Spontaneous Regression of Intracranial Aneurysms—Case Report and Systematic Review of the Literature
Abstract
1. Introduction
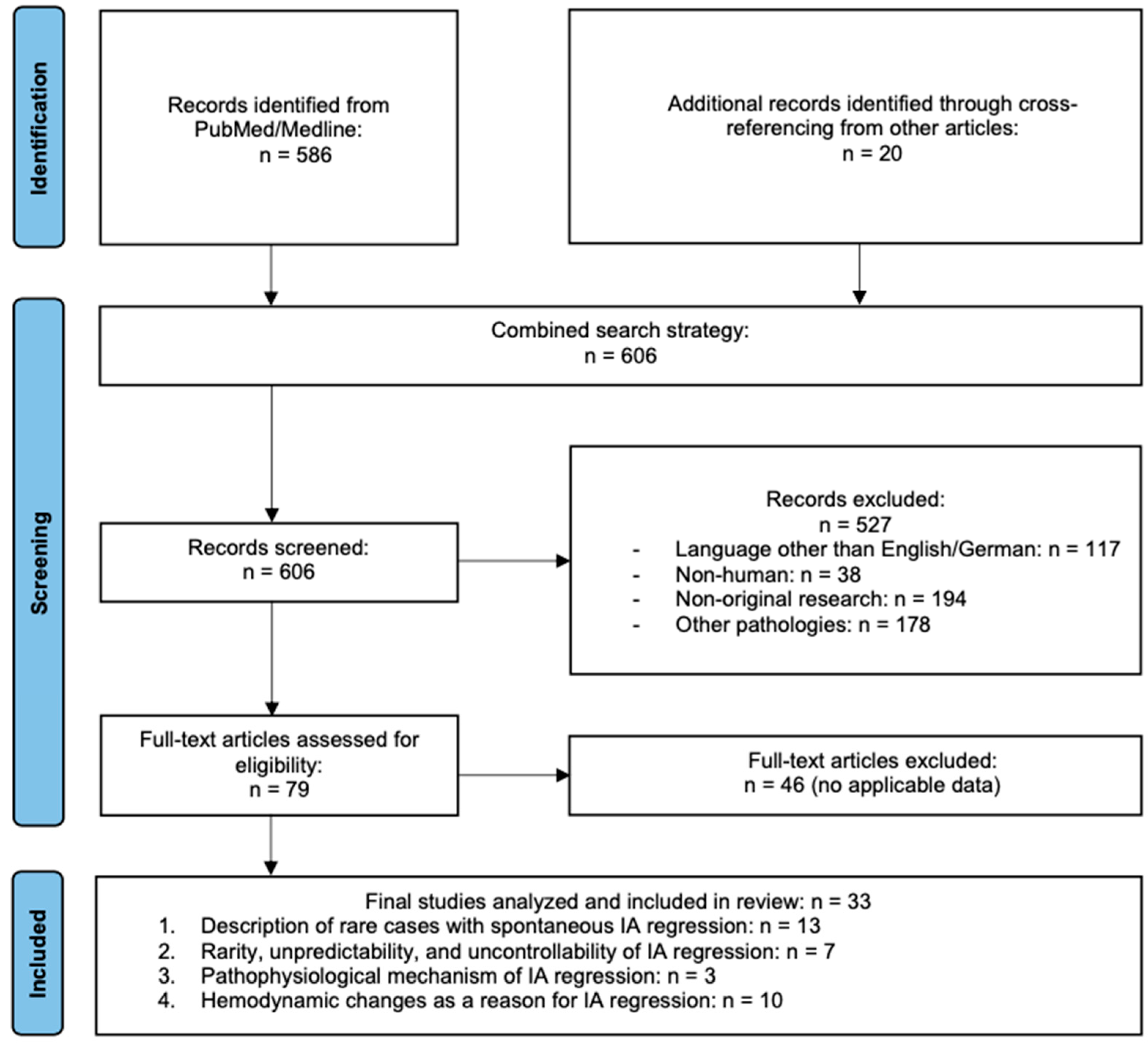
2. Materials and Methods
2.1. Definition of Spontaneous Regression
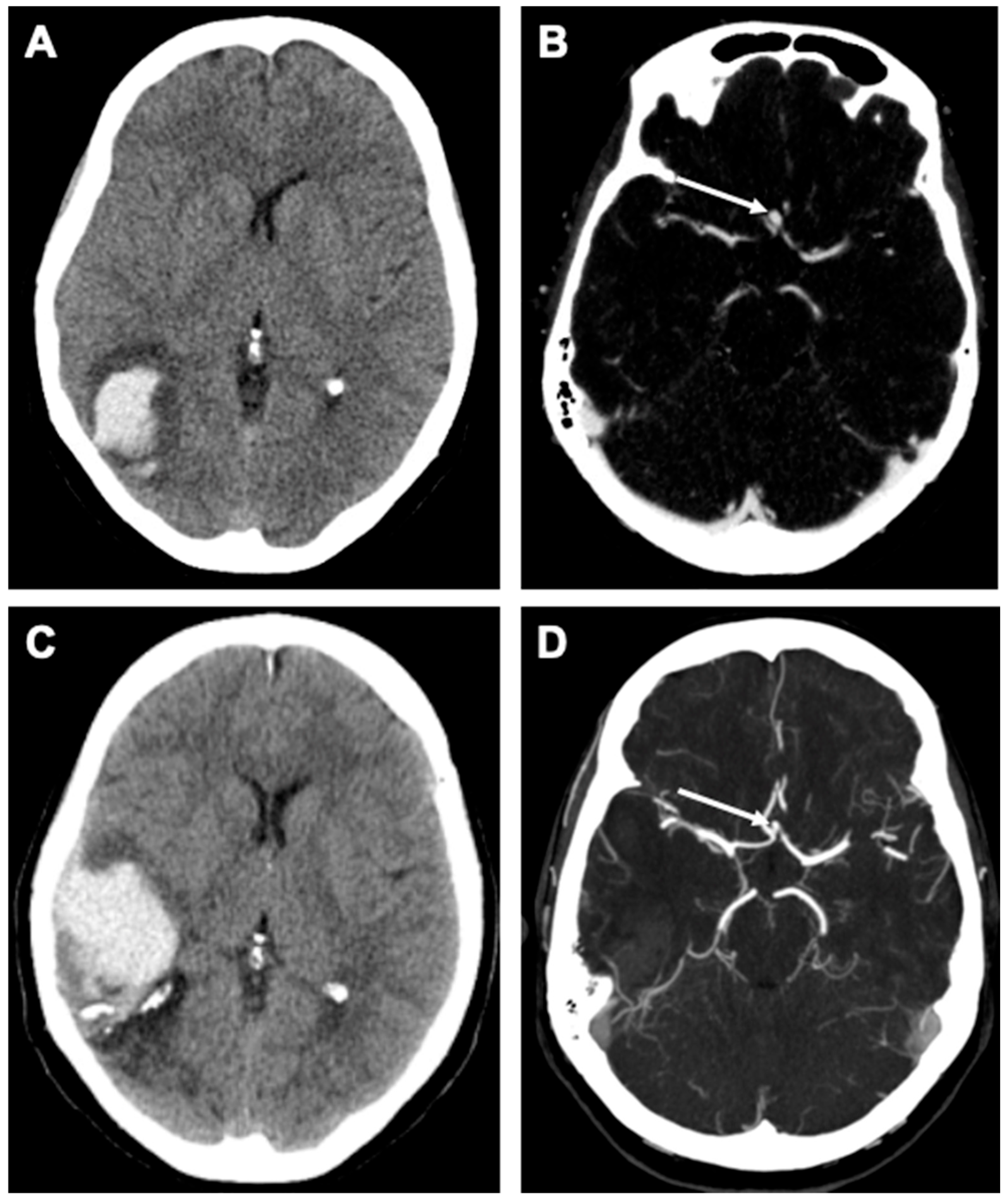
2.2. Case Report
2.3. Literature Review
2.4. Case Report
Case Description
2.5. Outcome and Follow-Up
3. Results
3.1. Quantitative Summary of Included Cases
3.2. Literature Review
4. Discussion
4.1. Spontaneous Regression as a Rare and Unpredictable Phenomenon
4.2. Rarity, Unpredictability, and Uncontrollability of IA Regression
4.3. Pathophysiological Mechanisms Leading to IA Regression
4.4. Hemodynamic Changes as a Driver of Spontaneous Regression
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACom | anterior communicating artery |
| CT/A | computed tomography angiography |
| CFD | computational fluid dynamics |
| DSA | digital subtraction angiography |
| EC-IC | extracranial–intracranial |
| FU | follow-up |
| IA | intracranial aneurysm |
| ICA | internal carotid artery |
| iDSA | intraoperative digital subtraction angiography |
| MCA | middle cerebral artery |
| MRI/MRA | magnetic resonance imaging / angiography |
| PCom | posterior communicating artery |
| SAH | subarachnoid hemorrhage |
References
- Bairstow, P.; Dodgson, A.; Linto, J.; Khangure, M. Comparison of cost and outcome of endovascular and neurosurgical procedures in the treatment of ruptured intracranial aneurysms. Australas. Radiol. 2002, 46, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Fischer, U.; Rothwell, P.M.; Luengo-Fernandez, R.; Bervini, D.; Goldberg, J.; Trelle, S.; Gralla, J.; Beck, J.; Zubak, I. Decision-Making for Preventive Interventions in Asymptomatic Patients. Stroke 2024, 55, 1951–1955. [Google Scholar] [CrossRef] [PubMed]
- Bekelis, K.; Missios, S.; MacKenzie, T.A.; Labropoulos, N.; Roberts, D.W. A predictive model of hospitalization cost after cerebral aneurysm clipping. J. Neurointerv. Surg. 2016, 8, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Bandhauer, B.; Gruber, P.; Andereggen, L.; Berberat, J.; Wanderer, S.; Cattaneo, M.; Schubert, G.A.; Remonda, L.; Marbacher, S.; Gruter, B.E. From conservative to interventional management in unruptured intracranial aneurysms. J. Neurosurg. 2024, 142, 619–625. [Google Scholar] [CrossRef]
- Rinaldo, L.; Rabinstein, A.A.; Cloft, H.J.; Knudsen, J.M.; Lanzino, G.; Rangel Castilla, L.; Brinjikji, W. Racial and economic disparities in the access to treatment of unruptured intracranial aneurysms are persistent problems. J. Neurointerv. Surg. 2019, 11, 833–836. [Google Scholar] [CrossRef]
- Hackett, A.M.; Adereti, C.O.; Walker, A.P.; Nico, E.; Scherschinski, L.; Rhodenhiser, E.G.; Eberle, A.T.; Naik, A.; Giraldo, J.P.; Hartke, J.N.; et al. Racial and Socioeconomic Status among a Patient Population Presenting with Aneurysmal Subarachnoid Hemorrhage versus Unruptured Intracranial Aneurysm: A Single-Center Study. Brain Sci. 2024, 14, 394. [Google Scholar] [CrossRef]
- Kandregula, S.; Savardekar, A.; Beyl, R.; Caskey, J.; Terrell, D.; Adeeb, N.; Whipple, S.G.; Newman, W.C.; Toms, J.; Kosty, J.; et al. Health inequities and socioeconomic factors predicting the access to treatment for unruptured intracranial aneurysms in the USA in the last 20 years: Interaction effect of race, gender, and insurance. J. Neurointerv. Surg. 2023, 15, 1251–1256. [Google Scholar] [CrossRef]
- Spetzler, R.F.; Winestock, D.; Newton, H.T.; Boldrey, E.B. Disappearance and reappearance of cerebral aneurysm in serial arteriograms. Case report. J. Neurosurg. 1974, 41, 508–510. [Google Scholar] [CrossRef]
- Amagasaki, K.; Higa, T.; Takeuchi, N.; Kakizawa, T.; Shimizu, T. Late recurrence of subarachnoid hemorrhage due to regrowth of aneurysm after neck clipping surgery. Neurol. Med. Chir. 2002, 42, 496–500. [Google Scholar] [CrossRef]
- Gruter, B.E.; Marbacher, S. The importance of wall degeneration in preclinical aneurysm models. J. Neurointerv. Surg. 2021, 13, 200–201. [Google Scholar] [CrossRef]
- Gruter, B.E.; Wanderer, S.; Strange, F.; Boillat, G.; Taschler, D.; Rey, J.; Croci, D.M.; Grandgirard, D.; Leib, S.L.; von Gunten, M.; et al. Patterns of Neointima Formation After Coil or Stent Treatment in a Rat Saccular Sidewall Aneurysm Model. Stroke 2021, 52, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Gruter, B.E.; Wanderer, S.; Strange, F.; Sivanrupan, S.; von Gunten, M.; Widmer, H.R.; Coluccia, D.; Andereggen, L.; Fandino, J.; Marbacher, S. Comparison of Aneurysm Patency and Mural Inflammation in an Arterial Rabbit Sidewall and Bifurcation Aneurysm Model under Consideration of Different Wall Conditions. Brain Sci. 2020, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Rison, R.A.; Kidd, M.R.; Koch, C.A. The CARE (CAse REport) guidelines and the standardization of case reports. J. Med. Case Rep. 2013, 7, 261. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Dandy, W.E. Intracranial Arterial Aneurysms; Hafner: Saint-Galmier, France, 1944. [Google Scholar]
- Epstein, B.S. The roentgenographic aspects of thrombosis of aneurysms of the anterior communicating and anterior cerebral arteries. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1953, 70, 211–217. [Google Scholar]
- Lindgren, S.O. Course and prognosis in spontaneous occlusions of cerebral arteries. Acta Psychiatr. Neurol. Scand. 1958, 33, 343–358. [Google Scholar] [CrossRef]
- Hemmer, R.; Umbach, W. The clinical course of intracranial saccular aneurysms. Arch. Psychiatr. Nervenkr. Z. Gesamte Neurol. Psychiatr. 1960, 200, 612–625. [Google Scholar] [CrossRef]
- af Bjorkesten, G.; Troupp, H. Changes in the size of intracranial arterial aneurysms. J. Neurosurg. 1962, 19, 583–588. [Google Scholar] [CrossRef]
- Schunk, H. Spontaneous Thrombosis of Intracranial Aneurysms. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1964, 91, 1327–1338. [Google Scholar]
- Lodin, H. Spontaneous thrombosis of cerebral aneurysms. Br. J. Radiol. 1966, 39, 701–703. [Google Scholar] [CrossRef]
- Edner, G.; Forster, D.M.; Steiner, L.; Bergvall, U. Spontaneous healing of intracranial aneurysms after subarachnoid hemorrhage. Case report. J. Neurosurg. 1978, 48, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.M.; Garrido, E. Spontaneous thrombosis of an intracranial aneurysm during treatment with epsilon aminocaproic acid. Surg. Neurol. 1977, 7, 21–23. [Google Scholar] [PubMed]
- Spallone, A.; Peresedov, V.V.; Kandel, E.I. Spontaneous cure of ruptured intracranial arterial aneurysms. Surg. Neurol. 1981, 16, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, A.; Curri, C.; Colombo, F. Rebleeding of an angiographically healed aneurysm. Surg. Neurol. 1983, 20, 206–208. [Google Scholar] [CrossRef]
- Ueta, T.; Ichi, S.; Ochi, T.; Suzuki, I. Spontaneous regression of an aneurysm at a nonbranching site of the supraclinoid internal carotid artery. Case report. J. Neurosurg. 2004, 101, 1070–1072. [Google Scholar] [CrossRef]
- Hassan, F.; Taschner, C.A.; Thines, L.; Lejeune, J.P.; Pruvo, J.P.; Leclerc, X. Spontaneous thrombosis of a recurrent clipped intracranial aneurysm. J. Neuroradiol. 2009, 36, 153–157. [Google Scholar] [CrossRef]
- Scott, R.M.; Ballantine, H.T., Jr. Spontaneous thrombosis in a giant middle cerebral artery aneurysm. Case report. J. Neurosurg. 1972, 37, 361–363. [Google Scholar] [CrossRef]
- Davila, S.; Oliver, B.; Molet, J.; Bartumeus, F. Spontaneous thrombosis of an intracranial aneurysm. Surg. Neurol. 1984, 22, 29–32. [Google Scholar] [CrossRef]
- Tan, C.B.; Rodesh, G.; Lasjaunias, P. Growth and spontaneous regression of a middle cerebral artery aneurysm after surgical clipping. Interv. Neuroradiol. 2001, 7, 147–151. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, D.W.; Jang, S.J.; Kang, S.D. Recanalization of completely thrombosed non-giant saccular aneurysm mimicking as de novo aneurysm. J. Korean Neurosurg. Soc. 2010, 48, 354–356. [Google Scholar] [CrossRef]
- Kalin-Hajdu, E.; Guilbert, F. Transformation of a cranial fusiform aneurysm into a pseudotumoral-like mass prior to spontaneous occlusion and regression. Interv. Neuroradiol. 2011, 17, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.S.; Ahn, J.; Song, J.H.; Chang, I.B. Spontaneous Regression of Aneurysm Remnant after Incomplete Surgical Clipping in a Patient with Ruptured Cerebral Aneurysm. J. Cerebrovasc. Endovasc. Neurosurg. 2016, 18, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Fodstad, H.; Liliequist, B. Spontaneous thrombosis of ruptured intracranial aneurysms during treatment with tranexamic acid (AMCA). Report of three cases. Acta Neurochir. 1979, 49, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Moritake, K.; Handa, H.; Ohtsuka, S.; Hashimoto, N. Vanishing cerebral aneurysm in serial angiography. Surg. Neurol. 1981, 16, 36–40. [Google Scholar] [CrossRef]
- Warschewske, G.; Benndorf, G.; Lehmann, T.H.; Lanksch, W. Spontaneous thrombosis of an intracranial giant aneurysm. Interv. Neuroradiol. 1999, 5, 327–332. [Google Scholar] [CrossRef]
- Marguth, F.; Schiefer, W. Spontaneous healing of an intracranial aneurysm detected by angiography. Acta Neurochir. 1957, 5, 38–45. [Google Scholar] [CrossRef]
- Hook, O.; Norlen, G. Aneurysms of the Internal Carotid Artery. Acta Neurol. Scand. 1964, 40, 200–218. [Google Scholar] [CrossRef]
- Yeh, H.; Tomsick, T.A. Obliteration of a giant carotid aneurysm after extracranial-to-intracranial bypass surgery: Case report. Surg. Neurol. 1997, 48, 473–476. [Google Scholar] [CrossRef]
- Cantore, G.; Santoro, A.; Da Pian, R. Spontaneous occlusion of supraclinoid aneurysms after the creation of extra-intracranial bypasses using long grafts: Report of two cases. Neurosurgery 1999, 44, 216–219, discussion 219–220. [Google Scholar] [CrossRef]
- Senn, P.; Krauss, J.K.; Remonda, L.; Godoy, N.; Schroth, G. The formation and regression of a flow-related cerebral artery aneurysm. Clin. Neurol. Neurosurg. 2000, 102, 168–172. [Google Scholar] [CrossRef]
- Hans, F.J.; Krings, T.; Reinges, M.H.; Mull, M. Spontaneous regression of two supraophthalmic internal cerebral artery aneurysms following flow pattern alteration. Neuroradiology 2004, 46, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.M.; Thorell, W.E.; Rasmussen, P.A. Aneurysm regression after coil embolization of a concurrent aneurysm. AJNR Am. J. Neuroradiol. 2005, 26, 917–921. [Google Scholar] [PubMed]
- Li, Y.; Payner, T.D.; Cohen-Gadol, A.A. Spontaneous regression of an intracranial aneurysm after carotid endarterectomy. Surg. Neurol. Int. 2012, 3, 66. [Google Scholar] [CrossRef] [PubMed]
- Tsimpas, A.; Ashley, W.W.; Germanwala, A.V. Spontaneous regression of intracranial aneurysm following remote ruptured aneurysm treatment with pipeline stent assisted coiling. J. Neurointerv. Surg. 2016, 8, e39. [Google Scholar] [CrossRef]
- Kim, S.; Kang, M.; Jo, J.; Kim, D. Spontaneous Regression of an Intracranial Aneurysm Following Remote Aneurysm Clipping: Evaluation with High-Resolution Vessel Wall MRI. CardioVasc. Interv. Radiol. 2018, 41, 660–663. [Google Scholar] [CrossRef]
- Boillat, G.; Franssen, T.; Wanderer, S.; Grüter, B.E.; Catalano, K.; Casoni, D.; Widmer, H.R.; Remonda, L.; Andereggen, L.; Fandino, J.; et al. Development and characterization of a dual saccular elastase digested aneurysms rabbit model. Brain Spine 2021, 1, 100336. [Google Scholar] [CrossRef]
- Xiang, J.; Yu, J.; Choi, H.; Dolan Fox, J.M.; Snyder, K.V.; Levy, E.I.; Siddiqui, A.H.; Meng, H. Rupture Resemblance Score (RRS): Toward risk stratification of unruptured intracranial aneurysms using hemodynamic-morphological discriminants. J. Neurointerv. Surg. 2015, 7, 490–495. [Google Scholar] [CrossRef]

| Year | Authors | Location and Number of IAs | Treatment | Peculiarity | Conclusion |
|---|---|---|---|---|---|
| |||||
| 1944 | Dandy et al. [15] | Different IA locations | Clipping | Summary of different locations of IAs and their surgical accessibility. | Six surgical methods to possibly cure an IA, as spontaneous thrombosis occurs only rarely. |
| 1953 | Epstein et al. [16] | ACom, n = 1 | None | Roentgenological demonstration of IA by pneumo-encephalogram. | Cerebral angiography as the most reliable method of diagnosis for the detection of IAs. |
| 1958 | Lindgren et al. [17] | MCA, n = 1 | None | SAH patient. | Regression over three years. |
| 1960 | Hemmer and Umbach [18] | MCA/ posterior cerebral artery (PCA), n = 1 | None | Visible IA two and nine weeks after hemorrhage. Not visible after six months. | Rarity of follow-ups in cases with arteriographically confirmed obliteration. |
| 1962 | Björkesten and Troupp [19] | Different IA locations, n = 25 | Unsuccessful treatment by clipping | Two-year follow-up showed an occlusion of the IA. | Rare occurrence of spontaneous thrombosis of an IA after attempted clipping. |
| 1964 | Schunk et al. [20] | Literature review and case report, different IA locations | None | Contrast retention within IA in all three phases as a predisposing factor of thrombus formation. | Displacement of vessels adjacent to the partially thrombosed IA as an indicator of size and location of the thrombus. |
| 1966 | Lodin et al. [21] | PCom (posterior communicating artery), n = 1 Left superior cerebellar artery, n = 1 | Clipping | Unsuccessful treatment by clipping. | Follow-up angiography in incidental and non-operated ruptured IA is justified, since they tend to increase in size. |
| 1978 | Edner et al. [22] | ACom, n = 1 | None | Administration of dexa-methasone and EACA. | Impossibility of prediction of the spontaneous thrombosis of ruptured IA without intervention due to its rare occurrence. |
| 1977 | Scott and Garrido [23] | MCA, n = 1 | Epsilon amino-caproic acid | Giant IA. | Spontaneous thrombosis of an IA is a rare event. |
| 1981 | Spallone et al. [24] | Supraclinoid ICA (internal carotid artery), n = 1 | None | Spontaneous cure of an IA after recurrent SAH. | Rarity of spontaneous cure of IAs. |
| 1983 | Benedetti et al. [25] | ACom, n = 1 | Clipping | Despite negative angiographic control, the patient was operated, and the aneurysmal sac was the parent. | An IA, revealed at operation, failed to opacify in the absence of vasospasm and thrombosis of parent vessels. |
| 2004 | Ueta et al. [26] | ICA, n = 1 | None | First reported case of a ruptured IA at non-branching site of the supraclinoid ICA with complete spontaneous regression. | Spontaneous regression of IA can occur even at non-branching sites without intervention or treatment. |
| 2009 | Hassan et al. [27] | Anterior cerebral artery (ACA), n = 1 SAH | Clipping | Spontaneous thrombosis of a recurrent clipped IA. | Spontaneous thrombosis of an IA remnant may occur even without further treatment. |
| |||||
| 1972 | Scott and Ballantine [28] | MCA, n = 1 | None | Progressive thrombosis of a giant IA and the underlying MCA branch. | Probably non-operative treatment should be preferred. |
| 1974 | Spetzler et al. [8] | Left frontopolar artery, n = 1 | Clipping | Disappearance and reappearance of an IA. | Terms such as “complete” and “spontaneous” cure must be used with caution, since the natural history of IA is not understood yet. |
| 1984 | Davila et al. [29] | ACom, n = 1 | None | Spontaneous thrombosis of an IA. | Three conditions necessary for spontaneous cure: (1) favorable neurological status, (2) complete disappearance of the IA on imaging, and (3) confirmation of its regression without intervention. |
| 2001 | Tan et al. [30] | MCA, n = 1 | Clipping | Re-clipping of a remnant five months post initial operation. Further regrowth due to an unsuccessful bypass. | Unpredictability of growth and regression of IAs. |
| 2010 | Choi et al. [31] | MCA, n = 1 | Wrapping using pericranium and surgical glue | Stability for one year, regression after three years. | Recanalization may occur also in completely thrombosed IA, with possibility of growth or rupture. |
| 2011 | Kalin-Hajdu et al. [32] | MCA, n = 1 | None | Angiography on scheduled embolization revealed that the IA had already undergone complete thrombosis. | Spontaneous occlusion and regression of a IA is exceptional and unpredictable. |
| 2016 | Jun et al. [33] | ACom, n = 1 | Clipping | Reluctance from the patient regarding reoperation. | Absence of a reliable methods to predict whether an IA remnant will spontaneously regress or grow. |
| |||||
| 1979 | Fodstad et al. [34] | MCA, n = 1 | AMCA (tranexamic acid) | Spontaneous thrombosis might occur more often when anti-fibrinolytic drugs are administered. | Possible local inhibition of plasminogen activators in and around the IA wall when using AMCA. |
| 1981 | Moritake et al. [35] | PCom, n = 1 | None | Ruptured IA. | Crucial roles of vasospasms and relative narrowness of the aneurysmal neck in cases of spontaneous IA thrombosis. |
| 1999 | Warschewske et al. [36] | Anterior cerebral artery (ACA), n = 1 | None | Spontaneous thrombosis of a giant IA. | Spontaneous thrombosis may occur under certain anatomic and hemodynamic conditions. Conservative management and follow-up examinations in asymptomatic patients is suggested. |
| |||||
| 1957 | Marguth et al. [37] | ICA, n = 1 | None | Spontaneous SAH at age thirteen yeats, regression on control thirteen years later. | Fully visible IA sac in the arterial phase, only contrast residuals visible in the early and late venous phases. |
| 1964 | Höök and Norlen [38] | MCA, n = 1 | Ligation of the aneurysmal neck | Impossibility of contrast filling of the carotid artery through an acute cerebral edema. | Intracranial treatment methods for IA are more effective and should be preferred. |
| 1997 | Yeh et al. [39] | Terminal ICA, n = 1 | EC–IC bypass | Spontaneous thrombosis of the IA at two-year follow-up. | Hemodynamic changes in the blood flow of the parent artery after EC–IC bypass caused obliteration. |
| 1999 | Cantore et al. [40] | Supraclinoid ICA, n = 2 | Clipping/ Bypass | Postoperative angiography demonstrated spontaneous occlusion of the IAs. | Spontaneous occlusion of an IA may be induced or favoured by hemodynamic vascular alterations that take place inside the IA after a high-flow EC-IC bypass has been created. |
| 2000 | Senn et al. [41] | PCom, n = 1 | Carotid end-arterectomy (CEA) | Regression after ipsilateral carotid endarterectomy. | Formation and regression of the ‘flow-related’ IA was associated with hemodynamic changes in blood flow of the right posterior communicating artery (PCoA) and the right ICA. |
| 2004 | Hans et al. [42] | ICA, n = 3 (1 giant cavernous ICA aneurysm, 2 supra-ophthalmic IAs) | Endo-vascular closure | Spontaneous regression of two supraophthalmic IAs following flow pattern alteration by proximal ICA IA treatment. | Changes in cerebral hemodynamics might potentially lead to plastic changes in the vessel architecture, therefore IAs can be flow related. |
| 2005 | Chow et al. [43] | Paraclinoidal ICA, n = 2 | Endovascular treatment | Disappearance of an ICA aneurysm after endovascular treatment of a concurrent ipsilateral ICA aneurysm. | Hemodynamic alterations may contribute to regression. |
| 2012 | Li et al. [44] | ICA, n = 1 | Carotid end-arterectomy (CEA) | Spontaneous regression of an intracranial IA after carotid endarterectomy. | Regression and potential formation of IAs may correlate with hemodynamic factors associated with stenosis of the contralateral ICA. |
| 2015 | Tsimpas et al. [45] | ICA, n = 3 right MCA, n = 1 left MCA, n = 1 | Coiling/ Flow Diverter | Regression of a downstream M2-aneurysm after Flow Diverter treatment of ICA aneurysms. | Changing flow dynamics of a proximal vessel may eventually lead to spontaneous regression of a downstream IA. |
| 2018 | Kim et al. [46] | Right anterior temporal artery, n = 1 | Clipping | Regression after contralateral MCA clipping. | Spontaneous occlusion may occur even without direct treatment. High-resolution MRI may provide insights into morphology and IA changes over time. |
| Patient Age | Aneurysm Location | Aneurysm Size | Follow-Up Duration |
|---|---|---|---|
| 56 y/o | ACom | 3 × 4 mm (diagnosis) 2 × 2.5 mm (FU) | 9 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, K.; Andereggen, L.; Schubert, G.A.; Marbacher, S.; Grüter, B.E. Spontaneous Regression of Intracranial Aneurysms—Case Report and Systematic Review of the Literature. Brain Sci. 2025, 15, 488. https://doi.org/10.3390/brainsci15050488
Catalano K, Andereggen L, Schubert GA, Marbacher S, Grüter BE. Spontaneous Regression of Intracranial Aneurysms—Case Report and Systematic Review of the Literature. Brain Sciences. 2025; 15(5):488. https://doi.org/10.3390/brainsci15050488
Chicago/Turabian StyleCatalano, Kristina, Lukas Andereggen, Gerrit A. Schubert, Serge Marbacher, and Basil E. Grüter. 2025. "Spontaneous Regression of Intracranial Aneurysms—Case Report and Systematic Review of the Literature" Brain Sciences 15, no. 5: 488. https://doi.org/10.3390/brainsci15050488
APA StyleCatalano, K., Andereggen, L., Schubert, G. A., Marbacher, S., & Grüter, B. E. (2025). Spontaneous Regression of Intracranial Aneurysms—Case Report and Systematic Review of the Literature. Brain Sciences, 15(5), 488. https://doi.org/10.3390/brainsci15050488





