Sphingosine-1-Phosphate Modulation in Neurological Disorders: Insights from MS and Stroke
Abstract
1. Introduction
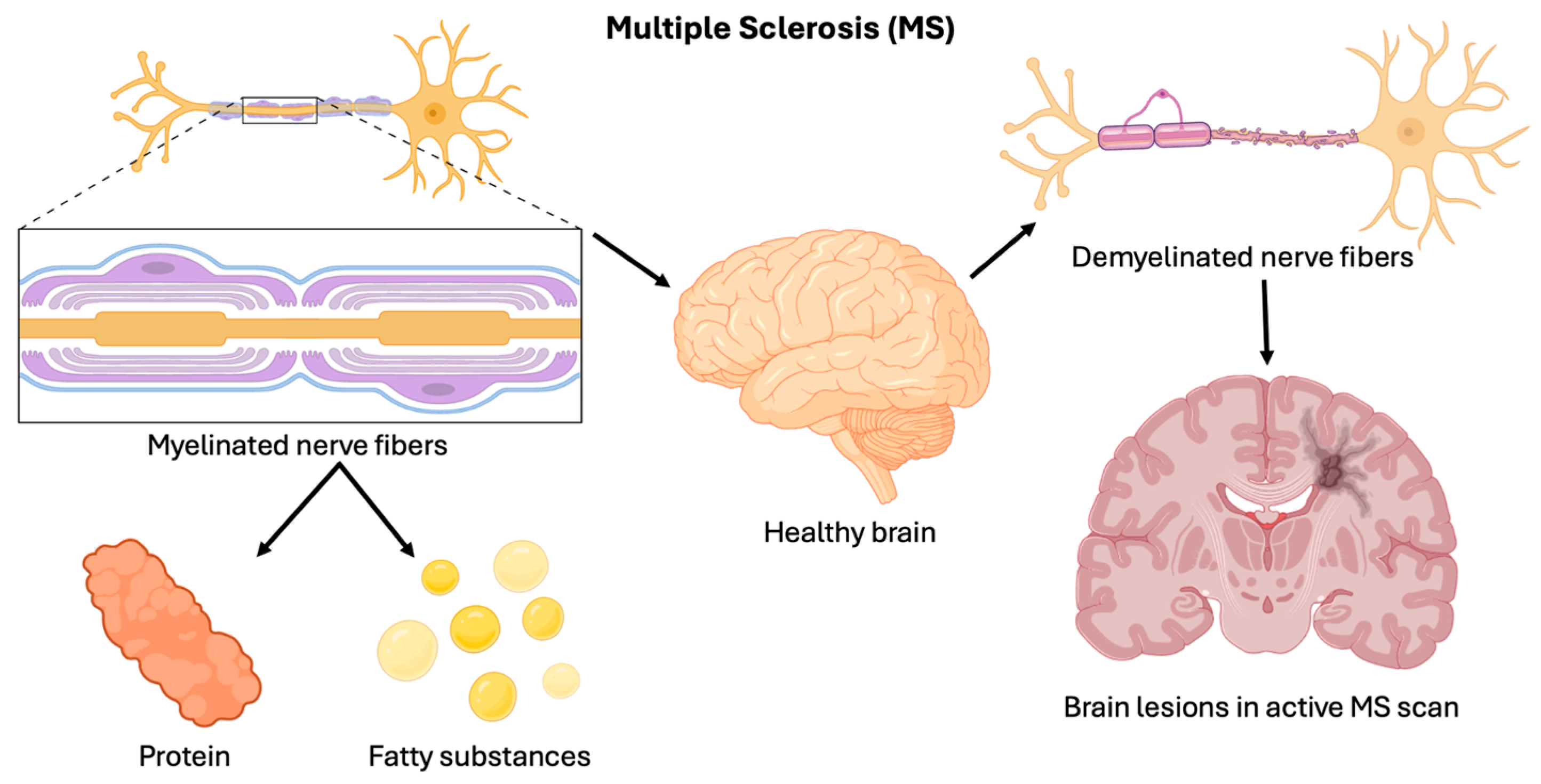
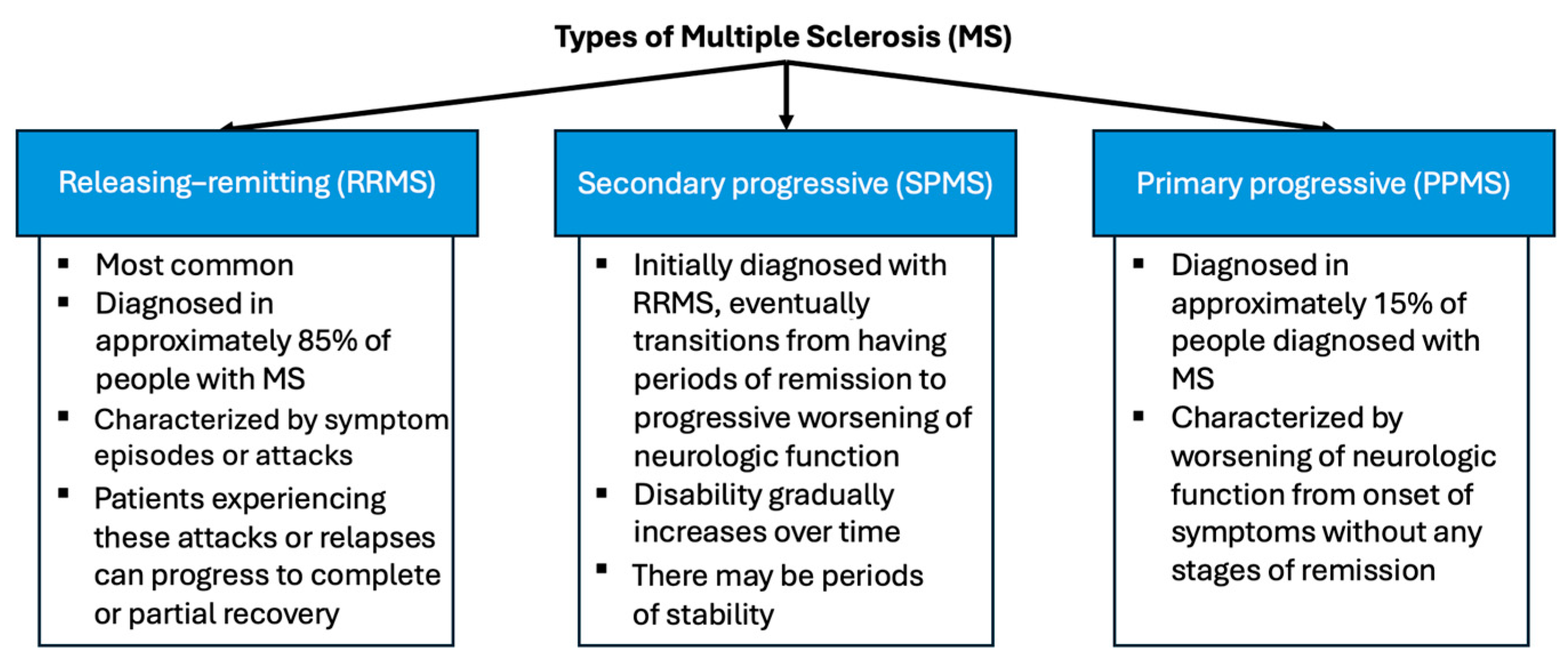
1.1. Multiple Sclerosis
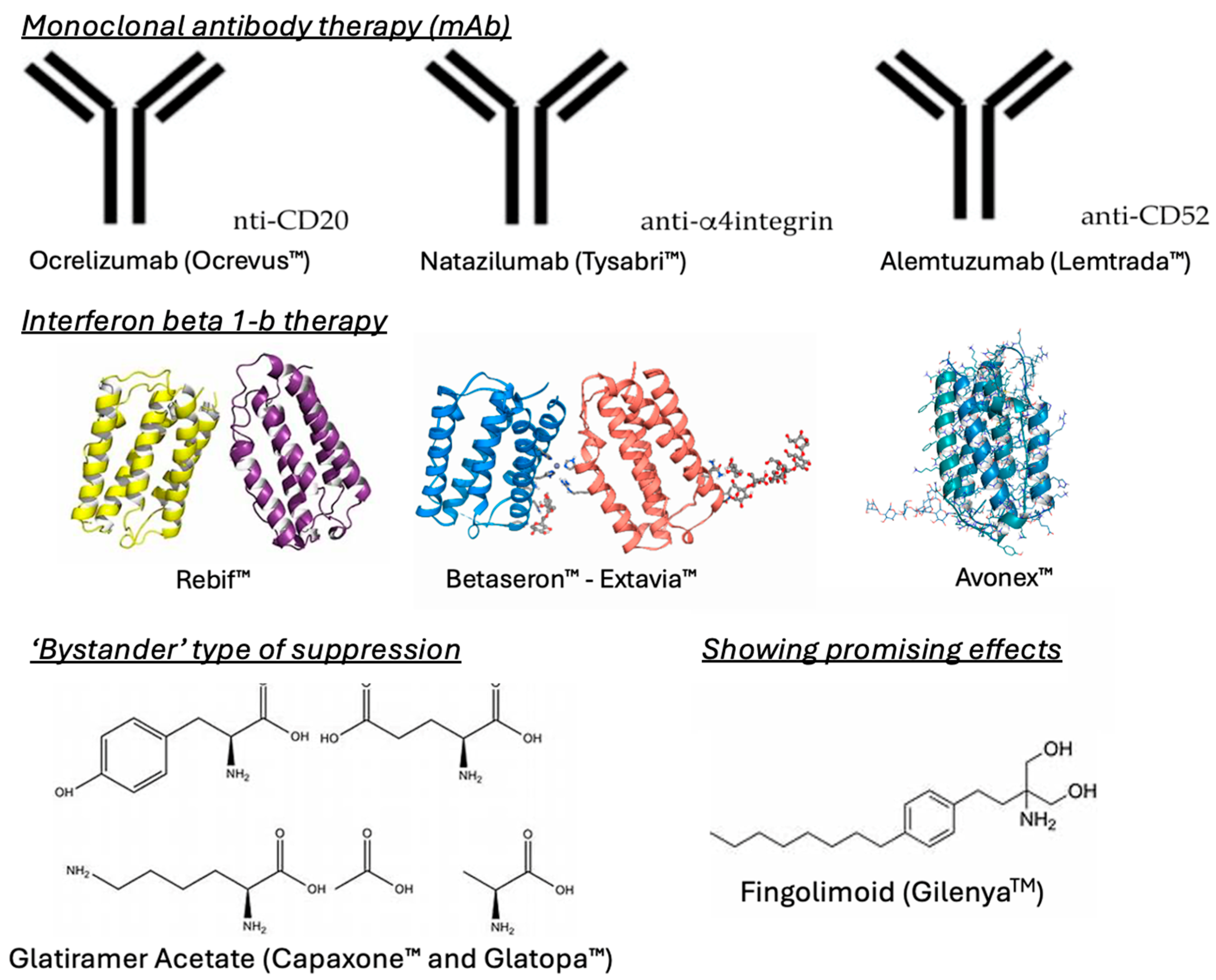
1.2. Current MS Therapies
1.3. Immune System Involvement in MS
- i.
- Adaptive Immune System:
- ii.
- Innate Immune System:
2. Stroke

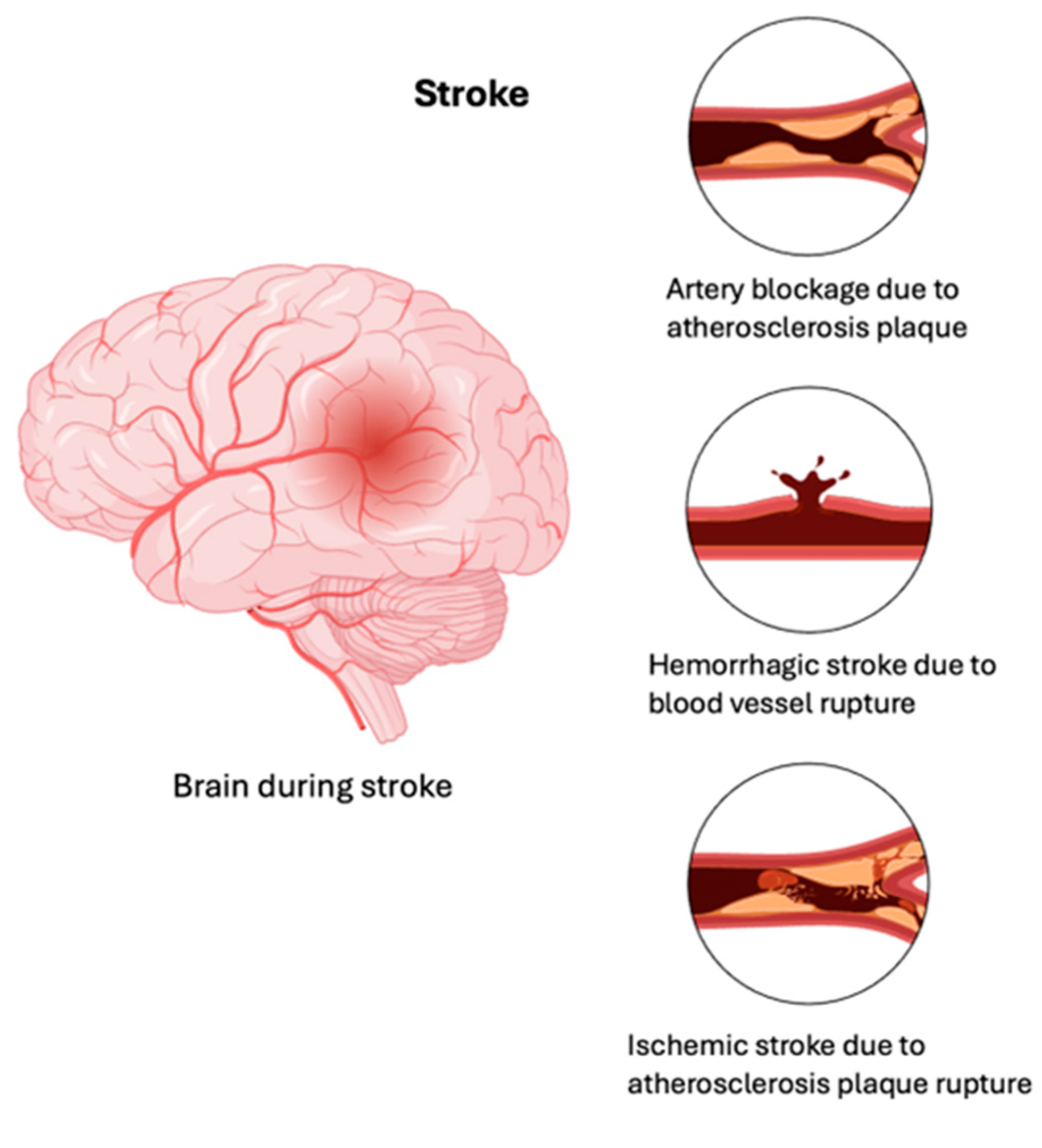
Current Stroke Therapies
3. S1P Receptors as Targets for MS and Stroke
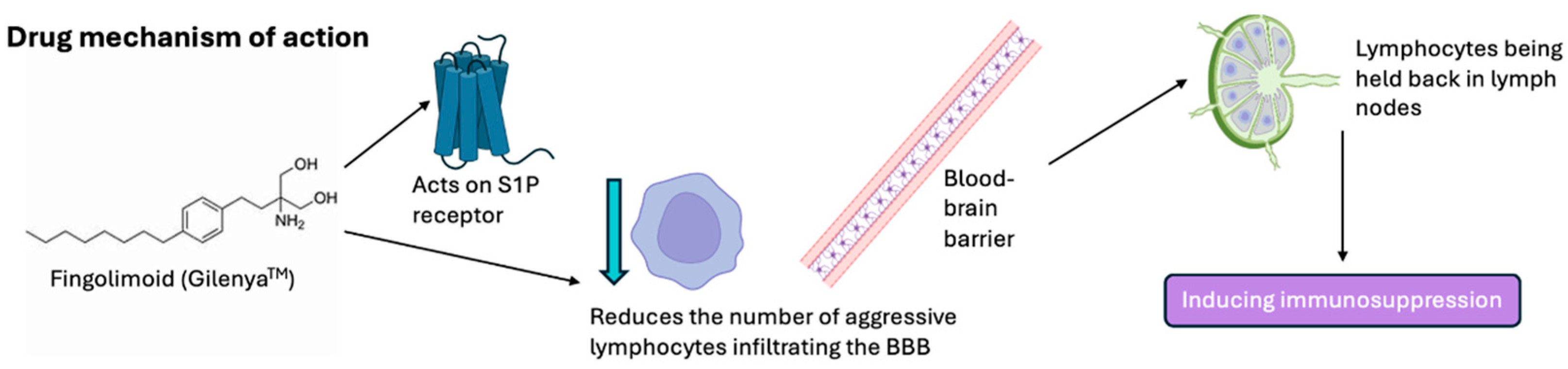
4. Fingolimod Mechanism of Action
5. Ongoing Clinical Trials
6. Limitations
7. Future Prospects and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2002, 359, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Pouly, S.; Antel, J.P. Multiple sclerosis and central nervous system demyelination. J. Autoimmun. 1999, 13, 297–306. [Google Scholar] [CrossRef] [PubMed]
- National Multiple Sclerosis Society [Internet]. What Causes MS? Available online: https://www.nationalmssociety.org/What-is-MS/What-Causes-MS (accessed on 24 September 2023).
- Weiner, H.L. A shift from adaptive to innate immunity: A potential mechanism of disease progression in multiple sclerosis. J. Neurol. 2008, 255 (Suppl. S1), 3–11. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N. The Perspectives of Mesenchymal Stem Cell Therapy in the Treatment of Multiple Sclerosis. Iran. J. Pharm. Res. IJPR 2015, 14, 1–2. [Google Scholar]
- Li, L.; Xie, T. Stem cell niche: Structure and function. Annu. Rev. Cell Dev. Biol. 2005, 21, 605–631. [Google Scholar] [CrossRef]
- Franklin, R.J.M. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 2002, 3, 705–714. [Google Scholar] [CrossRef]
- McTigue, D.M.; Tripathi, R.B. The life, death, and replacement of oligodendrocytes in the adult CNS. J. Neurochem. 2008, 107, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.S.; Blinkenberg, M. The potential role for ocrelizumab in the treatment of multiple sclerosis: Current evidence and future prospects. Ther. Adv. Neurol. Disord. 2016, 9, 44–52. [Google Scholar] [CrossRef]
- Selewski, D.T.; Shah, G.V.; Segal, B.M.; Rajdev, P.A.; Mukherji, S.K. Natalizumab (Tysabri). AJNR Am. J. Neuroradiol. 2010, 31, 1588–1590. [Google Scholar] [CrossRef]
- Ruck, T.; Bittner, S.; Wiendl, H.; Meuth, S.G. Alemtuzumab in Multiple Sclerosis: Mechanism of Action and Beyond. Int. J. Mol. Sci. 2015, 16, 16414–16439. [Google Scholar] [CrossRef]
- Filippi, M.; Rocca, M.A.; Camesasca, F.; Cook, S.; O’Connor, P.; Arnason, B.G.W.; Kappos, L.; Goodin, D.; Jeffery, D.; Hartung, H.-P.; et al. Interferon β-1b and glatiramer acetate effects on permanent black hole evolution. Neurology 2011, 76, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.; Cohen, J.A. Combination therapy in multiple sclerosis. Lancet Neurol. 2010, 9, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Hartung, H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef]
- Volpi, C.; Orabona, C.; Macchiarulo, A.; Bianchi, R.; Puccetti, P.; Grohmann, U. Preclinical discovery and development of fingolimod for the treatment of multiple sclerosis. Expert. Opin. Drug Discov. 2019, 14, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Li, X.K.; Enosawa, S.; Shinomiya, T. A new immunosuppressant, FTY720, induces bcl-2-associated apoptotic cell death in human lymphocytes. Immunology 1996, 89, 518–523. [Google Scholar] [CrossRef]
- Chiba, K.; Hoshino, Y.; Suzuki, C.; Masubuchi, Y.; Yanagawa, Y.; Ohtsuki, M.; Sasaki, S.; Fujita, T. FTY720, a novel immunosuppressant possessing unique mechanisms. I. Prolongation of skin allograft survival and synergistic effect in combination with cyclosporine in rats. Transplant. Proc. 1996, 28, 1056–1059. [Google Scholar]
- Gilenya.pdf [Internet]. Available online: https://www.novartis.com/us-en/sites/novartis_us/files/gilenya.pdf (accessed on 4 February 2024).
- Cohen, J.A.; Chun, J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann. Neurol. 2011, 69, 759–777. [Google Scholar] [CrossRef]
- San-Juan-Rodriguez, A.; Good, C.B.; Heyman, R.A.; Parekh, N.; Shrank, W.H.; Hernandez, I. Trends in Prices, Market Share, and Spending on Self-administered Disease-Modifying Therapies for Multiple Sclerosis in Medicare Part D. JAMA Neurol. 2019, 76, 1386–1390. [Google Scholar] [CrossRef]
- Murúa, S.R.; Farez, M.F.; Quintana, F.J. The Immune Response in Multiple Sclerosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 121–139. [Google Scholar] [CrossRef]
- Haase, S.; Linker, R.A. Inflammation in multiple sclerosis. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211007687. [Google Scholar] [CrossRef]
- Conti, P.; Kempuraj, D. Important role of mast cells in multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 5, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Jian, C.; Liao, Y.; Huang, Q.; Wu, Y.; Liu, X.; Zou, D.; Wu, Y. The role of microglia in multiple sclerosis. Neuropsychiatr. Dis. Treat. 2017, 13, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Alari-Pahissa, E.; Munteis, E.; Vera, A.; Zabalza, A.; Llop, M.; Villarrubia, N.; Costa-García, M.; Álvarez-Lafuente, R.; Villar, L.M.; et al. Adaptive Features of Natural Killer Cells in Multiple Sclerosis. Front. Immunol. 2019, 10, 2403. [Google Scholar] [CrossRef]
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef]
- Dong, Y.F.; Guo, R.B.; Ji, J.; Cao, L.L.; Zhang, L.; Chen, Z.Z.; Huang, J.Y.; Wu, J.; Lu, J.; Sun, X.L. S1PR3 is essential for phosphorylated fingolimod to protect astrocytes against oxygen-glucose deprivation-induced neuroinflammation via inhibiting TLR2/4-NFκB signalling. J. Cell Mol. Med. 2018, 22, 3159–3166. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S.V. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef] [PubMed]
- Primary Stroke Prevention Worldwide: Translating Evidence Into Action—PubMed [Internet]. Available online: https://pubmed.ncbi.nlm.nih.gov/34756176/ (accessed on 3 June 2024).
- Yousufuddin, M.; Young, N. Aging and ischemic stroke. Aging 2019, 11, 2542–2544. [Google Scholar] [CrossRef]
- George, M.G. Risk Factors for Ischemic Stroke in Younger Adults: A Focused Update. Stroke 2020, 51, 729–735. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Khatri, P. Stroke. The Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Magid-Bernstein, J.; Girard, R.; Polster, S.; Srinath, A.; Romanos, S.; Awad, I.A.; Sansing, L.H. Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circ. Res. 2022, 130, 1204–1229. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Owolabi, M.O.; World Stroke Organization–Lancet Neurology Commission Stroke Collaboration Group. Pragmatic solutions to reduce the global burden of stroke: A World Stroke Organization-Lancet Neurology Commission. Lancet Neurol. 2023, 22, 1160–1206. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Thundyil, J.; Tang, S.C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Ischaemic Stroke | Stroke Association [Internet]. Available online: https://www.stroke.org.uk/stroke/types/ischaemic (accessed on 3 June 2024).
- Unnithan, A.K.A.; Das, J.M.; Mehta, P. Hemorrhagic Stroke. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK559173/ (accessed on 3 June 2024).
- Perry, J.J.; Yadav, K.; Syed, S.; Shamy, M. Transient ischemic attack and minor stroke: Diagnosis, risk stratification and management. CMAJ Can. Med. Assoc. J. 2022, 194, E1344–E1349. [Google Scholar] [CrossRef]
- Amin, H.P.; Madsen, T.E.; Bravata, D.M.; Wira, C.R.; Johnston, S.C.; Ashcraft, S.; Burrus, T.M.; Panagos, P.D.; Wintermark, M.; Esenwa, C.; et al. Diagnosis, Workup, Risk Reduction of Transient Ischemic Attack in the Emergency Department Setting: A Scientific Statement From the American Heart Association. Stroke 2023, 54, e109–e121. [Google Scholar] [CrossRef] [PubMed]
- Mracsko, E.; Veltkamp, R. Neuroinflammation after intracerebral hemorrhage. Front. Cell. Neurosci. 2014, 8, 388. [Google Scholar] [CrossRef]
- Jurcau, A.; Ardelean, A.I. Oxidative Stress in Ischemia/Reperfusion Injuries following Acute Ischemic Stroke. Biomedicines 2022, 10, 574. [Google Scholar] [CrossRef]
- Hui, C.; Tadi, P.; Khan Suheb, M.Z.; Patti, L. Ischemic Stroke. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK499997/ (accessed on 3 June 2024).
- Li, Y.J.; Shi, S.X.; Liu, Q.; Shi, F.D.; Gonzales, R.J. Targeted role for sphingosine-1-phosphate receptor 1 in cerebrovascular integrity and inflammation during acute ischemic stroke. Neurosci. Lett. 2020, 735, 135160. [Google Scholar] [CrossRef]
- Hla, T.; Venkataraman, K.; Michaud, J. The vascular S1P gradient—Cellular sources and biological significance. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2008, 1781, 477–482. [Google Scholar] [CrossRef]
- Li, W.; Xu, H.; Testai, F.D. Mechanism of Action and Clinical Potential of Fingolimod for the Treatment of Stroke. Front. Neurol. 2016, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Hla, T.; Maciag, T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J. Biol. Chem. 1990, 265, 9308–9313. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.M.; Okada, T.; Matloubian, M.; Lo, C.G.; Cyster, J.G. S1P1 Receptor Signaling Overrides Retention Mediated by Gαi-Coupled Receptors to Promote T Cell Egress. Immunity 2008, 28, 122–133. [Google Scholar] [CrossRef]
- Xiang, P.; Chew, W.S.; Seow, W.L.; Lam, B.W.S.; Ong, W.Y.; Herr, D.R. The S1P2 receptor regulates blood-brain barrier integrity and leukocyte extravasation with implications for neurodegenerative disease. Neurochem. Int. 2021, 146, 105018. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, G.; Dejana, E. Endothelial Cell-to-Cell Junctions: Molecular Organization and Role in Vascular Homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef]
- Cruz-Orengo, L.; Daniels, B.P.; Dorsey, D.; Basak, S.A.; Grajales-Reyes, J.G.; McCandless, E.E.; Piccio, L.; Schmidt, R.E.; Cross, A.H.; Crosby, S.D.; et al. Enhanced sphingosine-1-phosphate receptor 2 expression underlies female CNS autoimmunity susceptibility. J. Clin. Investig. 2014, 124. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Bui, Q.; Badeanlou, L.; Hester, K.; Chun, J.; Ruf, W.; Ciaraldi, T.P.; Samad, F. S1P/S1PR3 signalling axis protects against obesity-induced metabolic dysfunction. Adipocyte 2022, 11, 69–83. [Google Scholar] [CrossRef]
- Bravo, G.Á.; Cedeño, R.R.; Casadevall, M.P.; Ramió-Torrentà, L. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives. Cells 2022, 11, 2058. [Google Scholar] [CrossRef]
- Chatzikonstantinou, S.; Poulidou, V.; Arnaoutoglou, M.; Kazis, D.; Heliopoulos, I.; Grigoriadis, N.; Boziki, M. Signaling through the S1P?S1PR Axis in the Gut, the Immune and the Central Nervous System in Multiple Sclerosis: Implication for Pathogenesis and Treatment. Cells 2021, 10, 3217. [Google Scholar] [CrossRef]
- Jaillard, C.; Harrison, S.; Stankoff, B.; Aigrot, M.S.; Calver, A.R.; Duddy, G.; Walsh, F.S.; Pangalos, M.N.; Arimura, N.; Kaibuchi, K.; et al. Edg8/S1P5: An oligodendroglial receptor with dual function on process retraction and cell survival. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 1459–1469. [Google Scholar] [CrossRef]
- Quarta, S.; Camprubí-Robles, M.; Schweigreiter, R.; Matusica, D.; Haberberger, R.V.; Proia, R.L.; Bandtlow, C.E.; Ferrer-Montiel, A.; Kress, M. Sphingosine-1-Phosphate and the S1P3 Receptor Initiate Neuronal Retraction via RhoA/ROCK Associated with CRMP2 Phosphorylation. Front. Mol. Neurosci. 2017, 10, 317. [Google Scholar] [CrossRef]
- Noguchi, K.; Chun, J. Roles for lysophospholipid S1P receptors in multiple sclerosis. Crit. Rev. Biochem. Mol. Biol. 2011, 46, 2–10. [Google Scholar] [CrossRef]
- Stepanovska, B.; Huwiler, A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol. Res. 2020, 154, 104170. [Google Scholar] [CrossRef]
- Foster, C.A.; Howard, L.M.; Schweitzer, A.; Persohn, E.; Hiestand, P.C.; Balatoni, B.; Reuschel, R.; Beerli, C.; Schwartz, M.; Billich, A. Brain Penetration of the Oral Immunomodulatory Drug FTY720 and Its Phosphorylation in the Central Nervous System during Experimental Autoimmune Encephalomyelitis: Consequences for Mode of Action in Multiple Sclerosis. J. Pharmacol. Exp. Ther. 2007, 323, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.E.; Schubart, A.; Antel, J.P. Central nervous system-directed effects of FTY720 (fingolimod). J. Neurol. Sci. 2008, 274, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kawabori, M.; Houkin, K. FTY720 (Fingolimod) Ameliorates Brain Injury through Multiple Mechanisms and is a Strong Candidate for Stroke Treatment. Curr. Med. Chem. 2020, 27, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Zhu, R.; Wang, P.; Jiang, F.; Zhen, J.; Yao, Y.; Zhao, C.; Liang, Z.; Wang, M.; Liu, B.; et al. The efficacy and safety of fingolimod plus standardized treatment versus standardized treatment alone for acute ischemic stroke: A systematic review and meta-analysis. Pharmacol. Res. Perspect. 2022, 10, e00972. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, N.; Ren, L.; Yan, Y.; Sun, N.; Li, Y.J.; Han, W.; Xue, R.; Liu, Q.; Hao, J.; et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc. Natl. Acad. Sci. USA 2014, 111, 18315–18320. [Google Scholar] [CrossRef]
- Guarnera, C.; Bramanti, P.; Mazzon, E. Alemtuzumab: A review of efficacy and risks in the treatment of relapsing remitting multiple sclerosis. Ther. Clin. Risk Manag. 2017, 13, 871–879. [Google Scholar] [CrossRef]
- Wei, Y.; Yemisci, M.; Kim, H.H.; Yung, L.M.; Shin, H.K.; Hwang, S.K.; Guo, S.; Qin, T.; Alsharif, N.; Brinkmann, V.; et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann. Neurol. 2011, 69, 119–129. [Google Scholar] [CrossRef]
- Naseh, M.; Vatanparast, J.; Rafati, A.; Bayat, M.; Haghani, M. The emerging role of FTY720 as a sphingosine 1-phosphate analog for the treatment of ischemic stroke: The cellular and molecular mechanisms. Brain Behav. 2021, 11, e02179. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Obermeier, B.; Cotleur, A.; Love, A.; Takeshita, Y.; Sano, Y.; Kanda, T.; Ransohoff, R.M. Sphingosine 1 Phosphate at the Blood Brain Barrier: Can the Modulation of S1P Receptor 1 Influence the Response of Endothelial Cells and Astrocytes to Inflammatory Stimuli? PLoS ONE 2015, 10, e0133392. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Secchiero, P.; Rimondi, E.; Gianesini, S.; Menegatti, E.; Casciano, F.; Zamboni, P.; Zauli, G. GM-CSF Exhibits Anti-Inflammatory Activity on Endothelial Cells Derived from Chronic Venous Disease Patients. Mediators Inflamm. 2013, 2013, 561689. [Google Scholar] [CrossRef][Green Version]
- Kraft, P.; Göb, E.; Schuhmann, M.K.; Göbel, K.; Deppermann, C.; Thielmann, I.; Herrmann, A.M.; Lorenz, K.; Brede, M.; Stoll, G.; et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke 2013, 44, 3202–3210. [Google Scholar] [CrossRef]
- Sanchez, T.; Estrada-Hernandez, T.; Paik, J.H.; Wu, M.T.; Venkataraman, K.; Brinkmann, V.; Claffey, K.; Hla, T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 2003, 278, 47281–47290. [Google Scholar] [CrossRef]
- Safarian, F.; Khallaghi, B.; Ahmadiani, A.; Dargahi, L. Activation of S1P1 receptor regulates PI3K/Akt/FoxO3a pathway in response to oxidative stress in PC12 cells. J. Mol. Neurosci. 2015, 56, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Tao, W.; Liu, M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. Int. J. Neurosci. 2013, 123, 163–169. [Google Scholar] [CrossRef]
- Malone, K.; Shearer, J.A.; Waeber, C.; Moore, A.C. The impact of fingolimod on Treg function in brain ischaemia. Eur. J. Immunol. 2023, 53, e2350370. [Google Scholar] [CrossRef]
- Liantao, Z.; Jing, Z.; Lingling, L.; Hua, L. Efficacy of fingolimod combined with alteplase in acute ischemic stroke and rehabilitation nursing. Pak. J. Pharm. Sci. 2019, 32, 413–419. [Google Scholar]
- Salas-Perdomo, A.; Miró-Mur, F.; Gallizioli, M.; Brait, V.H.; Justicia, C.; Meissner, A.; Urra, X.; Chamorro, A.; Planas, A.M. Role of the S1P pathway and inhibition by fingolimod in preventing hemorrhagic transformation after stroke. Sci. Rep. 2019, 9, 8309. [Google Scholar] [CrossRef]
- Tian, D.C.; Shi, K.; Zhu, Z.; Yao, J.; Yang, X.; Su, L.; Zhang, S.; Zhang, M.; Gonzales, R.J.; Liu, Q.; et al. Fingolimod enhances the efficacy of delayed alteplase administration in acute ischemic stroke by promoting anterograde reperfusion and retrograde collateral flow. Ann. Neurol. 2018, 84, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hao, J.; Zhang, N.; Ren, L.; Sun, N.; Li, Y.J.; Yan, Y.; Huang, D.; Yu, C.; Shi, F.D. Fingolimod for the treatment of intracerebral hemorrhage: A 2-arm proof-of-concept study. JAMA Neurol. 2014, 71, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Diaz Diaz, A.C.; Shearer, J.A.; Malone, K.; Waeber, C. Acute Treatment With Fingolimod Does Not Confer Long-Term Benefit in a Mouse Model of Intracerebral Haemorrhage. Front Pharmacol. 2020, 11, 613103. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maktabi, B.; Shehjar, F.; Senger, Z.; Kountz, L.; Hasan, S.; Maaieh, K.; Hoersten, K.; Duric, J.; Shah, Z.A. Sphingosine-1-Phosphate Modulation in Neurological Disorders: Insights from MS and Stroke. Brain Sci. 2025, 15, 436. https://doi.org/10.3390/brainsci15050436
Maktabi B, Shehjar F, Senger Z, Kountz L, Hasan S, Maaieh K, Hoersten K, Duric J, Shah ZA. Sphingosine-1-Phosphate Modulation in Neurological Disorders: Insights from MS and Stroke. Brain Sciences. 2025; 15(5):436. https://doi.org/10.3390/brainsci15050436
Chicago/Turabian StyleMaktabi, Briana, Faheem Shehjar, Zachary Senger, Logan Kountz, Syed Hasan, Kenan Maaieh, Kylee Hoersten, Jovana Duric, and Zahoor A. Shah. 2025. "Sphingosine-1-Phosphate Modulation in Neurological Disorders: Insights from MS and Stroke" Brain Sciences 15, no. 5: 436. https://doi.org/10.3390/brainsci15050436
APA StyleMaktabi, B., Shehjar, F., Senger, Z., Kountz, L., Hasan, S., Maaieh, K., Hoersten, K., Duric, J., & Shah, Z. A. (2025). Sphingosine-1-Phosphate Modulation in Neurological Disorders: Insights from MS and Stroke. Brain Sciences, 15(5), 436. https://doi.org/10.3390/brainsci15050436






