| Fremanezumab -> Atogepant | 1 | Pain and redness at injection site |
| Galcanezumab -> discontinuation (after the 6th dose) | 1 | Symptom improvement |
| Fremanezumab -> discontinuation (after the 8th dose) | 1 | Symptom improvement |
| Side Effect | Number of Patients | Types of Side Effects |
| Galcanezumab | 3 | Pain and redness at injection site |
| 1 | myalgia | |
| Fremanezumab | 2 | Pain and redness at injection site |
| Atogepant | 2 | dizziness |
| Serious adverse event | none |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, J.-H.; Jeon, H.; Shim, J.-E.; Lee, H.; Lee, Y.-M. Effectiveness and Safety of CGRP-Targeted Therapies Combined with Lifestyle Modifications for Chronic Migraine in Korean Pediatric Patients: A Retrospective Study. Brain Sci. 2025, 15, 493. https://doi.org/10.3390/brainsci15050493
Na J-H, Jeon H, Shim J-E, Lee H, Lee Y-M. Effectiveness and Safety of CGRP-Targeted Therapies Combined with Lifestyle Modifications for Chronic Migraine in Korean Pediatric Patients: A Retrospective Study. Brain Sciences. 2025; 15(5):493. https://doi.org/10.3390/brainsci15050493
Chicago/Turabian StyleNa, Ji-Hoon, Hayoon Jeon, Ji-Eun Shim, Hyunjoo Lee, and Young-Mock Lee. 2025. "Effectiveness and Safety of CGRP-Targeted Therapies Combined with Lifestyle Modifications for Chronic Migraine in Korean Pediatric Patients: A Retrospective Study" Brain Sciences 15, no. 5: 493. https://doi.org/10.3390/brainsci15050493
APA StyleNa, J.-H., Jeon, H., Shim, J.-E., Lee, H., & Lee, Y.-M. (2025). Effectiveness and Safety of CGRP-Targeted Therapies Combined with Lifestyle Modifications for Chronic Migraine in Korean Pediatric Patients: A Retrospective Study. Brain Sciences, 15(5), 493. https://doi.org/10.3390/brainsci15050493








