Intranasal Transplantation of Microbiota Derived from Parkinson’s Disease Mice Induced Astrocyte Activation and Neurodegenerative Pathology from Nose to Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of PD-Derived Fecal Bacteria
2.3. Microbiota Intranasal Transplantation and Animal Groups Design
2.4. Behavioral Experiments
2.5. Sample Collection and Tissue Preparation
2.6. 16S rRNA Gene Sequencing for Nasal Microbiota
2.7. Immunohistochemical and Immunofluorescent Staining
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
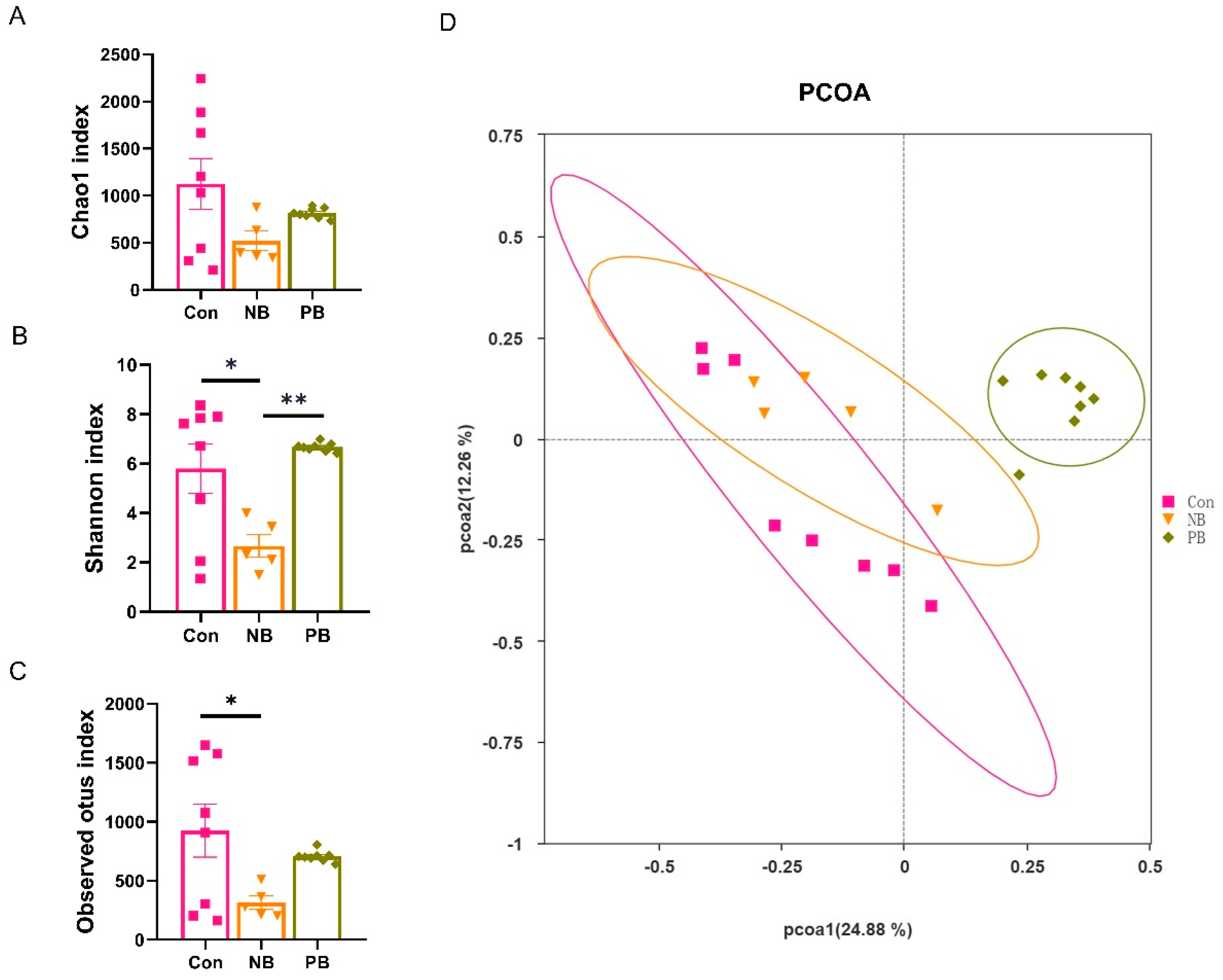
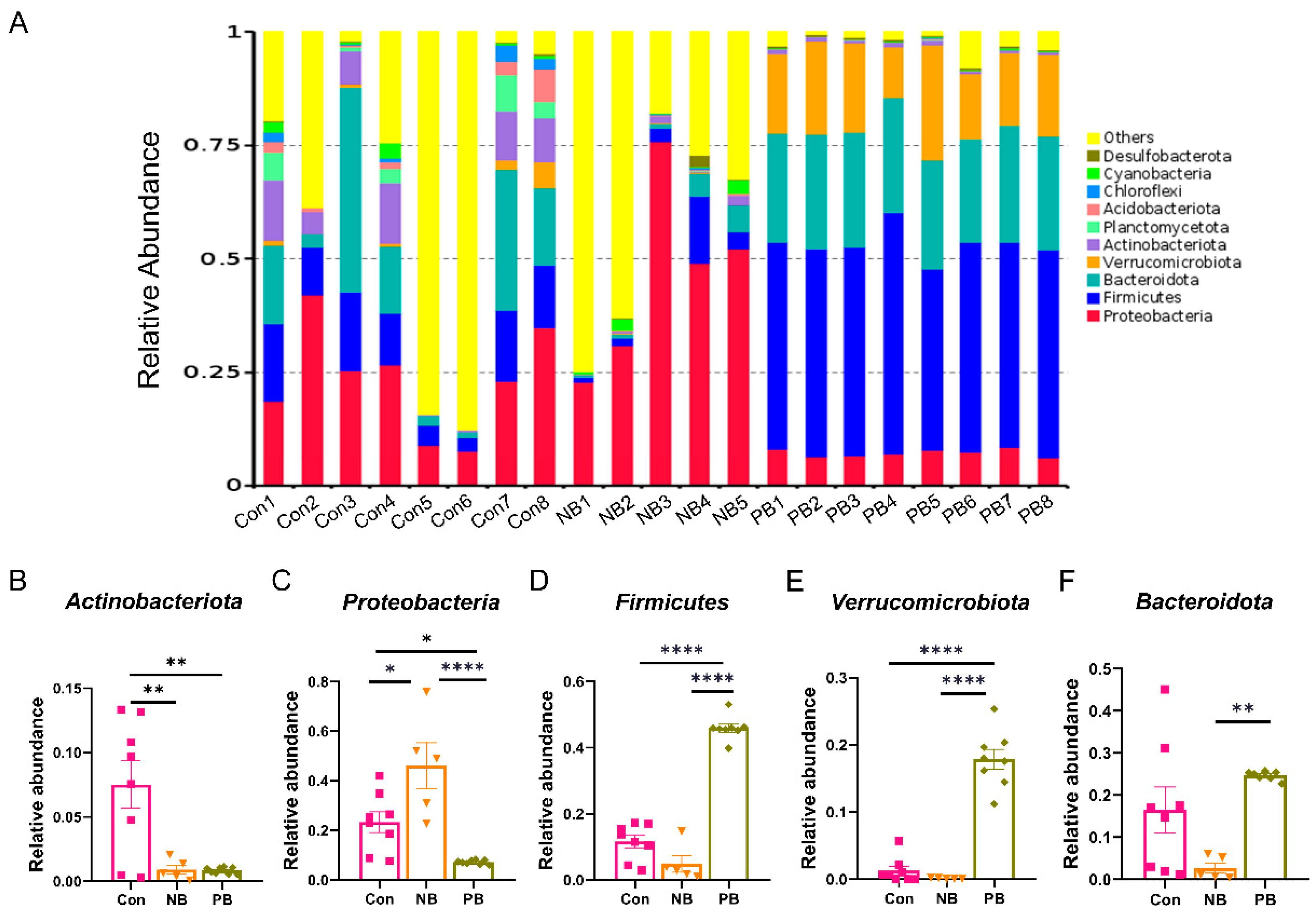
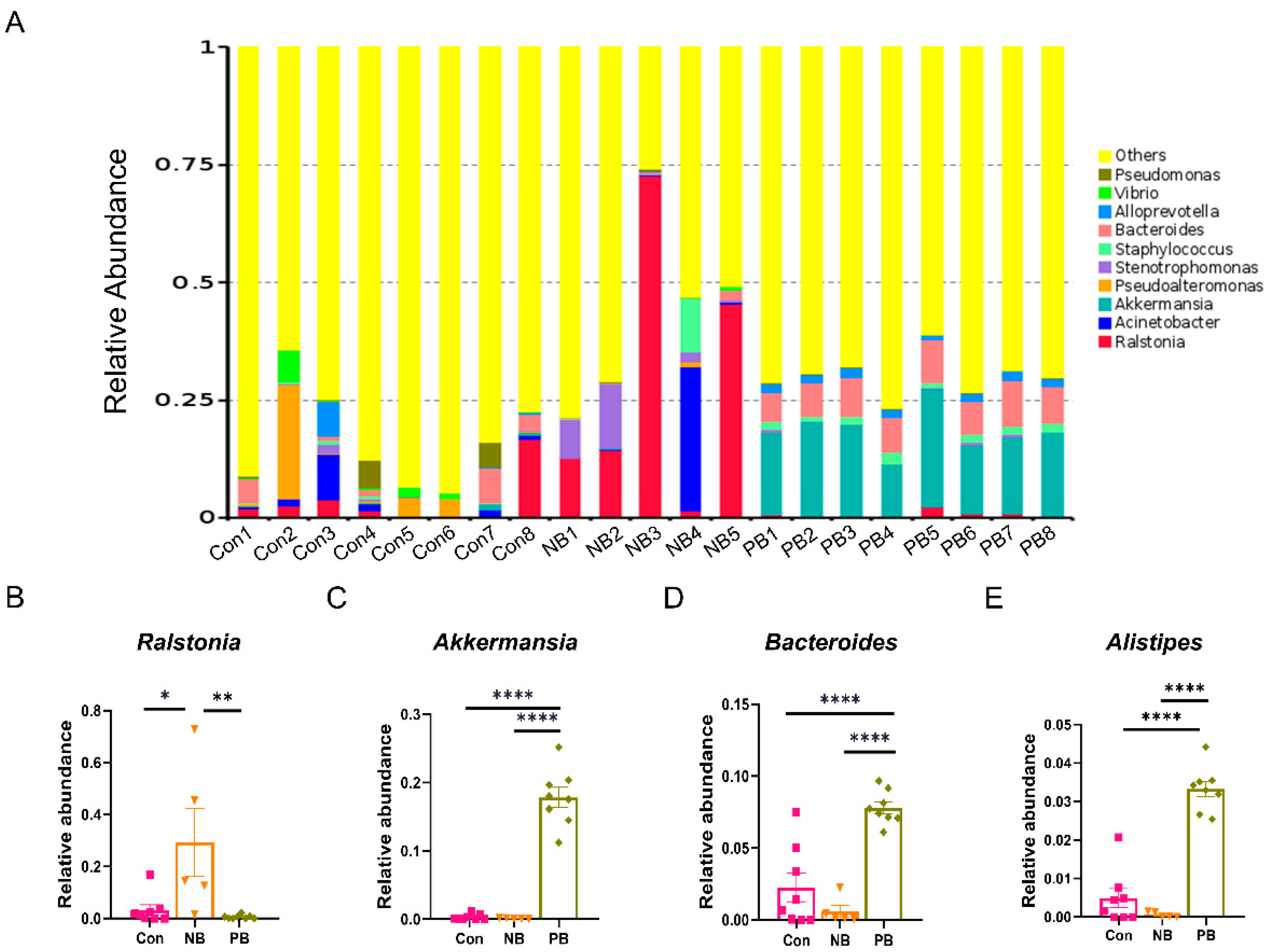
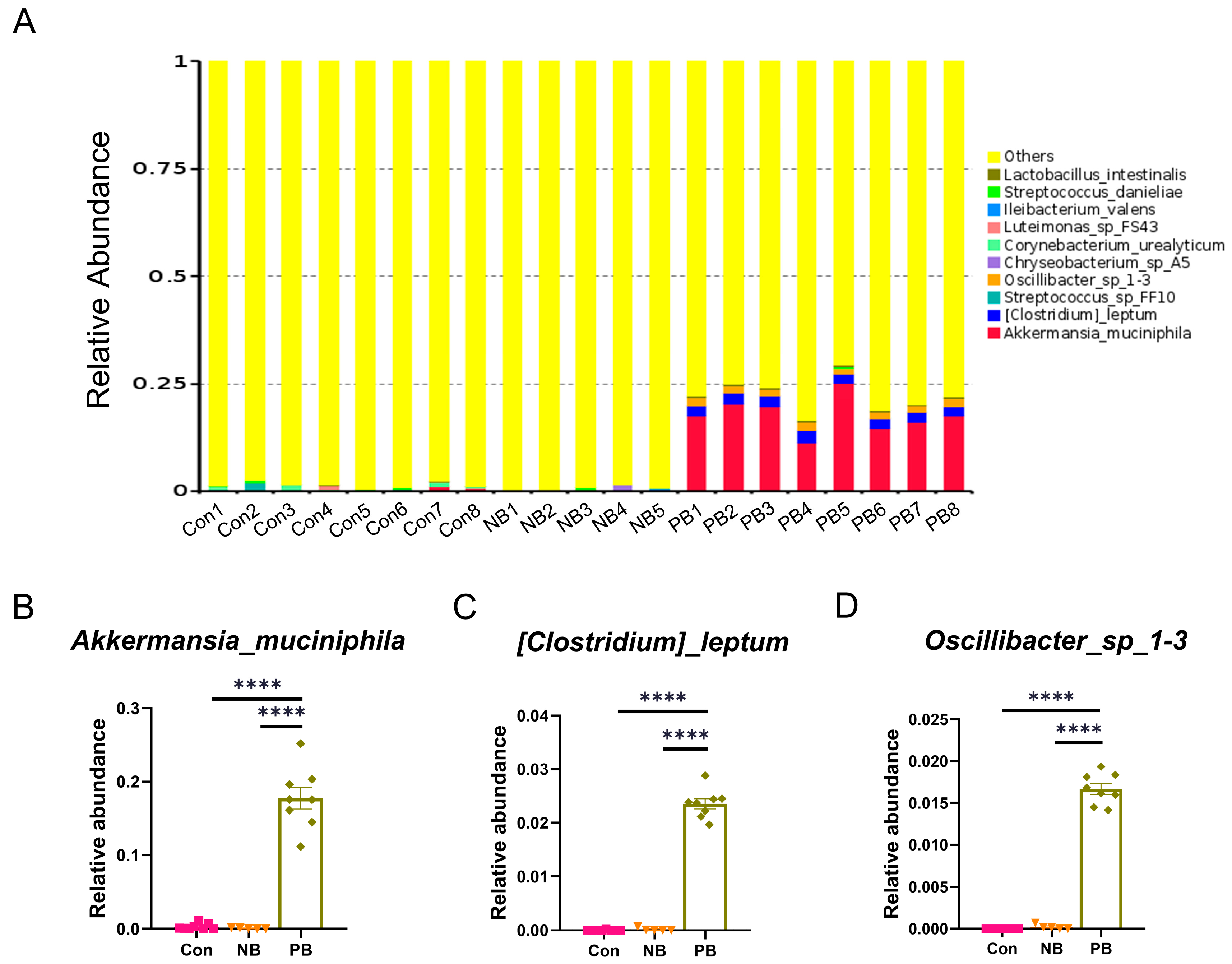
3.1. Intranasal Transplantation of Fecal Microbiota Led to Disorganized Nasal Microbiota in Mice
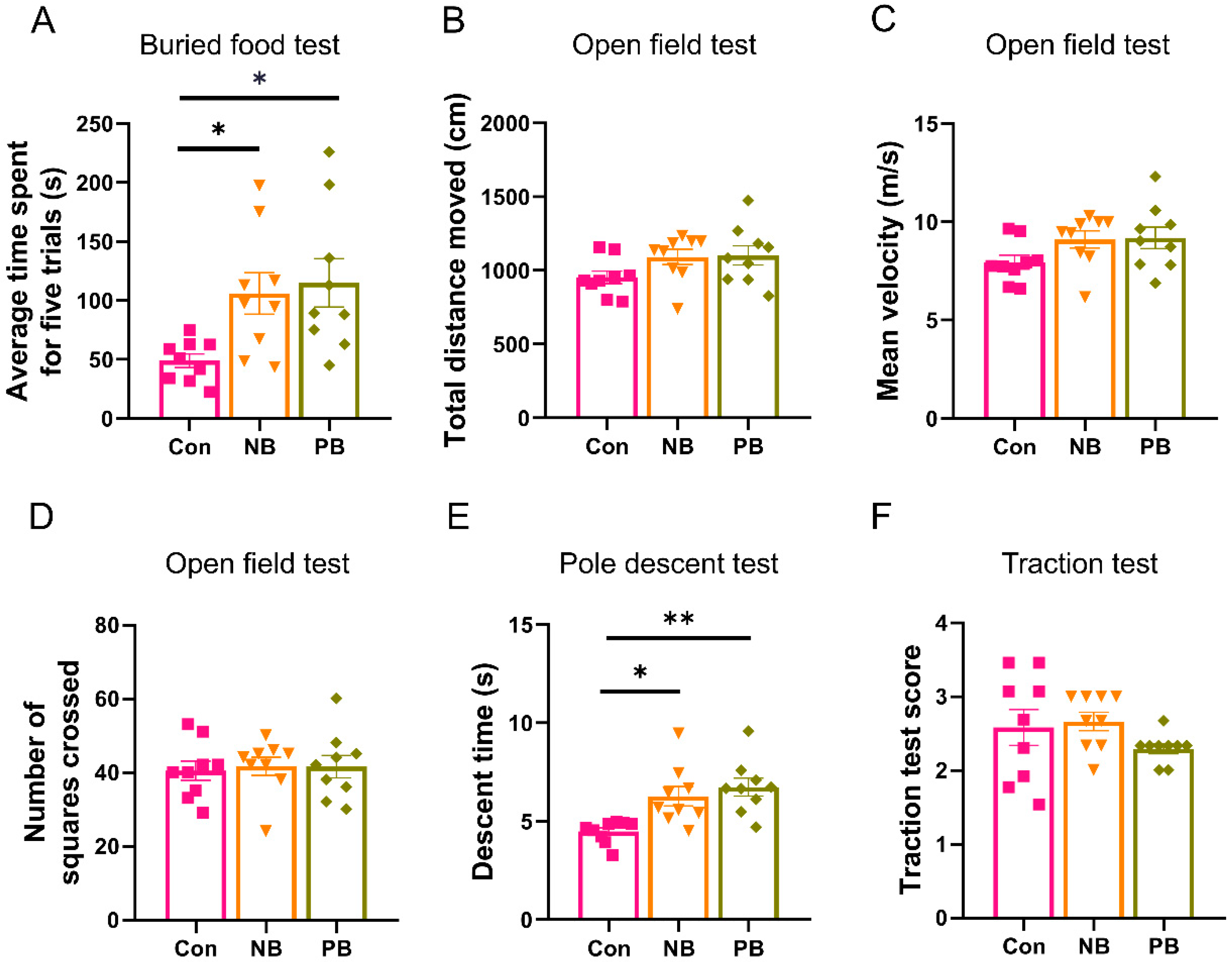
3.2. Nasal Microbiota Dysbiosis-Induced Olfactory and Motor Dysfunction
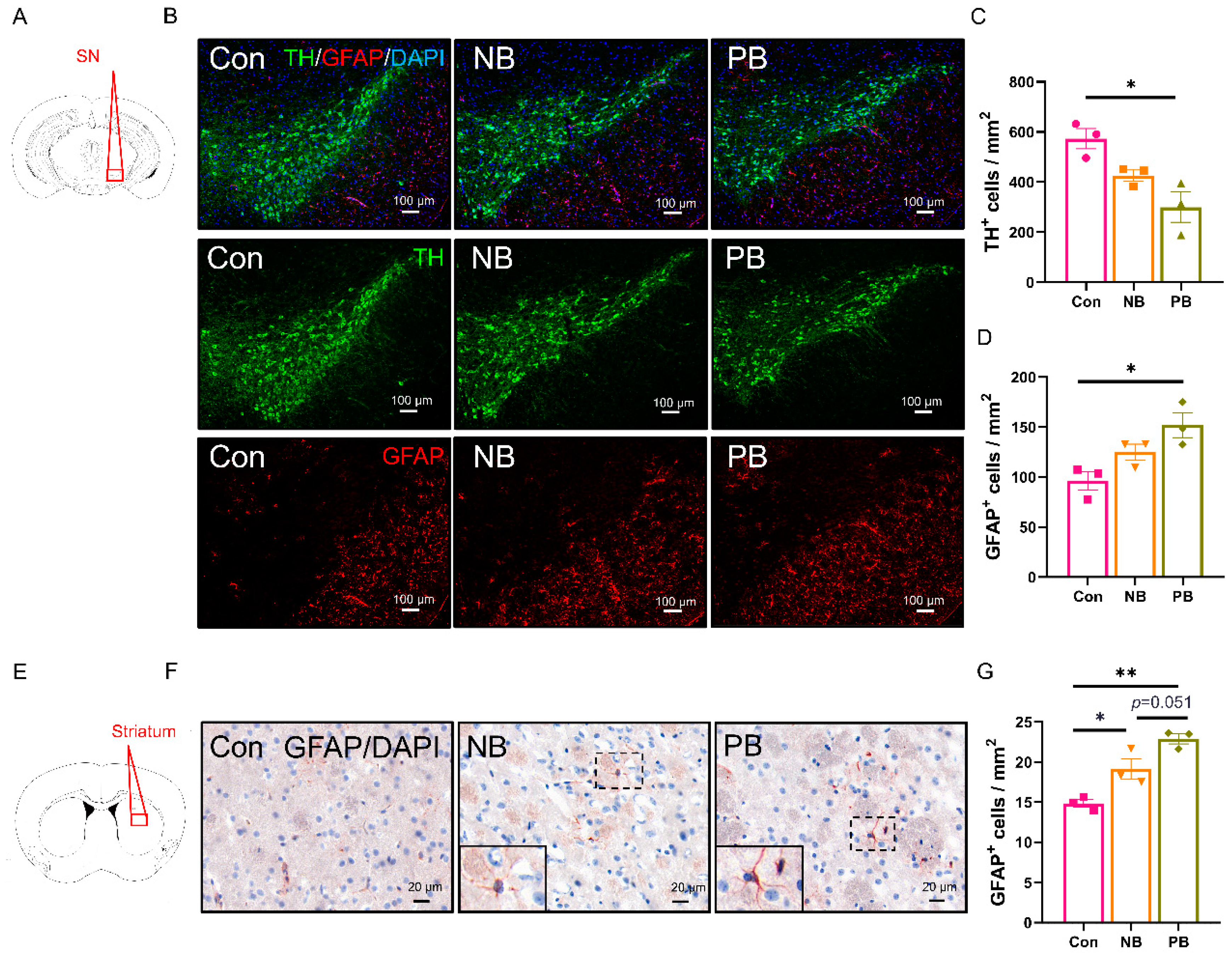
3.3. Neuronal Loss and Astrocyte Activation in the Olfactory-Related Brain Regions of the Mice with Nasal Microbiota Dysbiosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: Modelling study of Global Burden of Disease Study 2021. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Barresi, M.; Ciurleo, R.; Giacoppo, S.; Foti Cuzzola, V.; Celi, D.; Bramanti, P.; Marino, S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J. Neurol. Sci. 2012, 323, 16–24. [Google Scholar] [CrossRef]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef]
- Marin, C.; Vilas, D.; Langdon, C.; Alobid, I.; López-Chacón, M.; Haehner, A.; Hummel, T.; Mullol, J. Olfactory Dysfunction in Neurodegenerative Diseases. Curr. Allergy Asthma Rep. 2018, 18, 42. [Google Scholar] [CrossRef]
- Han, X.; He, X.; Zhan, X.; Yao, L.; Sun, Z.; Gao, X.; Wang, S.; Wang, Z. Disturbed microbiota-metabolites-immune interaction network is associated with olfactory dysfunction in patients with chronic rhinosinusitis. Front. Immunol. 2023, 14, 1159112. [Google Scholar] [CrossRef]
- Bachert, C.; Hicks, A.; Gane, S.; Peters, A.T.; Gevaert, P.; Nash, S.; Horowitz, J.E.; Sacks, H.; Jacob-Nara, J.A. The interleukin-4/interleukin-13 pathway in type 2 inflammation in chronic rhinosinusitis with nasal polyps. Front. Immunol. 2024, 15, 1356298. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Namkung, H.; Smith, A.; Sakamoto, S.; Zhu, X.; Ishizuka, K.; Lane, A.P.; Sawa, A.; Kamiya, A. Causal impact of local inflammation in the nasal cavity on higher brain function and cognition. Neurosci. Res. 2021, 172, 110–115. [Google Scholar] [CrossRef]
- Ebrahim Soltani, Z.; Badripour, A.; Haddadi, N.S.; Elahi, M.; Kazemi, K.; Afshari, K.; Dehpour, A.R. Allergic rhinitis in BALB/c mice is associated with behavioral and hippocampus changes and neuroinflammation via the TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2022, 108, 108725. [Google Scholar] [CrossRef] [PubMed]
- Francois, A.; Grebert, D.; Rhimi, M.; Mariadassou, M.; Naudon, L.; Rabot, S.; Meunier, N. Olfactory epithelium changes in germfree mice. Sci. Rep. 2016, 6, 24687. [Google Scholar] [CrossRef]
- Yang, S.; Wu, J.; Zhang, Q.; Li, X.; Liu, D.; Zeng, B.; Liu, Z.; Kang, H.; Zhong, Z. Allergic Rhinitis in Rats Is Associated with an Inflammatory Response of the Hippocampus. Behav. Neurol. 2018, 2018, 8750464. [Google Scholar] [CrossRef]
- Xie, J.; Tian, S.; Liu, J.; Cao, R.; Yue, P.; Cai, X.; Shang, Q.; Yang, M.; Han, L.; Zhang, D.K. Dual role of the nasal microbiota in neurological diseases-An unignorable risk factor or a potential therapy carrier. Pharmacol. Res. 2022, 179, 106189. [Google Scholar] [CrossRef]
- Little, C.S.; Hammond, C.J.; MacIntyre, A.; Balin, B.J.; Appelt, D.M. Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol. Aging 2004, 25, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Su, J.; Gao, X.; Yang, H.; Weng, R.; Ni, W.; Gu, Y. The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome 2022, 10, 62. [Google Scholar] [CrossRef]
- Lazarini, F.; Roze, E.; Lannuzel, A.; Lledo, P.M. The microbiome-nose-brain axis in health and disease. Trends Neurosci. 2022, 45, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, L.; Walkden, H.; Chacko, A.; Delbaz, A.; Shelper, T.; Armitage, C.W.; Reshamwala, R.; Trim, L.K.; St John, J.A.; Beagley, K.W.; et al. Chlamydia muridarum Can Invade the Central Nervous System via the Olfactory and Trigeminal Nerves and Infect Peripheral Nerve Glial Cells. Front. Cell Infect. Microbiol. 2020, 10, 607779. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Doring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef]
- Pereira, P.A.B.; Aho, V.T.E.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat. Disord. 2017, 38, 61–67. [Google Scholar] [CrossRef]
- Pal, G.; Ramirez, V.; Engen, P.A.; Naqib, A.; Forsyth, C.B.; Green, S.J.; Mahdavinia, M.; Batra, P.S.; Tajudeen, B.A.; Keshavarzian, A. Deep nasal sinus cavity microbiota dysbiosis in Parkinson’s disease. NPJ Parkinsons Dis. 2021, 7, 111. [Google Scholar] [CrossRef]
- van den Munckhof, E.H.A.; Hafkamp, H.C.; de Kluijver, J.; Kuijper, E.J.; de Koning, M.N.C.; Quint, W.G.V.; Knetsch, C.W. Nasal microbiota dominated by Moraxella spp. is associated with respiratory health in the elderly population: A case control study. Respir. Res. 2020, 21, 181. [Google Scholar] [CrossRef]
- McCauley, K.; Durack, J.; Valladares, R.; Fadrosh, D.W.; Lin, D.L.; Calatroni, A.; LeBeau, P.K.; Tran, H.T.; Fujimura, K.E.; LaMere, B.; et al. Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J. Allergy Clin. Immunol. 2019, 144, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; You, H.; Liu, J.F.; Ni, D.F.; Zhang, Z.X.; Guan, J. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am. J. Neuroradiol. 2011, 32, 677–681. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Gan, Q.F.; Kathivaloo, P.; Mohamad, N.A.; Dhamodharan, J.; Krishnan, A.; Sengodan, B.; Palanimuthu, V.R.; Marimuthu, K.; Rajandas, H.; et al. Deciduous DPSCs Ameliorate MPTP-Mediated Neurotoxicity, Sensorimotor Coordination and Olfactory Function in Parkinsonian Mice. Int. J. Mol. Sci. 2019, 20, 568. [Google Scholar] [CrossRef]
- Zhang, M.X.; Hong, H.; Shi, Y.; Huang, W.Y.; Xia, Y.M.; Tan, L.L.; Zhao, W.J.; Qiao, C.M.; Wu, J.; Zhao, L.P.; et al. A Pilot Study on a Possible Mechanism behind Olfactory Dysfunction in Parkinson’s Disease: The Association of TAAR1 Downregulation with Neuronal Loss and Inflammation along Olfactory Pathway. Brain Sci. 2024, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; de Sousa e Melo, F.; Roelofs, J.J.; de Boer, J.D.; Hoogendijk, A.J.; de Beer, R.; de Vos, A.; Belzer, C.; et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef]
- Zeppa, J.J.; Wakabayashi, A.T.; Kasper, K.J.; Xu, S.X.; Haeryfar, S.M.M.; McCormick, J.K. Nasopharyngeal Infection of Mice with Streptococcus pyogenes and In Vivo Detection of Superantigen Activity. Methods Mol. Biol. 2016, 1396, 95–107. [Google Scholar] [CrossRef]
- Yang, M.; Crawley, J.N. Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 2009, 48, 8.24.1–8.24.12. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.; Javaid, S.; Fawad Rasool, M.; Samad, N.; Rizwan Ahamad, S.; Alqahtani, F.; Imran, I. Amelioration of Scopolamine-Induced Amnesic, Anxiolytic and Antidepressant Effects of Ficus Benghalensis in Behavioral Experimental Models. Medicina 2020, 56, 144. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, F.; Wang, W. Vitexin protects dopaminergic neurons in MPTP-induced Parkinson’s disease through PI3K/Akt signaling pathway. Drug Des. Devel Ther. 2018, 12, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Hong, H.; Shi, Y.; Zhou, Y.; Qiao, C.M.; Zhao, W.J.; Zhao, L.P.; Wu, J.; Quan, W.; Niu, G.Y.; et al. Vancomycin Pretreatment on MPTP-Induced Parkinson’s Disease Mice Exerts Neuroprotection by Suppressing Inflammation Both in Brain and Gut. J. Neuroimmune Pharmacol. 2023, 18, 72–89. [Google Scholar] [CrossRef]
- Asakawa, T.; Fang, H.; Sugiyama, K.; Nozaki, T.; Hong, Z.; Yang, Y.; Hua, F.; Ding, G.; Chao, D.; Fenoy, A.J.; et al. Animal behavioral assessments in current research of Parkinson’s disease. Neurosci. Biobehav. Rev. 2016, 65, 63–94. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Feng, B.; Zhang, Q.; Tong, J.; Wang, M.; Lu, C.; Peng, S. Seizures in PPT1 Knock-In Mice Are Associated with Inflammatory Activation of Microglia. Int. J. Mol. Sci. 2022, 23, 5586. [Google Scholar] [CrossRef]
- Carroll, J.A.; Striebel, J.F.; Rangel, A.; Woods, T.; Phillips, K.; Peterson, K.E.; Race, B.; Chesebro, B. Prion Strain Differences in Accumulation of PrPSc on Neurons and Glia Are Associated with Similar Expression Profiles of Neuroinflammatory Genes: Comparison of Three Prion Strains. PLoS Pathog. 2016, 12, e1005551. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammat. 2019, 16, 129. [Google Scholar] [CrossRef]
- Zhao, Z.; Ning, J.; Bao, X.Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef]
- Hou, Y.F.; Shan, C.; Zhuang, S.Y.; Zhuang, Q.Q.; Ghosh, A.; Zhu, K.C.; Kong, X.K.; Wang, S.M.; Gong, Y.L.; Yang, Y.Y.; et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Bae, C.H.; Lee, Y.; Kim, H.Y.; Kim, S. Korean red ginseng suppresses 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced inflammation in the substantia nigra and colon. Brain Behav. Immun. 2021, 94, 410–423. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P.; Wullner, U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med. 2017, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Cheng, Y.; Gao, J.; Liu, X.; Shao, L.; Kong, Q.; Zheng, N.; Ling, Z.; Hu, W. Akkermansia muciniphila in neuropsychiatric disorders: Friend or foe? Front. Cell Infect. Microbiol. 2023, 13, 1224155. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Hassan, S.I.; Bigler, S.; Siegelbaum, S.A. Social odor discrimination and its enhancement by associative learning in the hippocampal CA2 region. Neuron 2023, 111, 2232–2246.e2235. [Google Scholar] [CrossRef]
- Stevenson, E.L.; Caldwell, H.K. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur. J. Neurosci. 2014, 40, 3294–3301. [Google Scholar] [CrossRef]
- Hitti, F.L.; Siegelbaum, S.A. The hippocampal CA2 region is essential for social memory. Nature 2014, 508, 88–92. [Google Scholar] [CrossRef]
- Liu, X.; Lei, Z.; Gilhooly, D.; He, J.; Li, Y.; Ritzel, R.M.; Li, H.; Wu, L.J.; Liu, S.; Wu, J. Traumatic brain injury-induced inflammatory changes in the olfactory bulb disrupt neuronal networks leading to olfactory dysfunction. Brain Behav. Immun. 2023, 114, 22–45. [Google Scholar] [CrossRef]
- Zhang, L.; Geng, C.; Li, S.; Tang, Q.; Liu, P.; Liu, W.; Qiu, G.; Li, A.; Hu, A.; Chen, F. Anterior piriform cortex dysfunction underlies autism spectrum disorders-related olfactory deficits in Fmr1 conditional deletion mice. Neuropsychopharmacology 2024, 50, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Cinar, E.; Yalcin-Cakmakli, G.; Saka, E.; Ulusoy, A.; Yuruker, S.; Elibol, B.; Tel, B.C. Modelling cognitive deficits in Parkinson’s disease: Is CA2 a gateway for hippocampal synucleinopathy? Exp. Neurol. 2020, 330, 113357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ruan, Z.; Jing, L.; Guo, Z.; Zhang, X.; Liu, J.; Tian, L.; Sun, W.; Song, S.; Hong, J.S.; et al. Complement receptor 3-mediated neurotoxic glial activation contributes to rotenone-induced cognitive decline in mice. Ecotoxicol. Environ. Saf. 2023, 266, 115550. [Google Scholar] [CrossRef]
- Delgado-Zabalza, L.; Mallet, N.P.; Glangetas, C.; Dabee, G.; Garret, M.; Miguelez, C.; Baufreton, J. Targeting parvalbumin-expressing neurons in the substantia nigra pars reticulata restores motor function in parkinsonian mice. Cell Rep. 2023, 42, 113287. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Gao, W.; Saiyin, H.; Li, Y.; Zeng, Y.; Zhang, Z.; Li, X.; Liu, Z.; Gao, Q.; An, P.; et al. MLKL deficiency alleviates neuroinflammation and motor deficits in the alpha-synuclein transgenic mouse model of Parkinson’s disease. Mol. Neurodegener. 2023, 18, 94. [Google Scholar] [CrossRef]
- De Boeck, I.; van den Broek, M.F.L.; Allonsius, C.N.; Spacova, I.; Wittouck, S.; Martens, K.; Wuyts, S.; Cauwenberghs, E.; Jokicevic, K.; Vandenheuvel, D.; et al. Lactobacilli Have a Niche in the Human Nose. Cell Rep. 2020, 31, 107674. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.-M.; Zhang, M.-X.; Ma, X.-Y.; Tan, L.-L.; Li, T.; Wu, J.; Li, M.-A.; Zhao, W.-J.; Qiao, C.-M.; Jia, X.-B.; et al. Intranasal Transplantation of Microbiota Derived from Parkinson’s Disease Mice Induced Astrocyte Activation and Neurodegenerative Pathology from Nose to Brain. Brain Sci. 2025, 15, 433. https://doi.org/10.3390/brainsci15050433
Xia Y-M, Zhang M-X, Ma X-Y, Tan L-L, Li T, Wu J, Li M-A, Zhao W-J, Qiao C-M, Jia X-B, et al. Intranasal Transplantation of Microbiota Derived from Parkinson’s Disease Mice Induced Astrocyte Activation and Neurodegenerative Pathology from Nose to Brain. Brain Sciences. 2025; 15(5):433. https://doi.org/10.3390/brainsci15050433
Chicago/Turabian StyleXia, Yi-Meng, Mei-Xuan Zhang, Xiao-Yu Ma, Lu-Lu Tan, Ting Li, Jian Wu, Ming-An Li, Wei-Jiang Zhao, Chen-Meng Qiao, Xue-Bing Jia, and et al. 2025. "Intranasal Transplantation of Microbiota Derived from Parkinson’s Disease Mice Induced Astrocyte Activation and Neurodegenerative Pathology from Nose to Brain" Brain Sciences 15, no. 5: 433. https://doi.org/10.3390/brainsci15050433
APA StyleXia, Y.-M., Zhang, M.-X., Ma, X.-Y., Tan, L.-L., Li, T., Wu, J., Li, M.-A., Zhao, W.-J., Qiao, C.-M., Jia, X.-B., Shen, Y.-Q., & Cui, C. (2025). Intranasal Transplantation of Microbiota Derived from Parkinson’s Disease Mice Induced Astrocyte Activation and Neurodegenerative Pathology from Nose to Brain. Brain Sciences, 15(5), 433. https://doi.org/10.3390/brainsci15050433





