Cognitive Impairment-Associated Risk Factors of Parkinson’s Disease: A Hospital-Based Study in a Cohort of Upper Egypt Parkinson’s Patients
Abstract
:1. Introduction
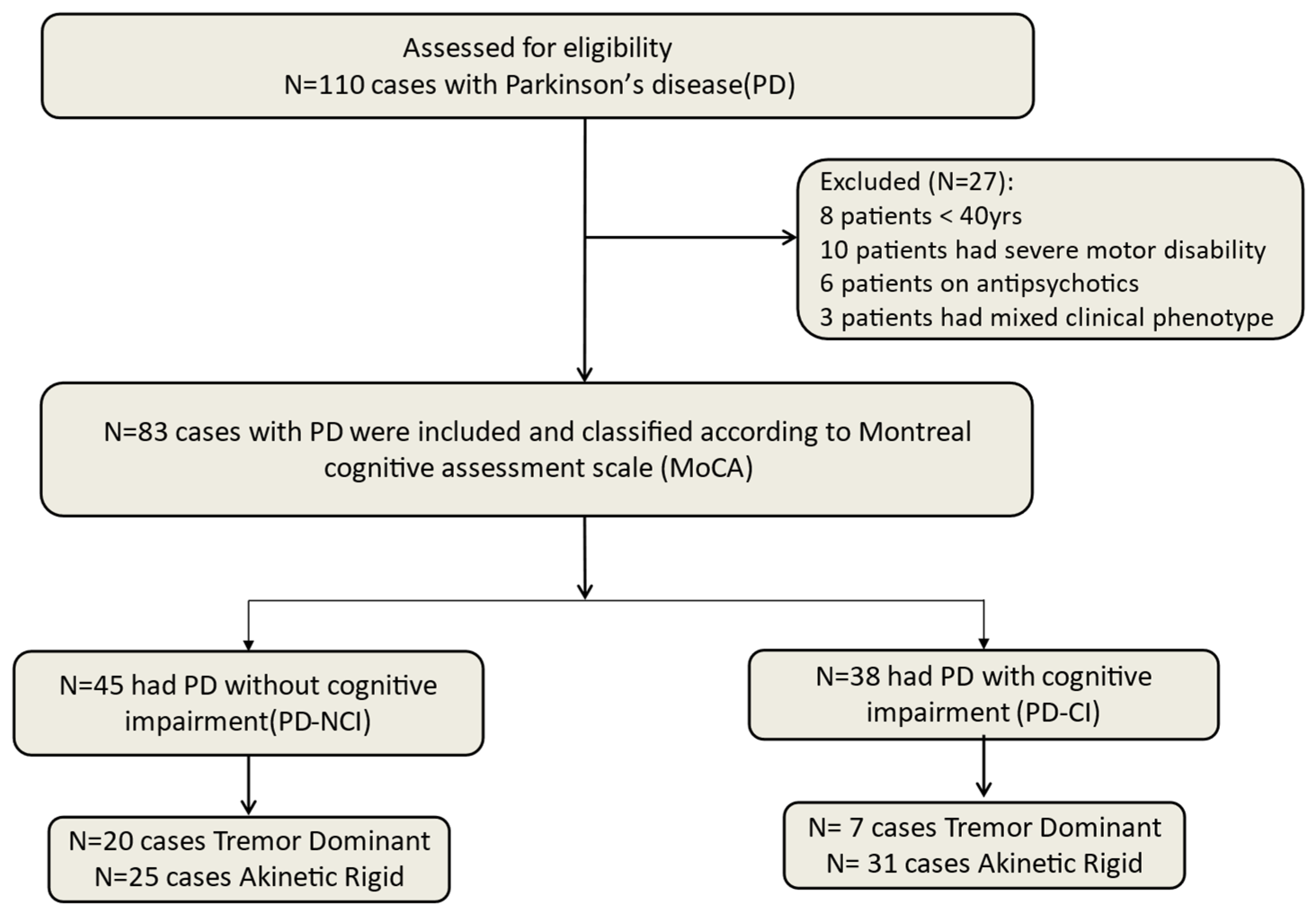
2. Materials and Methods
2.1. Clinical Scales
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Fawi, G.; Abbas, M.A.A.; Mohammed, T.A.; El-Fetoh, N.A.; Attar, G.A.; Zaki, A.F. Prevalence of Parkinsonism and Parkinson’s disease in Qena governorate/Egypt: A cross-sectional community-based survey. Neurol. Res. 2015, 37, 607–618. [Google Scholar] [CrossRef]
- Khedr, E.M.; Al Attar, G.S.; Kandil, M.R.; Kamel, N.F.; Abo Elfetoh, N.; Ahmed, M.A. Epidemiological Study and Clinical Profile of Parkinson’s Disease in the Assiut Governorate, Egypt: A Community-Based Study. Neuroepidemiology 2012, 38, 154–163. [Google Scholar] [CrossRef]
- William, M.B.; Hamed, S.; Shalash, A.; Khedr, E.M.; Yousef, M.H.; El-Jaafary, S.; Fawi, G.; Helmy, A.; Hamid, E.; Essam, M.; et al. The p.Gly2019Ser is a common LRRK2 pathogenic variant among Egyptians with familial and sporadic Parkinson’s disease. npj Park. Dis. 2024, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Andersen, K.; Larsen, J.P.; Lolk, A.; Nielsen, H.; Kragh-Sørensen, P. Risk of dementia in Parkinson’s disease: A community-based, prospective study. Neurology 2001, 56, 730–736. [Google Scholar] [CrossRef]
- Khedr, E.M.; El Fetoh, N.A.; Khalifa, H.; Ahmed, M.A.; El Beh, K.M.A. Prevalence of non motor features in a cohort of Parkinson’s disease patients. Clin. Neurol. Neurosurg. 2013, 115, 673–677. [Google Scholar] [CrossRef]
- Khedr, E.; Fawi, G.; Abbas, M.A.A.; Mohammed, T.A.; El-Fetoh, N.A.; Al Attar, G.; Noaman, M.; Zaki, A.F. Prevalence of Mild Cognitive Impairment and Dementia among the Elderly Population of Qena Governorate, Upper Egypt: A Community-Based Study. J. Alzheimer’s Dis. 2015, 45, 117–126. [Google Scholar] [CrossRef]
- Aarsland, D.; Zaccai, J.; Brayne, C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov. Disord. 2005, 20, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Lee, V.M.-Y.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef]
- Compta, Y.; Parkkinen, L.; O’Sullivan, S.S.; Vandrovcova, J.; Holton, J.L.; Collins, C.; Lashley, T.; Kallis, C.; Williams, D.R.; De Silva, R.; et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: Which is more important? Brain 2011, 134, 1493–1505. [Google Scholar] [CrossRef]
- Marinus, J.; Zhu, K.; Marras, C.; Aarsland, D.; Van Hilten, J.J. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018, 17, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.B.; Poewe, W.; Olanow, C.W.; Oertel, W.H.; Obeso, J.A.; Marek, K.L.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Stebbins, G.T.; Goetz, C.G.; Burn, D.J.; Jankovic, J.; Khoo, T.K.; Tilley, B.C. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov. Disord. 2013, 28, 668–670. [Google Scholar] [CrossRef]
- Adams, C.; Suescun, J.; Haque, A.; Block, K.; Chandra, S.; Ellmore, T.M.; Schiess, M.C. Updated Parkinson’s disease motor subtypes classification and correlation to cerebrospinal homovanillic acid and 5-hydroxyindoleacetic acid levels. Clin. Park. Relat. Disord. 2023, 8, 100187. [Google Scholar] [CrossRef]
- Giladi, N.; Shabtai, H.; Simon, E.S.; Biran, S.; Tal, J.; Korczyn, A.D. Construction of freezing of gait questionnaire for patients with Parkinsonism. Park. Relat. Disord. 2000, 6, 165–170. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Hollman, J.H.; Beckman, B.A.; Brandt, R.A.; Merriwether, E.N.; Williams, R.T.; Nordrum, J.T. Minimum Detectable Change in Gait Velocity during Acute Rehabilitation following Hip Fracture. J. Geriatr. Phys. Ther. 2008, 31, 53–56. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontol. 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Rosso, C.; Lamy, J.C. Does Resting Motor Threshold Predict Motor Hand Recovery After Stroke? Front. Neurol. 2018, 9, 1020. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, A.A.; Noaman, M.; Fawzy, M.; Moheb, A.; Karim, A.A.; Khedr, E.M. A double-blind randomized clinical trial of high frequency rTMS over the DLPFC on nicotine dependence, anxiety and depression. Sci. Rep. 2021, 11, 1640. [Google Scholar] [CrossRef]
- Wojtala, J.; Heber, I.A.; Neuser, P.; Heller, J.; Kalbe, E.; Rehberg, S.P.; Storch, A.; Linse, K.; Schneider, C.; Gräber, S.; et al. Cognitive decline in Parkinson’s disease: The impact of the motor phenotype on cognition. J. Neurol. Neurosurg. Psychiatry 2019, 90, 171–179. [Google Scholar] [CrossRef]
- Zhou, M.-Z.; Gan, J.; Wei, Y.-R.; Ren, X.-Y.; Chen, W.; Liu, Z.-G. The association between non-motor symptoms in Parkinson’s disease and age at onset. Clin. Neurol. Neurosurg. 2013, 115, 2103–2107. [Google Scholar] [CrossRef]
- Hughes, T.A.; Ross, H.F.; Musa, S.; Bhattacherjee, S.; Nathan, R.N.; Mindham, R.H.S.; Spokes, E.G.S. A 10-year study of the incidence of and factors predicting dementia in Parkinson’s disease. Neurology 2000, 54, 1596–1603. [Google Scholar] [CrossRef]
- Hurtig, H.I.; Trojanowski, J.Q.; Galvin, J.; Ewbank, D.; Schmidt, M.L.; Lee, V.M.-Y.; Clark, C.M.; Glosser, G.; Stern, M.B.; Gollomp, S.M.; et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology 2000, 54, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Mason, S.L.; Evans, J.R.; Foltynie, T.; Brayne, C.; Robbins, T.W.; Barker, R.A. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Setó-Salvia, N.; Clarimón, J.; Pagonabarraga, J.; Pascual-Sedano, B.; Campolongo, A.; Combarros, O.; Mateo, J.I.; Regaña, D.; Martínez-Corral, M.; Marquié, M.; et al. Dementia Risk in Parkinson Disease: Disentangling the Role of MAPT Haplotypes. Arch. Neurol. 2011, 68, 359–364. [Google Scholar] [CrossRef]
- Winder-Rhodes, S.E.; Hampshire, A.; Rowe, J.B.; Peelle, J.E.; Robbins, T.W.; Owen, A.M.; Barker, R.A. Association between MAPT haplotype and memory function in patients with Parkinson’s disease and healthy aging individuals. Neurobiol. Aging 2015, 36, 1519–1528. [Google Scholar] [CrossRef]
- Kann, S.J.; Chang, C.; Manza, P.; Leung, H.-C. Akinetic rigid symptoms are associated with decline in a cortical motor network in Parkinson’s disease. Npj Park. Dis. 2020, 6, 19. [Google Scholar] [CrossRef]
- Li, X.; Xing, Y.; Martin-Bastida, A.; Piccini, P.; Auer, D.P. Patterns of grey matter loss associated with motor subscores in early Parkinson’s disease. NeuroImage Clin. 2018, 17, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Spay, C.; Meyer, G.; Welter, M.-L.; Lau, B.; Boulinguez, P.; Ballanger, B. Functional imaging correlates of akinesia in Parkinson’s disease: Still open issues. NeuroImage Clin. 2019, 21, 101644. [Google Scholar] [CrossRef] [PubMed]
- Palermo, S.; Lopiano, L.; Zibetti, M.; Rosato, R.; Leotta, D.; Amanzio, M. A novel framework for understanding reduced awareness of dyskinesias in Parkinson’s Disease. Park. Relat. Disord. 2017, 39, 58–63. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Huang, Z.; Jiang, Y.; Ren, Z.; Liu, D.; Zhang, J.; La Piana, R.; Chen, Y. Cortical and subcortical morphological alterations in motor subtypes of Parkinson’s disease. Npj Park. Dis. 2022, 8, 167. [Google Scholar] [CrossRef]
- Rosenberg-Katz, K.; Herman, T.; Jacob, Y.; Giladi, N.; Hendler, T.; Hausdorff, J.M. Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology 2013, 80, 1476–1484. [Google Scholar] [CrossRef]
- Hensel, L.; Hoffstaedter, F.; Caspers, J.; Michely, J.; Mathys, C.; Heller, J.; Eickhoff, C.R.; Reetz, K.; Südmeyer, M.; Fink, G.R.; et al. Functional Connectivity Changes of Key Regions for Motor Initiation in Parkinson’s Disease. Cereb. Cortex 2019, 29, 383–396. [Google Scholar] [CrossRef]
- Chang, C.-W.; Tan, C.H.; Hong, W.-P.; Yu, R.L. GBA moderates cognitive reserve’s effect on cognitive function in patients with Parkinson’s disease. J. Neurol. 2024, 271, 4392–4405. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.L.; Tu, S.C.; Wu, R.-M.; Lu, P.A.; Tan, C.H. Interactions of COMT and ALDH2 Genetic Polymorphisms on Symptoms of Parkinson’s Disease. Brain Sci. 2021, 11, 361. [Google Scholar] [CrossRef]
- Dahodwala, N.; Dahodwala, N.; Siderowf, A.; Siderowf, A.; Xie, M.; Noll, E.; Stern, M.B.; Mandell, D.S.; Mandell, D.S. Racial differences in the diagnosis of Parkinson’s disease. Mov. Disord. 2009, 24, 1200–1205. [Google Scholar] [CrossRef]
- Zhang, Y.; Shu, L.; Sun, Q.; Zhou, X.; Pan, H.; Guo, J.; Tang, B. Integrated Genetic Analysis of Racial Differences of Common GBA Variants in Parkinson’s Disease: A Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 43. [Google Scholar] [CrossRef]
- Sousa, N.M.F.; Macedo, R.C. Relationship between cognitive performance and mobility in patients with Parkinson’s disease: A cross-sectional study. Dement. Neuropsychol. 2019, 13, 403–409. [Google Scholar] [CrossRef]
- Burn, D.J. Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry 2006, 77, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Oteng-Amoako, A.; Speechley, M.; Gopaul, K.; Beauchet, O.; Annweiler, C.; Muir-Hunter, S.W. The Motor Signature of Mild Cognitive Impairment: Results From the Gait and Brain Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 1415–1421. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S. Regional and cellular fractionation of working memory. Proc. Natl. Acad. Sci. USA 1996, 93, 13473–13480. [Google Scholar] [CrossRef] [PubMed]
- Schicktanz, N.; Schwegler, K.; Fastenrath, M.; Spalek, K.; Milnik, A.; Papassotiropoulos, A.; Nyffeler, T.; de Quervain, D.J. Motor threshold predicts working memory performance in healthy humans. Ann. Clin. Transl. Neurol. 2014, 1, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Ahmed, O.G.; Sayed, H.M.; Abo-Elfetoh, N.; Ali, A.M.; Gomaa, A.M. Electrophysiological differences in cortical excitability in different forms of dementia: A transcranial magnetic stimulation and laboratory biomarkers study. Neurophysiol. Clin. 2020, 50, 185–193. [Google Scholar] [CrossRef]
- Khedr, E.M.; Ahmed, M.A.; Darwish, E.S.; Ali, A.M. The relationship between motor cortex excitability and severity of Alzheimer’s disease: A transcranial magnetic stimulation study. Neurophysiol. Clin. 2011, 41, 107–113. [Google Scholar] [CrossRef]
- Chou, Y.H.; Sundman, M.; Ton That, V.; Green, J.; Trapani, C. Cortical excitability and plasticity in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis of transcranial magnetic stimulation studies. Ageing Res. Rev. 2022, 79, 101660. [Google Scholar] [CrossRef]
- Kamble, N.; Bhattacharya, A.; Hegde, S.; Vidya, N.; Gothwal, M.; Yadav, R.; Pal, P.K. Cortical excitability changes as a marker of cognitive impairment in Parkinson’s disease. Behav. Brain Res. 2022, 422, 113733. [Google Scholar] [CrossRef]
- Leroi, I.; McDonald, K.; Pantula, H.; Harbishettar, V. Cognitive Impairment in Parkinson Disease: Impact on Quality of Life, Disability, and Caregiver Burden. J. Geriatr. Psychiatry Neurol. 2012, 25, 208–214. [Google Scholar] [CrossRef]
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Breen, D.P.; Khoo, T.K.; Williams-Gray, C.H.; Barker, R.A.; Collerton, D.; Taylor, J.-P.; Burn, D.J. Cognitive decline and quality of life in incident Parkinson’s disease: The role of attention. Park. Relat. Disord. 2016, 27, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Buono, V.L.; Corallo, F.; Foti, M.; Lorenzo, G.D.; Bramanti, P.; Marino, S. Nonmotor Symptoms in Parkinson Disease: A Descriptive Review on Social Cognition Ability. J. Geriatr. Psychiatry Neurol. 2017, 30, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Pua, S.-Y.; Yu, R.-L. Effects of Executive Function on Age-related Emotion Recognition Decline Varied by Sex. Soc. Sci. Med. 2024, 361, 117392. [Google Scholar] [CrossRef] [PubMed]
| (A) | ||||
| PD-NCI Mean ± SD (n = 45) | PD-CI Mean ± SD (n = 38) | Chi or Z Value | p-Value | |
| Sex | ||||
| Male (61 males with 73.5%) | 38 (84.4%) | 23 (60.5%) | 6.05 | 0.024 |
| Female (22 females with 26.5%) | 7 (15.6%) | 15 (39.5%) | ||
| Age | 57.16 ± 9.66 | 62.37 ± 9.17 | −2.759 | 0.006 |
| Median (IQR) | 57 (11) | 63.5 (10) | ||
| Age of onset (years) | 52.38 ± 10.2 | 57.13 ± 10.57 | −2.369 | 0.018 |
| Median (IQR) | 52 (13) | 58 (11.25) | ||
| Education | ||||
| Yes | 40 (88.9%) | 21 (55.3%) | 11.958 | 0.001 |
| No (illiterate) | 5 (11.1%) | 17 (44.7%) | ||
| Duration of education (years) | 13.2 ± 5.08 | 7.53 ± 7.37 | −3.37 | 0.001 |
| Median (IQR) | 16 (4) | 7 (16) | ||
| Duration of disease (years) | 4.78 ± 3.71 | 5.24 ± 5.04 | −0.369 | 0.712 |
| Median (IQR) | 3 (5.5) | 4 (4) | ||
| Family history | ||||
| Yes | 25 (55.6%) | 16 (42.1%) | 1.491 | 0.273 |
| No | 20 (44.4%) | 22 (57.9%) | ||
| Clinical type | ||||
| Tremor-dominant | 20 (44.4%) | 7 (18.4%) | 6.357 | 0.01 |
| Akinetic–rigid | 25 (55.6%) | 31 (81.6%) | ||
| (B) | ||||
| Variable | PD-NCI (n = 32) | PD-CI (n = 29) | Chi or Z Value | p-Value |
| Sex | ||||
| Male | 24 (75%) | 20 (68.96%) | 0.276 | 0.77 |
| Female | 8 (25%) | 9 (31.04%) | ||
| Age | 54.75 ± 8.99 | 63.21 ± 9.65 | −3.34 | 0.001 |
| Median (IQR) | 55 (16) | 63 (13) | ||
| Age of onset | 49.64 ± 10.00 | 58.38 ± 10.50 | −3.063 | 0.002 |
| Median (IQR) | 51.25 (17) | 57 (12) | ||
| Education | ||||
| Yes | 27 (84.38%) | 12 (41.38%) | 12.19 | 0.001 |
| No | 5 (15.62%) | 17 (58.62%) | ||
| Years of education | 12.31 ± 5.37 | 5.38 ± 6.93 | −3.53 | 0.0001 |
| Median (IQR) | 15 (4) | 0 (12) | ||
| Duration of illness | 5.11 ± 3.83 | 4.59 ± 4.86 | −0.859 | 0.391 |
| Median (IQR) | 4 (6) | 3 (3) | ||
| Family history | ||||
| Yes | 14 (60.87%) | 10 (34.48%) | 0.547 | 0.60 |
| No | 18 (39.13%) | 19 (65.52%) | ||
| Total UPDRS | 73.66 ± 36.54 | 108.28 ± 38.03 | −3.438 | 0.001 |
| Median (IQR) | 69 (46) | 107 (43) | ||
| Clinical type | ||||
| Tremor-dominant | 15 (46.87%) | 5 (17.24%) | 6.06 | 0.016 |
| Akinetic–rigid | 17 (53.13%) | 24 (83.76%) | ||
| Resting motor threshold (RMTS) | 37.38 ± 7.71 | 42.55 ± 8.60 | −2.379 | 0.017 |
| Median (IQR) | 36 (13) | 43 (13) | ||
| Variables | PD-NCI Mean ± SD (n = 45) | PD-CI Mean ± SD (n = 38) | Chi or Z Value | p-Value |
|---|---|---|---|---|
| Freezing of Gait Questionnaire (FOG-Q) | 2.8 ± 5.91 | 3.95 ± 5.99 | −1.23 | 0.219 |
| Timed up and go (TUG-T) (in seconds) | 48.69 ± 41.02 | 87.71 ± 62.91 | −3.305 | 0.001 |
| Median (IQR) | 32 (53) | 71 (84.25) | ||
| The 10-Meter Walk Test (10MWT) | ||||
| Average self-selected velocity (m/s) | 0.836 ± 0.578 | 0.485 ± 0.441 | −3.099 | 0.002 |
| Median (IQR) | 0.67 (0.64) | 0.31 (0.51) | ||
| Average fast velocity (m/s) | 1.27 ± 0.764 | 0.735 ± 0.648 | −3.346 | 0.001 |
| Median (IQR) | 1.27 (1.22) | 0.58 (0.75) | ||
| MDS-UPDRS Part I: Non-Motor Aspects of Experiences of Daily Living (n-MEDL) | 11.756 ± 6.889 | 19.263 ± 8.15 | −4.267 | <0.001 |
| Median (IQR) | 11 (7) | 18 (9.50) | ||
| MDS-UPDRS Part II: Motor Aspects of Experiences of Daily Living | 19.711 ± 9.603 | 28.84 ± 11.103 | −3.599 | <0.001 |
| Median (IQR) | 19 (16.5) | 30 (16.25) | ||
| MDS-UPDRS Part III: Motor Examination | 50 ± 22.427 | 66.026 ± 24.18 | −2.880 | 0.004 |
| Median (IQR) | 47 (31.5) | 60.5 (36) | ||
| HOEHN and YAHR staging | 1.93 ± 0.963 | 2.5 ± 1.225 | −2.363 | 0.018 |
| Median (IQR) | 2 (2) | 2 (1) | ||
| Instrumental Activity of Daily Living [18] | 6.22 ± 1.704 | 4 ± 1.845 | −4.908 | <0.001 |
| Median (IQR) | 6 (3) | 4 (2) | ||
| Tremor-Dominant Mean ± SD (n = 27) | Akinetic–Rigid Mean ± SD (n = 56) | Chi or z Value | p-Value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 19 (70.4%) | 42 (75%) | 0.200 | 0.654 |
| Female | 8 (29.6%) | 14 (25%) | ||
| Age | 59.00 ± 9.94 | 59.8 ± 9.72 | −0.749 | 0.454 |
| Median (IQR) | 58 (13) | 60.5 (9) | ||
| Age group | ||||
| Younger than 50 yrs | 2 (7.4%) | 9 (16.1%) | 1.189 | 0.275 |
| Older or equal to 50 | 25 (92.6%) | 47 (83.9%) | ||
| Age of onset | 54.63 ± 10.34 | 54.52 ± 10.79 | −0.224 | 0.823 |
| Median (IQR) | 54 (15) | 55.5 (12.5) | ||
| Education | ||||
| Yes | 22 (81.5%) | 39 (69.6%) | 1.311 | 0.252 |
| No | 5 (18.5%) | 17 (30.4%) | ||
| Duration of education | 12.26 ± 6.25 | 9.8 ± 6.99 | −1.702 | 0.089 |
| Median (IQR) | 16 (4) | 12 (16) | ||
| Duration of disease | 4.37 ± 3.78 | 5.29 ± 4.6 | −1.195 | 0.232 |
| Median (IQR) | 3 (6) | 4 (4.5) | ||
| Family history | ||||
| Yes | 14 (51.9%) | 27 (48.2%) | 0.096 | 0.756 |
| No | 13 (48.1%) | 29 (51.8%) | ||
| PD-NCI vs. PD-CI | ||||
| PD-NCI | 20 (74.1%) | 25 (44.6%) | 6.357 | 0.018 |
| PD-CI | 7 (25.9%) | 31 (55.4%) |
| Variable | Positive Family History Mean ± SD (n = 41) | Negative Family History Mean ± SD (n = 42) | Chi or z Value | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 29 (70.7%) | 32 (76.2%) | 0.317 | 0.573 |
| Female | 12 (29.3%) | 10 (23.8%) | ||
| Age | 59.44 ± 10.57 | 59.64 ± 8.99 | −0.160 | 0.873 |
| Median (IQR) | 59 (11) | 50.5 (10) | ||
| Age group | ||||
| Younger than 50 yrs | 6 (14.6%) | 5 (11.9%) | 1.34 | 0.714 |
| Older or equal to 50 | 35 (85.4%) | 37 (88.1%) | ||
| Age of onset | 53.68 ± 11.4 | 55.4 ± 9.77 | −0.902 | 0.37 |
| Median (IQR) | 53 (16) | 55 (11.75) | ||
| Education | ||||
| Yes | 34 (82.9%) | 27 (64.3%) | 3.701 | 0.054 |
| No | 7 (17.1%) | 15 (35.7%) | ||
| Duration of education | 12.05 ± 6.07 | 9.19 ± 7.27 | −2.062 | 0.039 |
| Median (IQR) | 16 (6) | 12 (16) | ||
| Duration of disease | 5.76 ± 5.37 | 4.24 ± 2.92 | −1.116 | 0.265 |
| Median (IQR) | 4 (5) | 3 (4) | ||
| PD-NCI vs. PD-CI | ||||
| PD-NCI | 25 (61%) | 20 (47.6%) | 1.491 | 0.273 |
| PD-CI | 16 (39%) | 22 (52.4%) | ||
| Clinical type | ||||
| Tremor-dominant | 14 (34.1%) | 13 (31%) | 0.096 | 0.756 |
| Akinetic–rigid | 27 (65.9%) | 29 (69%) |
| MoCA Score | ||
|---|---|---|
| Demographic Data | ||
| Gender | r | −0.202 |
| p value | 0.067 | |
| Age in years | r | −0.381 |
| p value | <0.0001 | |
| Age of onset | r | −0.323 |
| p value | 0.003 | |
| Years of education | r | 0.428 |
| p value | 0.001 | |
| Family history | r | −0.096 |
| p value | 0.386 | |
| Duration of disease (in years) | r | −0.044 |
| p value | 0.690 | |
| Gait Scales | ||
| Freezing of Gait Questionnaire (FOG-Q) | r | 0.279 |
| p value | 0.089 | |
| Timed up and go (TUG-T) (in seconds) | r | −0.357 |
| p value | 0.001 | |
| The 10-Meter Walk Test (10MWT) Average self-selected velocity (m/s) | r | 0.384 |
| p value | <0.0001 | |
| Average fast velocity (m/s) | r | 0.406 |
| p value | <0.0001 | |
| MDS-UPDRS, IADL, and Clinical Phenotype | ||
| Total MDS-UPDRS | r | −0.429 |
| p value | <0.0001 | |
| Tremor Score | r | −0.151 |
| p value | 0.173 | |
| Akinetic–rigid Score | r | −0.432 |
| p value | <0.0001 | |
| Cortical Excitability for 61 Patients | ||
| Resting motor threshold (rMT) | r | −0.334 |
| p value | 0.009 | |
| Predictors | Model 1 Nag R2 = 0.287 | ||
|---|---|---|---|
| p-Value | Unstandardized Coefficients (B) | 95.0% Confidence Interval for B | |
| Total MDS-UPDRS | <0.0001 | −0.094 | −0.139–0.048 |
| Model 2 Nag R2 = 0.505 | |||
| Total MDS-UPDRS | <0.0001 | −0.101 | −0.139–0.062 |
| Age of onset | <0.0001 | −0.324 | −0.477–0.170 |
| Model 3 Nag R2 = 0.559 | |||
| Total MDS-UPDRS | <0.0001 | −0.089 | −0.128–−0.050 |
| Age of onset | <0.0001 | −0.280 | −0.432–−0.127 |
| Resting motor threshold (stimulation in percentage) | 0.033 | −0.232 | −0.445–−0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khedr, E.M.; Aboshaera, K.; Karim, A.A.; Korayem, M.A.; Ahmed, G.K.; Mahmoud, D.M. Cognitive Impairment-Associated Risk Factors of Parkinson’s Disease: A Hospital-Based Study in a Cohort of Upper Egypt Parkinson’s Patients. Brain Sci. 2025, 15, 459. https://doi.org/10.3390/brainsci15050459
Khedr EM, Aboshaera K, Karim AA, Korayem MA, Ahmed GK, Mahmoud DM. Cognitive Impairment-Associated Risk Factors of Parkinson’s Disease: A Hospital-Based Study in a Cohort of Upper Egypt Parkinson’s Patients. Brain Sciences. 2025; 15(5):459. https://doi.org/10.3390/brainsci15050459
Chicago/Turabian StyleKhedr, Eman M., Khaled Aboshaera, Ahmed A. Karim, Mohammad A. Korayem, Gellan K. Ahmed, and Doaa M. Mahmoud. 2025. "Cognitive Impairment-Associated Risk Factors of Parkinson’s Disease: A Hospital-Based Study in a Cohort of Upper Egypt Parkinson’s Patients" Brain Sciences 15, no. 5: 459. https://doi.org/10.3390/brainsci15050459
APA StyleKhedr, E. M., Aboshaera, K., Karim, A. A., Korayem, M. A., Ahmed, G. K., & Mahmoud, D. M. (2025). Cognitive Impairment-Associated Risk Factors of Parkinson’s Disease: A Hospital-Based Study in a Cohort of Upper Egypt Parkinson’s Patients. Brain Sciences, 15(5), 459. https://doi.org/10.3390/brainsci15050459







