The Neural Mechanisms of Private Speech in Second Language Learners’ Oral Production: An fNIRS Study
Abstract
:1. Introduction
1.1. Understanding Private Speech: Perspectives from Piaget and Vygotsky
1.2. The Role of Private Speech in Adults’ Second Language Learning
1.3. Neural Mechanisms of Private Speech: Insights from Inner and Outer Speech
1.4. The Current Study
2. Experiment 1: The Impact of Private Speech on L2 Oral Production
2.1. Objectives of Experiment 1
2.2. Materials and Methods
2.2.1. Participants
2.2.2. Materials
2.2.3. Task and Procedure
2.2.4. Data Coding
- “em and in the……it is the tree, the tree, grow up, the tree……faster than the boy……shorter, than the record before”.
- “等到了等到了春天还是夏天到了树叶再次茂盛的时候他们要站在这个树的下面像刚开始……似的……第一……的树然后发现钉子比他现在高了一截了” (When, when spring arrives, or summer arrives, the leaves grow lush again, they will stand beneath this tree, just like when they first……as the very first……tree, and then realize that the nail has risen a little higher than him).
2.3. Results
2.3.1. Preparation Phase: Private Speech
2.3.2. Execution Phase: Oral Production
2.4. Discussion
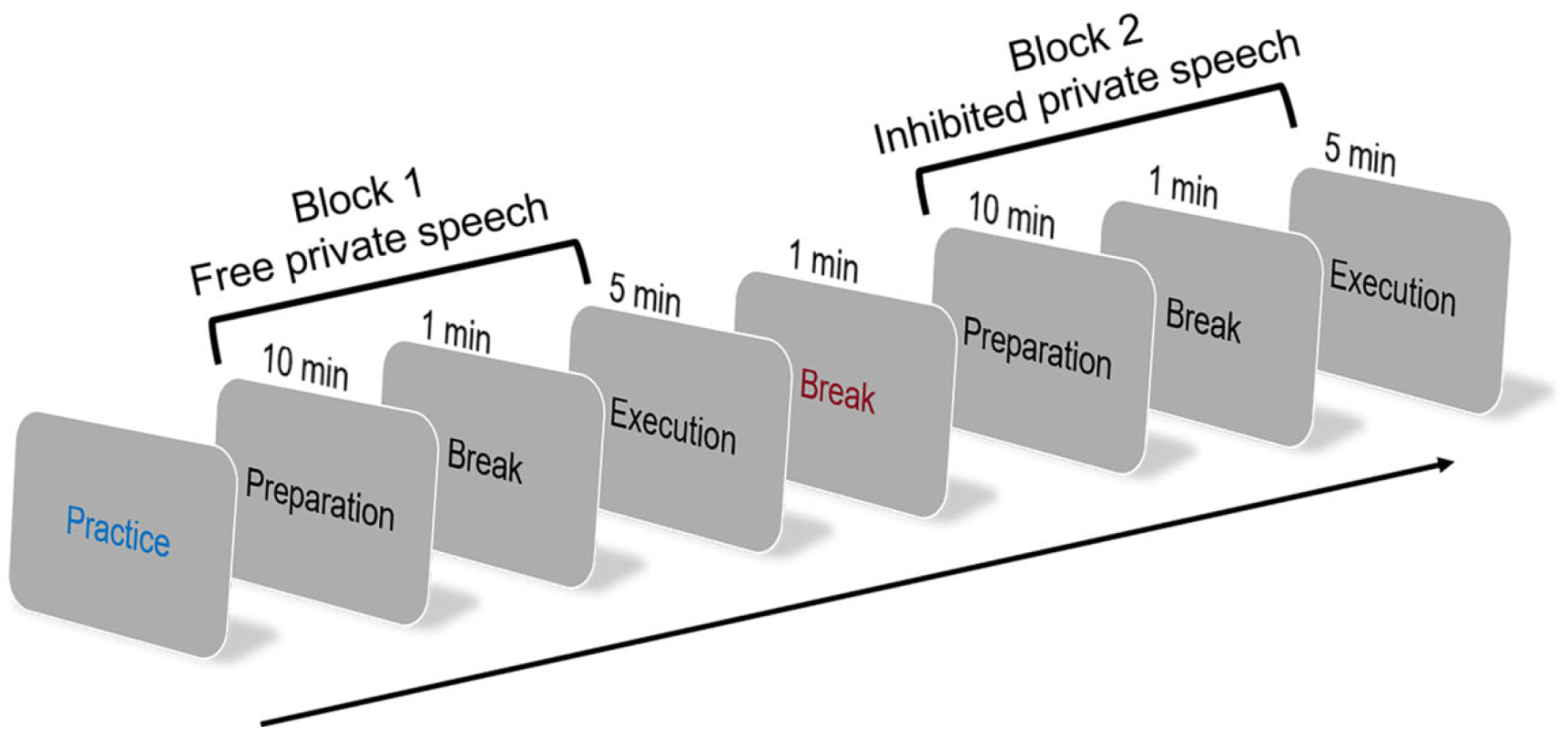
3. Experiment 2: Investigating the Neural Mechanisms of Private Speech in L2 Oral Production
3.1. Objectives of Experiment 2
3.2. Materials and Methods
3.2.1. Participants
3.2.2. Materials
3.2.3. Task and Procedure
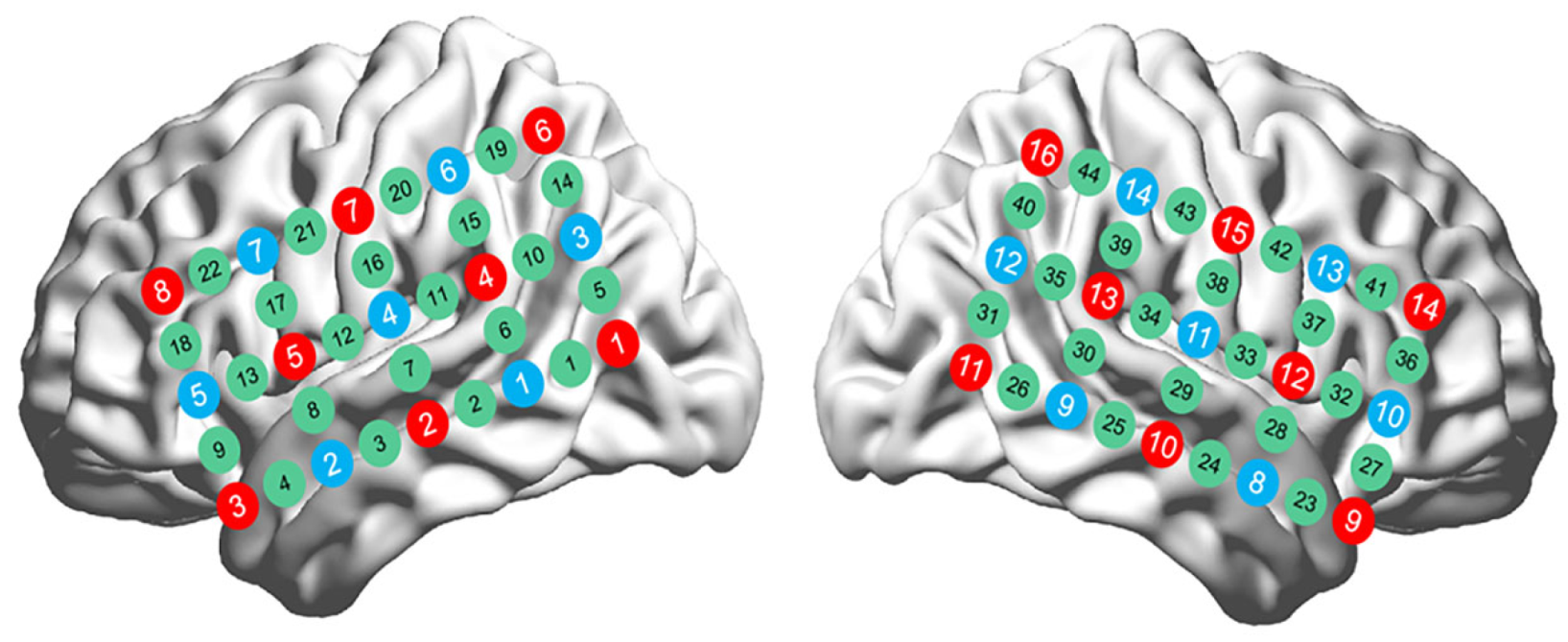
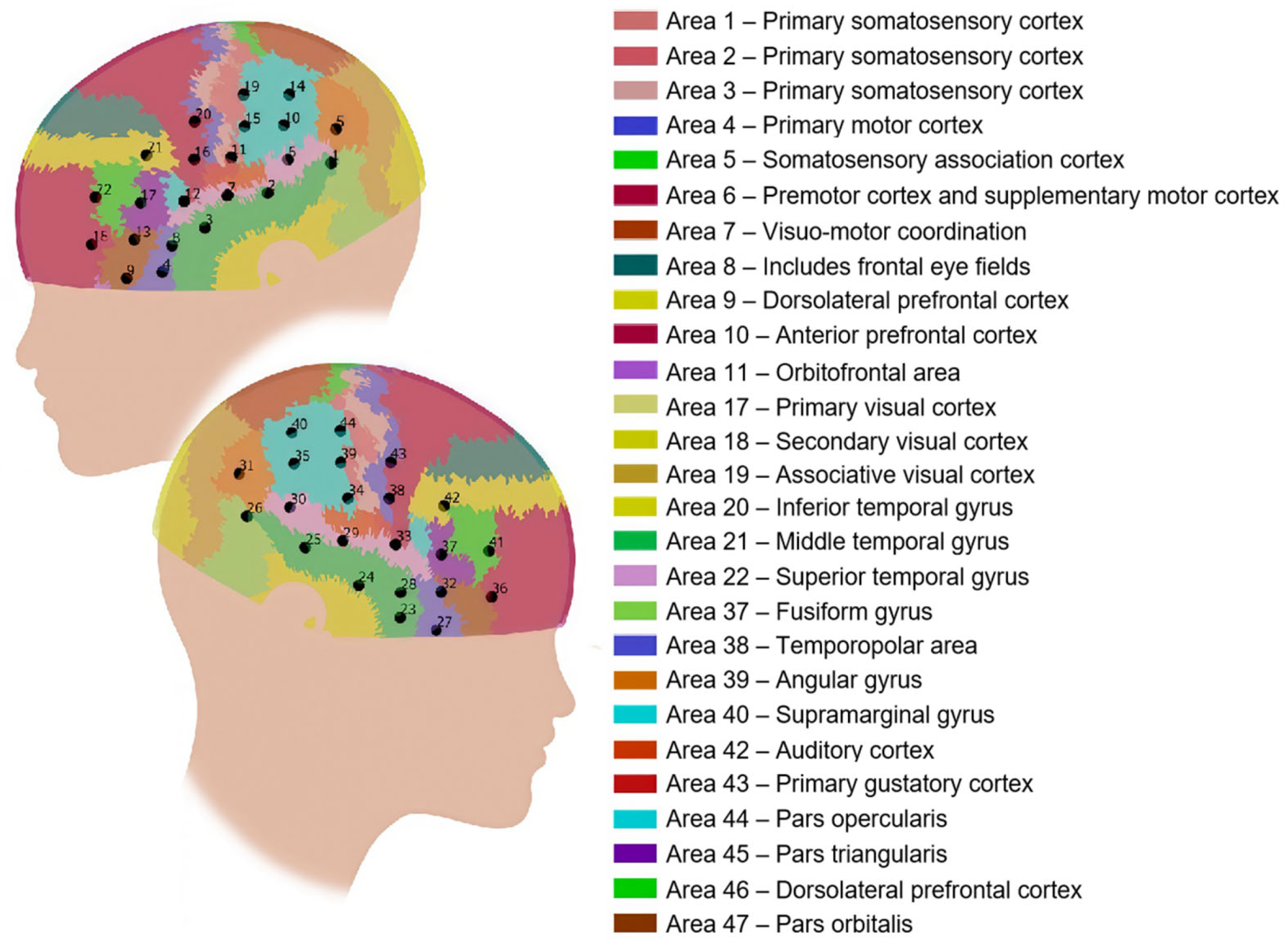
3.2.4. Data Acquisition
3.2.5. Data Preprocessing
3.2.6. Data Analysis
3.3. Results
3.3.1. Activation Analysis
3.3.2. Functional Connectivity Analysis
3.4. Discussion
3.4.1. Activation of Different Speech Types
3.4.2. Private as a Thought-Regulation Tool Similar to Inner Speech
3.4.3. Private Speech as a Transitional Form Bridging Outer and Inner Speech
3.4.4. Summary and Pedagogical Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| L2 | Second language |
| STG | Superior temporal gyrus |
| IFG | Inferior frontal gyrus |
| MTG | Middle temporal gyrus |
| SMG | Supramarginal gyrus |
| DLPFC | Dorsolateral prefrontal cortex |
| fNIRS | Functional near-infrared spectroscopy |
| U | Number of utterances |
| W_PS | Number of words (during private speech) |
| U/S | Average number of utterances per second |
| W/U | Average number of words per utterance |
| CAF | Complexity, accuracy, and fluency |
| W_OP | Number of words (during oral production) |
| AS | Analysis of speech |
| W/AS | Average number of words per AS unit |
| CAS | Complex analysis of speech |
| EW | Number of erroneous words |
| HbO | Oxygenated hemoglobin |
| HbR | Deoxygenated hemoglobin |
| tHb | Total hemoglobin |
| FDR | False discovery rate |
| CH | Channel |
| OFC | Orbitofrontal cortex |
| AG | Angular gyrus |
References
- Martínez, C.S.M.; i Calbet, H.B.; Feigenbaum, P. Private and inner speech and the regulation of social speech communication. Cogn. Dev. 2011, 26, 214–229. [Google Scholar] [CrossRef]
- Alarcón-Rubio, D.; Sánchez-Medina, J.A.; Prieto-García, J.R. Executive Function and Verbal Self-Regulation in Childhood: Developmental linkages between partially internalized private speech and cognitive flexibility. Early Child. Res. Q. 2014, 29, 95–105. [Google Scholar] [CrossRef]
- Damianova, M.K.; Lucas, M.; Sullivan, G.B. Verbal mediation of problem solving in pre-primary and junior primary school children. S. Afr. J. Psychol. 2012, 42, 445–455. [Google Scholar] [CrossRef]
- McGonigle-Chalmers, M.; Slater, H.; Smith, A. Rethinking private speech in preschoolers: The effects of social presence. Dev. Psychol. 2014, 50, 829–836. [Google Scholar] [CrossRef]
- Lidstone, J.; Meins, E.; Fernyhough, C. The roles of private speech and inner speech in planning during middle childhood: Evidence from a dual task paradigm. J. Exp. Child Psychol. 2010, 107, 438–451. [Google Scholar] [CrossRef]
- Lidstone, J.; Meins, E.; Fernyhough, C. Individual differences in children’s private speech: Consistency across tasks, Timepoints, and contexts. Cogn. Dev. 2011, 26, 203–213. [Google Scholar] [CrossRef]
- Day, K.L.; Smith, C.L. Understanding the role of private speech in children’s emotion regulation. Early Child. Res. Q. 2013, 28, 405–414. [Google Scholar] [CrossRef]
- Piaget, J. The Language and Thought of the Child; Harcourt, Brace & Company: New York, NY, USA, 1926. [Google Scholar]
- Vygotsky, L.S. Thought and Language; MIT Press: Cambridge, MA, USA, 1986. [Google Scholar]
- De Guerrero, M.C.M. Going Covert: Inner and private speech in language learning. Lang. Teach. 2018, 51, 1–35. [Google Scholar] [CrossRef]
- McCafferty, S.G. The use of private speech by adult ESL learners at different levels of proficiency. In Vygotskian Approaches to Second Language Research; Lantolf, J.P., Appel, G., Eds.; Ablex: Norwood, NJ, USA, 1994; pp. 117–134. [Google Scholar]
- Ohta, A.S. Second Language Acquisition Processes in the Classroom: Learning Japanese; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2001. [Google Scholar]
- Yoshida, R. Learners in Japanese Language Classrooms: Overt and Covert Participation; Continuum International Pub. Group: London, UK, 2009. [Google Scholar]
- Duncan, R.M.; Cheyne, J.A. Incidence and functions of self-reported private speech in young adults: A self-verbalization questionnaire. Can. J. Behav. Sci. Rev. Can. Sci. Comport. 1999, 31, 133–136. [Google Scholar] [CrossRef]
- Lee, J. Gesture and private speech in second language acquisition. Stud. Sec. Lang. Acq. 2008, 30, 169–190. [Google Scholar] [CrossRef]
- Lupyan, G.; Swingley, D. Self-directed speech affects visual search performance. Q. J. Exp. Psychol. 2012, 65, 1068–1085. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, M.; Farrokh, P. Investigating Iranian English learners’ private speech across proficiency levels and gender based on Vygotsky’s sociocultural theory. J. Psycholinguist. Res. 2022, 51, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Appel, G.; Lantolf, J.P. Speaking as mediation: A study of L1 and L2 text recall tasks. Mod. Lang. J. 1994, 78, 437–452. [Google Scholar] [CrossRef]
- Centeno-Cortés, B.; Jiménez Jiménez, A.F. Problem-solving tasks in a foreign language: The importance of the L1 in private verbal thinking. Int. J. Appl. Linguist. 2004, 14, 7–35. [Google Scholar] [CrossRef]
- De Guerrero, M.C.M. Form and functions of inner speech in adult second language learning. In Vygotskian Approaches to Second Language Research; Lantolf, J.P., Appel, G., Eds.; Ablex Press: Norwood, NJ, USA, 1994; pp. 83–115. [Google Scholar]
- De Guerrero, M.C.M. Inner speech as mental rehearsal: The case of advanced L2 learners. Issues Appl. Linguist. 1999, 10, 27–55. [Google Scholar] [CrossRef]
- McGuire, P.K.; Silbersweig, D.A.; Murray, R.M.; David, A.S.; Frackowiak, R.S.J.; Frith, C.D. Functional anatomy of inner speech and auditory verbal imagery. Psychol. Med. 1996, 26, 29–38. [Google Scholar] [CrossRef]
- Price, C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 2012, 62, 816–847. [Google Scholar] [CrossRef]
- Ackermann, H.; Riecker, A. The contribution of the insula to motor aspects of speech production: A review and a hypothesis. Brain Lang. 2004, 89, 320–328. [Google Scholar] [CrossRef]
- Huang, J.; Carr, T.H.; Cao, Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Hum. Brain Mapp. 2002, 15, 39–53. [Google Scholar] [CrossRef]
- Bookheimer, S.Y.; Zeffiro, T.A.; Blaxton, T.; Gaillard, W.; Theodore, W. Regional cerebral blood flow during object naming and word reading. Hum. Brain Mapp. 1995, 3, 93–106. [Google Scholar] [CrossRef]
- Shergill, S.S.; Brammer, M.J.; Williams, S.C.R.; Murray, R.M.; McGuire, P.K. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch. Gen. Psychiatry 2000, 57, 1033. [Google Scholar] [CrossRef] [PubMed]
- Frawley, W.; Lantolf, J.P. Second language discourse: A Vygotskyan perspective. Appl. Lingusit. 1985, 6, 19–44. [Google Scholar] [CrossRef]
- Lantolf, J.P.; Frawley, W. Second language performance and Vygotskyan psycholinguistics: Implications for L2 instruction. In The Tenth LACUS Forum 1983; Manning, A., Martin, P., McCalla, K., Eds.; Hornbeam Press: Columbia, SC, USA, 1984; pp. 425–440. [Google Scholar]
- McCafferty, S.G. The use of private speech by adult second language learners: A cross-cultural study. Mod. Lang. J. 1992, 76, 179–189. [Google Scholar]
- Xu, J.; Fu, H. Thirty years of research on international L2-private speech. Foreign Lang. Res. 2019, 49–54. [Google Scholar] [CrossRef]
- Notice of the General Office of the Ministry of Education on the Issuance of the “China’s English Proficiency Level Scale”. Available online: http://www.moe.gov.cn/srcsite/A19/s229/201804/t20180416_333315.html (accessed on 25 March 2022).
- Garbaj, M.M. Thinking through the non-native language: The role of private speech in mediating cognitive functioning in problem solving among proficient non-native speakers. Lang. Sociocult. Theory 2018, 5, 108–129. [Google Scholar] [CrossRef]
- Diaz, R.M. Methodological concerns in the study of private speech. In Private Speech: From Social Interaction to Self-Regulation; Diaz, R.M., Berk, L.E., Eds.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1992; pp. 55–81. [Google Scholar]
- Feigenbaum, P. Development of the syntactic and discourse structures of private speech. In Private Speech: From Social Interaction to Self-Regulation; Diaz, R.M., Berk, L.E., Eds.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1992; pp. 181–198. [Google Scholar]
- Norris, J.M.; Ortega, L. Towards an organic approach to investigating CAF in instructed SLA: The case of complexity. Appl. Lingusit. 2009, 30, 555–578. [Google Scholar] [CrossRef]
- Foster, P. Measuring spoken language: A unit for all reasons. Appl. Lingusit. 2000, 21, 354–375. [Google Scholar] [CrossRef]
- Li, J.; Zou, W.; Peng, Y. A study of the effects of task sequence on learners’ oral output. Foreign Lang. China 2022, 19, 62–68. [Google Scholar]
- Lu, X. Automatic analysis of syntactic complexity in second language writing. Int. J. Corpus Linguist. 2010, 15, 474–496. [Google Scholar] [CrossRef]
- Lu, X. A corpus-based evaluation of syntactic complexity measures as indices of college-level ESL writers’ language development. TESOL Q. 2011, 45, 36–62. [Google Scholar] [CrossRef]
- Ai, H.; Lu, X. A corpus-based comparison of syntactic complexity in NNS and NS university students’ writing. In Studies in Corpus Linguistics; Díaz-Negrillo, A., Ballier, N., Thompson, P., Eds.; John Benjamins Publishing Company: Amsterdam, The Netherlands, 2013; Volume 59, pp. 249–264. [Google Scholar]
- Lu, X.; Ai, H. Syntactic complexity in college-level English writing: Differences among writers with diverse L1 backgrounds. J. Second. Lang. Writ. 2015, 29, 16–27. [Google Scholar] [CrossRef]
- Bulté, B.; Roothooft, H. Investigating the interrelationship between rated L2 proficiency and linguistic complexity in L2 speech. System 2020, 91, 102246. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y. The complexity of Chinese oral speeches by Korean native speakers. Appl. Linguist. 2016, 61–70. [Google Scholar] [CrossRef]
- Skehan, P. Limited attentional capacity, second language performance, and task-based pedagogy. In Processing Perspectives on Task Performance; Skehan, P., Ed.; John Benjamins Publishing Company: Amsterdam, The Netherlands, 2014; Volume 5, pp. 211–260. [Google Scholar]
- Hao, Y.; Xu, X.; Wang, X.; Lin, Y.; Liu, H. Typological characteristics of interlanguage: Across L2 modalities and proficiency levels. Front. Psychol. 2023, 13, 1071906. [Google Scholar] [CrossRef]
- Coyle, S.M.; Ward, T.E.; Markham, C.M. Brain–computer interface using a simplified functional near-infrared spectroscopy system. J. Neural Eng. 2007, 4, 219–226. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, X.; Zhang, Z.; Zhao, Y.; Jiang, Y.; Li, Z.; Yang, Y.; Zhu, C. Transcranial brain atlas. Sci. Adv. 2018, 4, eaar6904. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, T.; Zhai, Y.; Long, Y.; Wang, Z.; Lu, C. How mother–child interactions are associated with a child’s compliance. Cereb. Cortex 2021, 31, 4398–4410. [Google Scholar] [CrossRef]
- Fishburn, F.A.; Ludlum, R.S.; Vaidya, C.J.; Medvedev, A.V. Temporal Derivative Distribution Repair (TDDR): A motion correction method for fNIRS. Neuroimage 2019, 184, 171–179. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, M.; Zhang, Z.; Zhou, H.; Zhao, Y.; Duan, L.; Kreplin, U.; Xiao, X.; Zhu, C. Test–retest reliability of the prefrontal response to affective pictures based on functional near-infrared spectroscopy. J. Biomed. Opt. 2017, 22, 016011. [Google Scholar] [CrossRef]
- Chang, A.C.-S.; Millett, S. Improving reading rates and comprehension through audio-assisted extensive reading for beginner learners. System 2015, 52, 91–102. [Google Scholar] [CrossRef]
- Johnsrude, I.S.; Zatorre, R.J.; Milner, B.A.; Evans, A.C. Left-hemisphere specialization for the processing of acoustic transients. Neuroreport 1997, 8, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Holler, J.; Kokal, I.; Toni, I.; Hagoort, P.; Kelly, S.D.; Özyürek, A. Eye’m talking to you: Speakers’ gaze direction modulates co-speech gesture processing in the right MTG. Soc. Cogn. Affect. Neurosci. 2015, 10, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Fan, L.; Li, H.; Zhang, W.; Hu, Q.; Jiang, T. Tractography-based parcellation of the human middle temporal gyrus. Sci. Rep. 2015, 5, 18883. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Du, Y. TMS reveals dynamic interaction between inferior frontal gyrus and posterior middle temporal gyrus in gesture-speech semantic integration. J. Neurosci. 2021, 41, 10356–10364. [Google Scholar] [CrossRef]
- Oberhuber, M.; Hope, T.M.H.; Seghier, M.L.; Parker Jones, O.; Prejawa, S.; Green, D.W.; Price, C.J. Four functionally distinct regions in the left supramarginal gyrus support word processing. Cereb. Cortex 2016, 26, 4212–4226. [Google Scholar] [CrossRef]
- Yue, Q.; Martin, R.C. Phonological working memory representations in the left inferior parietal lobe in the face of distraction and neural stimulation. Front. Hum. Neurosci. 2022, 16, 890483. [Google Scholar] [CrossRef]
- Church, J.A.; Balota, D.A.; Petersen, S.E.; Schlaggar, B.L. Manipulation of length and lexicality localizes the functional neuroanatomy of phonological processing in adult readers. J. Cogn. Neurosci. 2011, 23, 1475–1493. [Google Scholar] [CrossRef]
- Li, H.; Booth, J.R.; Bélanger, N.N.; Feng, X.; Tian, M.; Xie, W.; Zhang, M.; Gao, Y.; Ang, C.; Yang, X.; et al. Structural correlates of literacy difficulties in the second language: Evidence from Mandarin-speaking children learning English. Neuroimage 2018, 179, 288–297. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, J.X.; Wang, X.; Wu, R.; Hu, X.; Weng, X.; Tan, L.H. Differential activity in left inferior frontal gyrus for pseudowords and real words: An event-related fMRI study on auditory lexical decision. Hum. Brain Mapp. 2005, 25, 212–221. [Google Scholar] [CrossRef]
- Golfinopoulos, E.; Tourville, J.A.; Bohland, J.W.; Ghosh, S.S.; Nieto-Castanon, A.; Guenther, F.H. fMRI investigation of unexpected somatosensory feedback perturbation during speech. Neuroimage 2011, 55, 1324–1338. [Google Scholar] [CrossRef]
- Basho, S.; Palmer, E.D.; Rubio, M.A.; Wulfeck, B.; Müller, R.-A. Effects of generation mode in fMRI adaptations of semantic fluency: Paced production and overt speech. Neuropsychologia 2007, 45, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Neef, N.E.; Hoang, T.N.L.; Neef, A.; Paulus, W.; Sommer, M. Speech dynamics are coded in the left motor cortex in fluent speakers but not in adults who stutter. Brain 2015, 138, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, L.; Zou, L.; Yan, X.; Zhang, J.; Yang, M.; Ding, G. “Antagonistic” cooperation of control regions in bilingual language production: An effective connectivity study. Neuropsychologia 2022, 167, 108165. [Google Scholar] [CrossRef]
- Chang, Y.; Peng, D.; Zhao, Y.; Chen, X.; Li, J.; Wu, X.; Liu, P.; Liu, H. Transcranial direct current stimulation over left dorsolateral prefrontal cortex facilitates auditory-motor integration for vocal pitch regulation. Front. Neurosci. 2023, 17, 1208581. [Google Scholar] [CrossRef]
- Liu, D.; Dai, G.; Liu, C.; Guo, Z.; Xu, Z.; Jones, J.A.; Liu, P.; Liu, H. Top–down inhibitory mechanisms underlying auditory–motor integration for voice control: Evidence by TMS. Cereb. Cortex 2020, 30, 4515–4527. [Google Scholar] [CrossRef]
- Hertrich, I.; Dietrich, S.; Blum, C.; Ackermann, H. The role of the dorsolateral prefrontal cortex for speech and language processing. Front. Hum. Neurosci. 2021, 15, 645209. [Google Scholar] [CrossRef]
- Komeilipoor, N.; Cesari, P.; Daffertshofer, A. Involvement of superior temporal areas in audiovisual and audiomotor speech integration. Neuroscience 2017, 343, 276–283. [Google Scholar] [CrossRef]
- Nagle, S.; Musiek, F.E.; Kossoff, E.H.; Jallo, G.; Boatman-Reich, D. Auditory processing following consecutive right temporal lobe resections: A prospective case study. J. Am. Acad. Audiol. 2013, 24, 535–543. [Google Scholar] [CrossRef]
- McGettigan, C.; Tremblay, P. Links between perception and production: Examining the roles of motor and premotor cortices in understanding speech. In The Oxford Handbook of Psycholinguistics; Rueschemeyer, S.-A., Gaskell, M.G., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 306–332. [Google Scholar]
- Jiang, R. A study on the cognitive mechanism of Chinese language learners in vocabulary acquisition. Lang. Teach. Linguist. Stud. 2013, 9–15. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFD2013&filename=YYJX201301005 (accessed on 16 December 2024).
- Wagner, J.; Rusconi, E. Causal involvement of the left angular gyrus in higher functions as revealed by transcranial magnetic stimulation: A systematic review. Brain Struct. Funct. 2023, 228, 169–196. [Google Scholar] [CrossRef] [PubMed]
- Beaty, R.E.; Silvia, P.J.; Benedek, M. Brain networks underlying novel metaphor production. Brain Cogn. 2017, 111, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bellana, B.; Ladyka-Wojcik, N.; Lahan, S.; Moscovitch, M.; Grady, C.L. Recollection and prior knowledge recruit the left angular gyrus during recognition. Brain Struct. Funct. 2023, 228, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wu, D.H.; Duann, J.-R. Dynamic brain connectivity attuned to the complexity of relative clause sentences revealed by a single-trial analysis. Neuroimage 2020, 217, 116920. [Google Scholar] [CrossRef]
- Roux, F.-E.; Minkin, K.; Durand, J.-B.; Sacko, O.; Réhault, E.; Tanova, R.; Démonet, J.-F. Electrostimulation mapping of comprehension of auditory and visual words. Cortex 2015, 71, 398–408. [Google Scholar] [CrossRef]
- Barbeau, E.B.; Chai, X.J.; Chen, J.-K.; Soles, J.; Berken, J.; Baum, S.; Watkins, K.E.; Klein, D. The role of the left inferior parietal lobule in second language learning: An intensive language training fMRI study. Neuropsychologia 2017, 98, 169–176. [Google Scholar] [CrossRef]
- Hakonen, M.; May, P.J.C.; Jääskeläinen, I.P.; Jokinen, E.; Sams, M.; Tiitinen, H. Predictive processing increases intelligibility of acoustically distorted speech: Behavioral and neural correlates. Brain Behav. 2017, 7, e00789. [Google Scholar] [CrossRef]
- Hope, T.M.H.; Prejawa, S.; Parker Jones, O.; Oberhuber, M.; Seghier, M.L.; Green, D.W.; Price, C.J. Dissecting the functional anatomy of auditory word repetition. Front. Hum. Neurosci. 2014, 8, 246. [Google Scholar] [CrossRef]
- Uchida-Ota, M.; Arimitsu, T.; Tsuzuki, D.; Dan, I.; Ikeda, K.; Takahashi, T.; Minagawa, Y. Maternal speech shapes the cerebral frontotemporal network in neonates: A hemodynamic functional connectivity study. Dev. Cogn. Neurosci. 2019, 39, 100701. [Google Scholar] [CrossRef]



| Measures | High Proficiency | Low Proficiency |
|---|---|---|
| U | 99.750 (42.278) 1 | 106.500 (33.269) |
| W_PS | 714.250 (371.590) | 671.125 (216.766) |
| U/S | 0.166 (0.070) | 0.178 (0.055) |
| W/U | 6.857 (1.800) | 6.424 (1.341) |
| Measures | Inhibition | Private Speech Users | Non-Users of Private Speech | |||
|---|---|---|---|---|---|---|
| High | Low | High | Low | |||
| Fluency | W_OP | Free | 311.313 (106.71) 1 | 300.000 (99.416) | 287.813 (62.778) | 283.563 (83.814) |
| Inhibited | 282.000 (127.436) | 277.125 (100.601) | 294.313 (72.621) | 289.000 (112.140) | ||
| AS | Free | 22.938 (8.790) | 27.813 (9.354) | 21.938 (5.196) | 23.375 (7.898) | |
| Inhibited | 23.000 (9.913) | 27.000 (8.892) | 24.688 (7.245) | 24.688 (9.700) | ||
| Complexity | CAS | Free | 7.500 (2.733) | 7.250 (5.209) | 7.625 (4.455) | 6.250 (3.088) |
| Inhibited | 6.813 (3.563) | 5.625 (3.810) | 8.125 (3.364) | 7.375 (4.365) | ||
| W/AS | Free | 14.024 (2.982) | 10.874 (1.258) | 13.357 (2.074) | 12.426 (1.930) | |
| Inhibited | 14.021 (7.584) | 10.358 (1.546) | 12.225 (2.255) | 12.108 (3.106) | ||
| Accuracy | EW | Free | 30.188 (19.181) | 29.125 (19.131) | 21.313 (12.700) | 30.250 (10.208) |
| Inhibited | 27.250 (18.237) | 30.000 (23.232) | 20.375 (10.059) | 26.938 (11.066) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.; Xiao, Z.; Jiang, Y.; Jiang, X. The Neural Mechanisms of Private Speech in Second Language Learners’ Oral Production: An fNIRS Study. Brain Sci. 2025, 15, 451. https://doi.org/10.3390/brainsci15050451
Jiang R, Xiao Z, Jiang Y, Jiang X. The Neural Mechanisms of Private Speech in Second Language Learners’ Oral Production: An fNIRS Study. Brain Sciences. 2025; 15(5):451. https://doi.org/10.3390/brainsci15050451
Chicago/Turabian StyleJiang, Rong, Zhe Xiao, Yihan Jiang, and Xueqing Jiang. 2025. "The Neural Mechanisms of Private Speech in Second Language Learners’ Oral Production: An fNIRS Study" Brain Sciences 15, no. 5: 451. https://doi.org/10.3390/brainsci15050451
APA StyleJiang, R., Xiao, Z., Jiang, Y., & Jiang, X. (2025). The Neural Mechanisms of Private Speech in Second Language Learners’ Oral Production: An fNIRS Study. Brain Sciences, 15(5), 451. https://doi.org/10.3390/brainsci15050451





