Longitudinal Trajectories of Cognitive Function Among Chinese Middle-Aged and Older Adults: The Role of Sarcopenia and Depressive Symptoms
Abstract
1. Introduction
2. Method
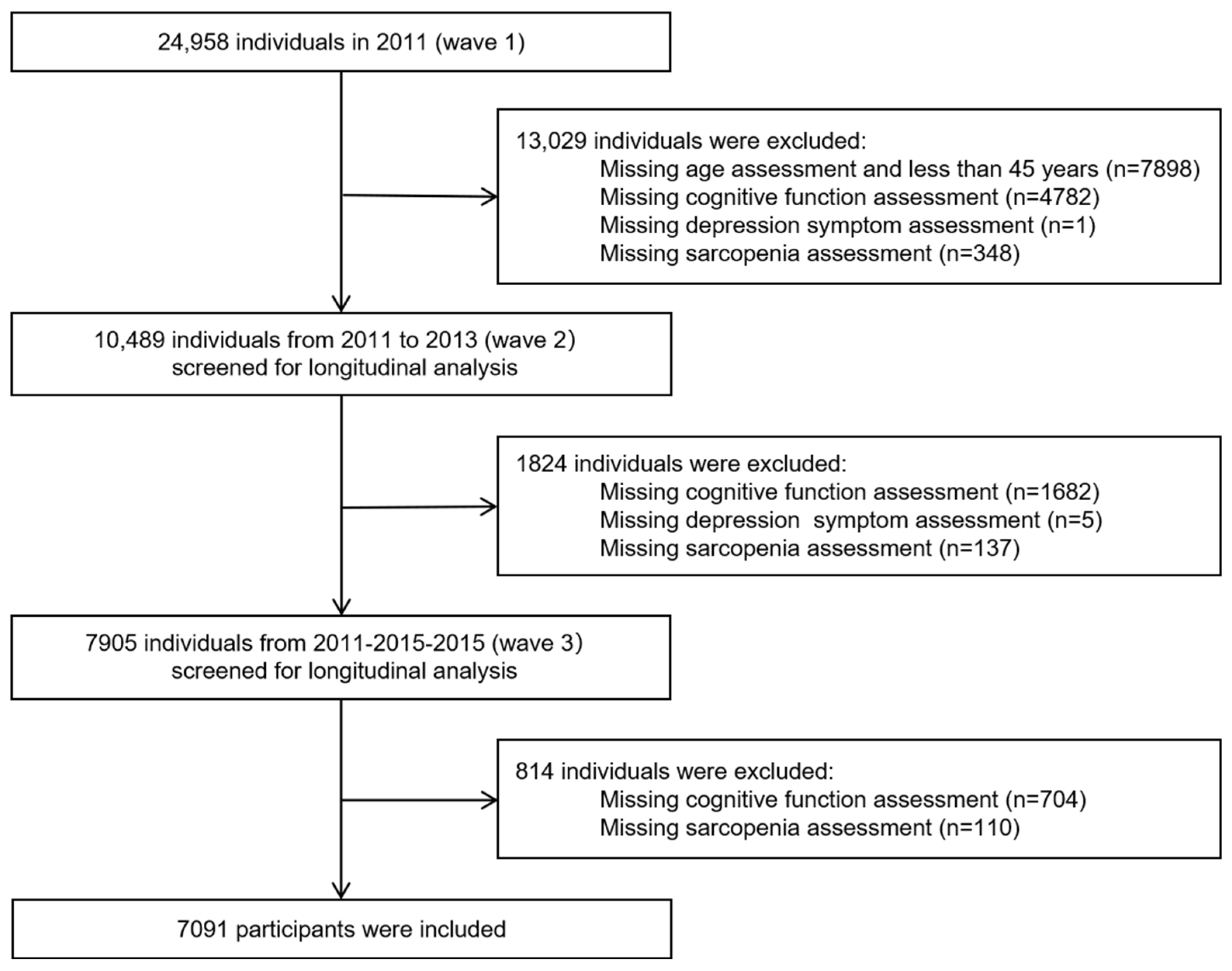
2.1. Participants and Procedure
2.2. Measurements
2.3. Potential Covariates
2.4. Statistical Analyses
3. Results
3.1. Cognitive Trajectory Modeling
3.2. Descriptive Statistics
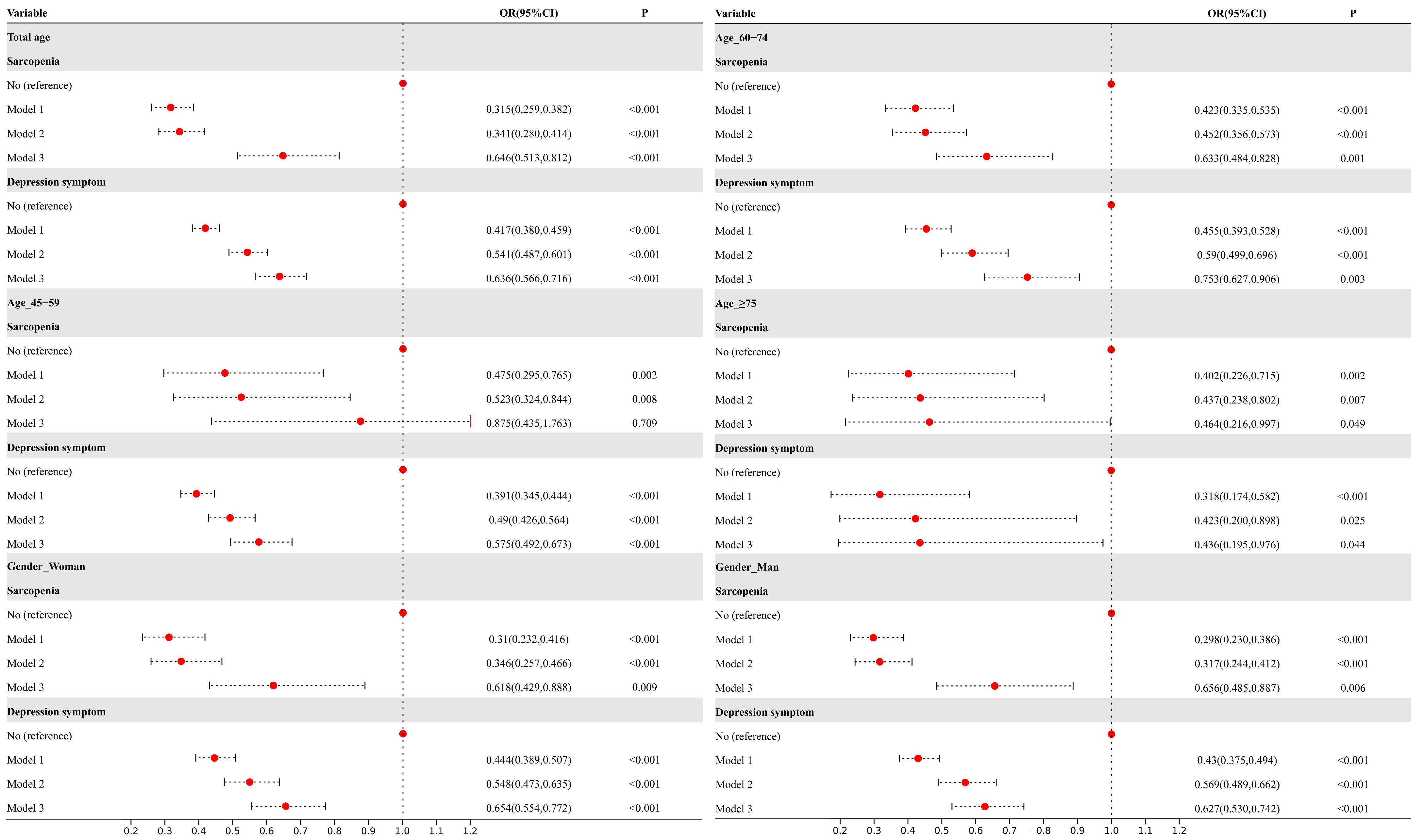
3.3. Association Between Sarcopenia and Depressive Symptoms with Cognitive Trajectories
3.4. Mediation Effect of Depression Symptoms Between Sarcopenia and Cognitive Trajectories
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| MCI | Mild cognitive impairment |
| AD | Alzheimer’s disease |
| AWGS | Asian Working Group for Sarcopenia |
| ASM | Appendicular skeletal muscle mass |
| BMI | Body mass index |
| ADL | Activities of Daily Living |
| IADL | Instrumental Activities of Daily Living |
| SRH | Self-rated health |
| GBTM | Group-based trajectory modeling |
| BIC | Bayesian Information Criterion |
| AIC | Akaike information criterion |
| AvePP | Average posterior probability |
| CLPM | Cross-lagged panel modeling |
References
- Womack, C.L.; Perkins, A.; Arnold, J.M. Cognitive Impairment in the Primary Care Clinic. Prim. Care Clin. Off. Pract. 2024, 51, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Song, W.X.; Wu, W.W.; Zhao, Y.Y.; Xu, H.L.; Chen, G.C.; Jin, S.Y.; Chen, J.; Xian, S.X.; Liang, J.H. Evidence from a meta-analysis and systematic review reveals the global prevalence of mild cognitive impairment. Front. Aging Neurosci. 2023, 15, 1227112. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Ruano, L.; Carvalho, O.P.; Barros, H. Global Cognitive Impairment Prevalence and Incidence in Community Dwelling Older Adults—A Systematic Review. Geriatrics 2020, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.; Li, T.; Xu, M.; Yang, D.; Lv, H.; Gassara, G.; Ibrahim, T.I.; Radman, B.A.; Wang, J. Emerging trends in cognitive impairment and dementia among older populations in Asia: A systematic review. J. Glob. Health 2024, 14, 4233. [Google Scholar] [CrossRef]
- Zaninotto, P.; Batty, G.D.; Allerhand, M.; Deary, I.J. Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. J. Epidemiol. Community Health 2018, 72, 685–694. [Google Scholar] [CrossRef]
- Clare, L.; Wu, Y.T.; Teale, J.C.; MacLeod, C.; Matthews, F.; Brayne, C.; Woods, B. Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study. PLoS Med. 2017, 14, e1002259. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef]
- Beeri, M.S.; Leugrans, S.E.; Delbono, O.; Bennett, D.A.; Buchman, A.S. Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. (Jags) 2021, 69, 1826–1835. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, W.; Ren, R.; Wang, Y.; Wang, G. Sarcopenia and mild cognitive impairment among elderly adults: The first longitudinal evidence from CHARLS. J. Cachexia Sarcopenia Muscle 2022, 13, 2944–2952. [Google Scholar] [CrossRef]
- Jacob, L.; Kostev, K.; Smith, L.; Oh, H.; López-Sánchez, G.F.; Shin, J.I.; Abduljabbar, A.S.; Haro, J.M.; Koyanagi, A. Sarcopenia and Mild Cognitive Impairment in Older Adults from Six Low- and Middle-Income Countries. J. Alzheimer’s Dis. 2021, 82, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiao, M.; Leng, L.; Jiang, S.; Feng, L.; Pan, G.; Li, Z.; Wang, Y.; Wang, J.; Wen, Y.; et al. A systematic review and meta-analysis of the prevalence and correlation of mild cognitive impairment in sarcopenia. J. Cachexia Sarcopenia Muscle 2023, 14, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Chen, W.; Wu, L.; Chang, Y.; Kao, T. Sarcopenia and cognitive impairment: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Cabett Cipolli, G.; Sanches Yassuda, M.; Aprahamian, I. Erratum to: Sarcopenia Is Associated with Cognitive Impairment in Older Adults: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2023, 27, 687. [Google Scholar] [CrossRef]
- Amini, N.; Ibn, H.M.; Lapauw, L.; Dupont, J.; Vercauteren, L.; Verschueren, S.; Tournoy, J.; Gielen, E. Meta-analysis on the interrelationship between sarcopenia and mild cognitive impairment, Alzheimer’s disease and other forms of dementia. J. Cachexia Sarcopenia Muscle 2024, 15, 1240–1253. [Google Scholar] [CrossRef]
- Chang, K.M.P.; Hsu, T.M.; Wu, W.M.; Huang, K.M.P.; Han, D.M.P. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164–1167. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. 2017. Available online: https://iris.who.int/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf? (accessed on 24 November 2024).
- Kim, N.H.; Kim, H.S.; Eun, C.R.; Seo, J.A.; Cho, H.J.; Kim, S.G.; Choi, K.M.; Baik, S.H.; Choi, D.S.; Park, M.H.; et al. Depression Is Associated with Sarcopenia, Not Central Obesity, in Elderly Korean Men. J. Am. Geriatr. Soc. (Jags) 2011, 59, 2062–2068. [Google Scholar] [CrossRef]
- Kurita, N.; Wakita, T.; Fujimoto, S.; Yanagi, M.; Koitabashi, K.; Suzuki, T.; Yazawa, M.; Kawarazaki, H.; Shibagaki, Y.; Ishibashi, Y. Hopelessness and Depression Predict Sarcopenia in Advanced CKD and Dialysis: A Multicenter Cohort Study. J. Nutr. Health Aging 2021, 25, 593–599. [Google Scholar] [CrossRef]
- Wang, L.T.; Huang, W.C.; Hung, Y.C.; Park, J.H. Association between Depressive Symptoms and Risk of Sarcopenia in Taiwanese Older Adults. J. Nutr. Health Aging 2021, 25, 790–794. [Google Scholar] [CrossRef]
- Wang, H.; Hai, S.; Liu, Y.; Cao, L.; Liu, Y.; Liu, P.; Zhou, J.; Yang, Y.; Dong, B. Association between depressive symptoms and sarcopenia in older Chinese community-dwelling individuals. Clin. Interv. Aging 2018, 13, 1605–1611. [Google Scholar] [CrossRef]
- Li, Z.Z.; Tong, X.; Ma, Y.; Bao, T.; Yue, J.R. Prevalence of depression in patients with sarcopenia and correlation between the two diseases: Systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 128–144. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, J.; Cao, L.; Stubbendorff, A.; Zhang, S. Association of depression with incident sarcopenia and modified effect from healthy lifestyle: The first longitudinal evidence from the CHARLS. J. Affect. Disord. 2024, 344, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Thomas, A.J. Depression and dementia: Cause, consequence or coincidence? Maturitas 2014, 79, 184–190. [Google Scholar] [CrossRef]
- Wagner, S.; Doering, B.; Helmreich, I.; Lieb, K.; Tadic, A. A meta-analysis of executive dysfunctions in unipolar major depressive disorder without psychotic symptoms and their changes during antidepressant treatment. Acta Psychiatr. Scand. 2012, 125, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, P.; Yu, X.; Zhang, Y.; Song, P.; Liu, Y.; Jiang, Z.; Tao, Z.; Shen, S.; Wu, Y.; et al. Relationships between sarcopenia, depressive symptoms, and mild cognitive impairment in Chinese community-dwelling older adults. J. Affect. Disord. 2021, 286, 71–77. [Google Scholar] [CrossRef]
- Wu, X.; Hou, G.; Han, P.; Yu, X.; Chen, X.; Song, P.; Zhang, Y.; Zhao, Y.; Xie, F.; Niu, S.; et al. Association Between Physical Performance and Cognitive Function in Chinese Community-Dwelling Older Adults: Serial Mediation of Malnutrition and Depression. Clin. Interv. Aging 2021, 16, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Li, X.; Xiao, Y.; Song, K.; Wang, L.; Shen, J.; Aimaiti, M.; Ma, X.; Shi, C.; Li, G. Relationship between Social Support, Sarcopenia, and Cognitive Impairment in Chinese Community-Dwelling Older Adults. J. Nutr. Health Aging 2023, 27, 726–733. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort Profile: The China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef]
- Chen, L.; Woo, J.; Assantachai, P.; Auyeung, T.; Chou, M.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Wen, X.; Wang, M.; Jiang, C.M.; Zhang, Y.M. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac. J. Clin. Nutr. 2011, 20, 551–556. [Google Scholar]
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Newman, A.B. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Luo, Y.; Yang, F.; Zhu, X.; Gao, X.; Wang, W.; Qiao, G.; Zhou, J. The mediating role of children’s intergenerational support in association between grandparenting and cognitive function among middle-aged and older Chinese: Findings from the CHARLS cohort study. BMC Public. Health 2024, 24, 597. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Xu, C.; Fang, Y. The transitions and predictors of cognitive frailty with multi-state Markov model: A cohort study. Bmc Geriatr. 2022, 22, 550. [Google Scholar] [CrossRef]
- Cheng, S.T.; Chan, A. The Center for Epidemiologic Studies Depression Scale in older Chinese: Thresholds for long and short forms. Int. J. Geriatr. Psychiatry 2005, 20, 465–470. [Google Scholar] [CrossRef]
- Chen, H.; Mui, A.C. Factorial validity of the Center for Epidemiologic Studies Depression Scale short form in older population in China. Int. Psychogeriatr. 2014, 26, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Naharci, M.I.; Tasci, I. Comment on “Association between depression and motoric cognitive risk syndrome among community-dwelling older adults in China: A 4-year prospective cohort study”. Eur. J. Neurol. 2023, 30, 558–559. [Google Scholar] [CrossRef]
- Luo, H.; Li, J.; Zhang, Q.; Cao, P.; Ren, X.; Fang, A.; Liao, H.; Liu, L. Obesity and the onset of depressive symptoms among middle-aged and older adults in China: Evidence from the CHARLS. Bmc Public. Health 2018, 18, 909. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Dugravot, A.; Shipley, M.; Brunner, E.J.; Elbaz, A.; Sabia, S.; Kivimaki, M. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimer’s Dement. 2018, 14, 178–186. [Google Scholar] [CrossRef]
- Nagin, D.S.; Odgers, C.L. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 2010, 6, 109–138. [Google Scholar]
- Zhang, W.; Chen, Y.; Chen, N. Body mass index and trajectories of the cognition among Chinese middle and old-aged adults. Bmc Geriatr. 2022, 22, 613. [Google Scholar]
- Li, G.; Liu, L.; Wang, M.; Li, Y.; Wu, H. The longitudinal mediating effect of rumination on the relationship between depressive symptoms and problematic smartphone use in Chinese university students: A three-wave cross-lagged panel analysis. Addict. Behav. 2024, 150, 107907. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, Y.; Shi, R. Association between obesity, physical activity, and cognitive decline in Chinese middle and old-aged adults: A mediation analysis. BMC Geriatr. 2024, 24, 54. [Google Scholar] [CrossRef]
- Gardeniers, M.; van Groenou, M.; Meijboom, E.J.; Huisman, M. Three-year trajectories in functional limitations and cognitive decline among Dutch 75+ year olds, using nine-month intervals. BMC Geriatr. 2022, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jia, X.; Shi, X.; Yang, C.; Wang, R.; Zhao, C.; Xin, X.; Yang, Y. Association between Sarcopenia and Cognitive Trajectories among Middle-Aged and Older Adults in China: A Nationally Representative Cohort Study. J. Nutr. Health Aging 2023, 27, 243–250. [Google Scholar]
- Li, H.; Li, C.; Wang, A.; Qi, Y.; Feng, W.; Hou, C.; Tao, L.; Liu, X.; Li, X.; Wang, W.; et al. Associations between social and intellectual activities with cognitive trajectories in Chinese middle-aged and older adults: A nationally representative cohort study. Alzheimer’s Res. Ther. 2020, 12, 115. [Google Scholar]
- Jo, D.; Yoon, G.; Kim, O.Y.; Song, J. A new paradigm in sarcopenia: Cognitive impairment caused by imbalanced myokine secretion and vascular dysfunction. Biomed. Pharmacother. 2022, 147, 112636. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, F.; Hsiao, Y. Myostatin Is Associated With Cognitive Decline in an Animal Model of Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 1984–1991. [Google Scholar] [CrossRef]
- Buchman, A.S.; Wang, T.; Yu, L.; Leurgans, S.E.; Schneider, J.A.; Bennett, D.A. Brain pathologies are associated with both the rate and variability of declining motor function in older adults. Acta Neuropathol. 2020, 140, 587–589. [Google Scholar] [CrossRef]
- Liu, X.; Hou, L.; Xia, X.; Liu, Y.; Zuo, Z.; Zhang, Y.; Zhao, W.; Hao, Q.; Yue, J.; Dong, B. Prevalence of sarcopenia in multi ethnics adults and the association with cognitive impairment: Findings from West-China health and aging trend study. BMC Geriatr. 2020, 20, 63. [Google Scholar] [CrossRef]
- Rubin, R. Exploring the Relationship Between Depression and Dementia. Jamajama 2018, 320, 961–962. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Xie, W.; Zhong, B.; Wu, Y.; Blumenthal, J.A. Trajectories of depressive symptoms and subsequent cognitive decline in older adults: A pooled analysis of two longitudinal cohorts. Age Ageing 2022, 51, 9. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, Y.; Hu, M.; Sun, Y.; Xu, M.; Hao, J.; Zhu, C. Associations between trajectories of depressive symptoms and rate of cognitive decline among Chinese middle-aged and older adults: An 8-year longitudinal study. J. Psychosom. Res. 2022, 160, 110986. [Google Scholar] [CrossRef] [PubMed]
- Kaup, A.R.; Byers, A.L.; Falvey, C.; Simonsick, E.M.; Satterfield, S.; Ayonayon, H.N.; Smagula, S.F.; Rubin, S.M.; Yaffe, K. Trajectories of Depressive Symptoms in Older Adults and Risk of Dementia. JAMA Psychiatry 2016, 73, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, X.; Qiu, Q.; Yan, F.; Fang, Y.; Shen, C.; Wang, H.; Chen, Y.; Xiao, S.; Yue, L.; et al. Cognitive trajectories in older adults and the role of depressive symptoms: A 7-year follow-up study. Asian J. Psychiatr. 2024, 95, 104007. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Niu, C.; Wei, Y.; Cai, Y.; Zhang, W.; Xiao, Y.; Yin, J. COVID-19 impacts on cross-border mobility of senior population between Shenzhen and Hong Kong. Front. Public. Health 2023, 11, 1285288. [Google Scholar] [CrossRef]
- Wang, D.K.; Li, Y.H.; Guo, X.M. Depression and sarcopenia-related traits: A Mendelian randomization study. World J. Psychiatry 2023, 13, 929–936. [Google Scholar] [CrossRef]
- Steffens, D.C. Depressive symptoms and mild cognitive impairment in the elderly: An ominous combination. Biol. Psychiatry 2012, 71, 762–764. [Google Scholar] [CrossRef]
- Fiske, A.; Wetherell, J.L.; Gatz, M. Depression in Older Adults. Annu. Rev. Clin. Psychol. 2009, 5, 363–389. [Google Scholar] [CrossRef]


| Variable | Polynomial Order by Group | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| −1 | (1, 2) | (1, 1, 2) | (1, 1, 1, 2) | (1, 1, 1, 1, 2) | (1, 2, 2, 2, 1, 2) | |
| BIC (n = 7091) | −54,983.53 | −51,959.34 | −51,282.61 | −51,161.54 | −51,091.78 | −51,034.94 |
| AIC | −54,973.23 | −51,935.31 | −51,248.28 | −51,116.91 | −51,036.85 | −50,959.41 |
| Proportion | ||||||
| Group 1 | 1.00 | 28.39 | 12.18 | 5.90 | 6.33 | 7.02 |
| Group 2 | 71.61 | 37.27 | 18.62 | 7.12 | 7.87 | |
| Group 3 | 50.55 | 38.76 | 12.75 | 5.79 | ||
| Group 4 | 36.73 | 39.11 | 8.52 | |||
| Group 5 | 34.69 | 39.00 | ||||
| Group 6 | 31.80 | |||||
| AvePP | ||||||
| Group 1 | — | 0.91 | 0.89 | 0.85 | 0.83 | 0.84 |
| Group 2 | 0.96 | 0.84 | 0.79 | 0.66 | 0.64 | |
| Group 3 | 0.90 | 0.76 | 0.69 | 0.65 | ||
| Group 4 | 0.85 | 0.76 | 0.65 | |||
| Group 5 | 0.84 | 0.76 | ||||
| Group 6 | 0.84 | |||||
| Baseline Characteristics | Overall | Low and Decline | Middle and Decline | Middle and Stable | High and Stable | x2/F |
|---|---|---|---|---|---|---|
| (n = 7091) | (n = 388) | (n = 1280) | (n = 2860) | (n = 2563) | ||
| Age, n (%) | ||||||
| 45–59 | 4156 (58.61) | 145 (3.49) | 631 (15.18) | 1643 (39.53) | 1737 (41.79) | 266.419 *** |
| 60–74 | 2717 (38.32) | 203 (7.47) | 587 (21.60) | 1139 (41.92) | 788 (29.00) | |
| ≥75 | 218 (3.07) | 40 (18.35) | 62 (28.44) | 78 (35.78) | 38 (17.43) | |
| Gender, n (%) | 124.372 *** | |||||
| Male | 3832 (54.04) | 145 (3.78) | 564 (14.72) | 1620 (42.28) | 1503 (39.22) | |
| Female | 3259 (45.96) | 243 (7.46) | 716 (21.97) | 1240 (38.05) | 1060 (32.53) | |
| Residence, n (%) | 585.923 *** | |||||
| Rural | 5354 (75.54) | 352 (6.57) | 1140 (21.29) | 2334 (43.59) | 1528 (28.54) | |
| Urban | 1734 (24.46) | 36 (2.08) | 139 (8.02) | 524 (30.22) | 1035 (59.69) | |
| Educational level, n (%) | 1547.554 *** | |||||
| Primary school or below | 4137 (58.37) | 370 (8.94) | 1118 (27.02) | 1834 (44.33) | 815 (19.70) | |
| Junior high school | 1892 (26.69) | 13 (0.69) | 130 (6.87) | 753 (39.80) | 996 (52.64) | |
| Senior high school or above | 1059 (14.94) | 5 (0.47) | 31 (2.93) | 272 (25.68) | 751 (70.92) | |
| Marital status, n (%) | 109.779 *** | |||||
| Married | 6467 (91.20) | 315 (4.87) | 1107 (17.12) | 2642 (40.85) | 2403 (37.16) | |
| Others | 624 (8.80) | 73 (11.70) | 173 (27.72) | 218 (34.94) | 160 (25.64) | |
| Medical insurance, n (%) | 6.612 | |||||
| Yes | 6710 (94.84) | 358 (5.34) | 1200 (17.88) | 2713 (40.43) | 2439 (36.35) | |
| No | 365 (5.16) | 27 (7.40) | 76 (20.82) | 147 (40.27) | 115 (31.51) | |
| Sleep, mean (SD) | 6.4 (1.7) | 276 (6.2) | 852 (19.1) | 1807 (40.6) | 1515 (34) | 21.093 *** |
| Smoke, n (%) | 19.001 *** | |||||
| Yes | 3068 (43.27) | 149 (4.86) | 500 (16.30) | 1303 (42.47) | 1116 (36.38) | |
| No | 4022 (56.73) | 238 (5.92) | 780 (19.39) | 1557 (38.71) | 1447 (35.98) | |
| Drink, n (%) | 34.383 *** | |||||
| Yes | 2641 (37.24) | 112 (4.24) | 428 (16.21) | 1053 (39.87) | 1048 (39.68) | |
| No | 4450 (62.76) | 276 (6.20) | 852 (19.15) | 1807 (40.61) | 1515 (34.04) | |
| BMI, n (%) | 117.134 *** | |||||
| Normal | 3173 (50.95) | 214 (6.74) | 633 (19.95) | 1321 (41.63) | 1005 (31.67) | |
| Underweight | 212 (3.40) | 26 (12.26) | 65 (30.66) | 88 (41.51) | 33 (15.57) | |
| Overweight | 2017 (32.39) | 79 (3.92) | 332 (16.46) | 819 (40.60) | 787 (39.02) | |
| Obese | 826 (13.26) | 33 (4.00) | 127 (15.38) | 332 (40.19) | 334 (40.44) | |
| Number of chronic diseases, n (%) | 21.282 ** | |||||
| 0 | 2271 (32.03) | 105 (4.62) | 402 (17.70) | 874 (38.49) | 890 (39.19) | |
| 1 | 2057 (29.01) | 105 (5.10) | 376 (18.28) | 839 (40.79) | 737 (35.83) | |
| ≥2 | 2763 (38.96) | 178 (6.44) | 502 (18.17) | 1147 (41.51) | 936 (33.88) | |
| ADL, n (%) | 171.642 *** | |||||
| Yes | 899 (12.79) | 100 (11.12) | 238 (26.47) | 378 (42.05) | 183 (20.36) | |
| No | 6132 (87.21) | 287 (4.68) | 1028 (16.76) | 2462 (40.15) | 2355 (38.41) | |
| IADL, n (%) | 232.434 *** | |||||
| Yes | 1042 (14.69) | 122 (11.71) | 276 (26.49) | 443 (42.51) | 201 (19.29) | |
| No | 6049 (85.31) | 266 (4.40) | 1004 (16.60) | 2417 (39.96) | 2362 (39.05) | |
| Pain, n (%) | 172.275 *** | |||||
| Yes | 2139 (30.19) | 174 (8.13) | 487 (22.77) | 924 (43.20) | 554 (25.90) | |
| No | 4946 (69.81) | 213 (4.31) | 793 (16.03) | 1935 (39.12) | 2005 (40.54) | |
| SRH, n (%) | 237.228 *** | |||||
| Very good | 500 (7.06) | 14 (2.80) | 69 (13.80) | 194 (38.80) | 223 (44.60) | |
| Good | 1311 (18.50) | 48 (3.66) | 172 (13.12) | 485 (36.99) | 606 (46.22) | |
| Normal | 3650 (51.50) | 172 (4.71) | 660 (18.08) | 1479 (40.52) | 1339 (36.68) | |
| Poor | 1390 (19.61) | 126 (9.06) | 314 (22.59) | 604 (43.45) | 346 (24.89) | |
| Very poor | 236 (3.33) | 28 (11.86) | 64 (27.12) | 96 (40.68) | 48 (20.34) |
| Baseline Characteristics | Sarcopenia, n (%) | CED-10, Mean ± SD | Depression Symptom, n (%) | Cognitive Function Scores (MMSE), Mean ± SD | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Wave | ||||||
| 2011 | 373 (5.26) | 6718 (94.74) | 7.60 ± 5.979 | 2240 (31.59) | 4851 (68.41) | 12.615 ± 3.101 |
| 2013 | 351 (4.95) | 6740 (95.05) | 7.31 ± 5.455 | 1983 (27.97) | 5108 (72.03) | 12.530 ± 3.230 |
| 2015 | 461 (6.50) | 6630 (93.50) | 7.44 ± 6.076 | 2158 (30.43) | 4933 (69.57) | 12.229 ± 3.284 |
| x2/F | 18.166 | 4.252 | 23.147 | 28.314 | ||
| p | <0.001 | 0.014 | <0.001 | <0.001 | ||
| Cognitive trajectory | ||||||
| Low and decline | 63 (16.89) | 325 (4.84) | 9.61 ± 6.535 | 216 (9.64) | 172 (3.55) | 6.914 ± 2.234 |
| Middle and decline | 113 (30.29) | 1167 (17.37) | 7.85 ± 5.891 | 562 (25.09) | 718 (14.80) | 9.633 ± 2.27 |
| Middle and stable | 153 (41.02) | 2707 (40.29) | 5.76 ± 4.994 | 958 (42.77) | 1902 (39.21) | 12.422 ± 2.008 |
| High and stable | 44 (11.80) | 2519 (37.50) | 7.60 ± 5.979 | 504 (22.50) | 2059 (42.44) | 15.182 ± 1.67 |
| x2/F | 191.131 | 192.512 | 367.436 | 3560.884 | ||
| p | <0.001 | <0.001 | <0.001 | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Chao, J.; Jin, Q.; Yang, B.; Tan, G.; Wang, L.; Wu, Y. Longitudinal Trajectories of Cognitive Function Among Chinese Middle-Aged and Older Adults: The Role of Sarcopenia and Depressive Symptoms. Brain Sci. 2025, 15, 408. https://doi.org/10.3390/brainsci15040408
Jin S, Chao J, Jin Q, Yang B, Tan G, Wang L, Wu Y. Longitudinal Trajectories of Cognitive Function Among Chinese Middle-Aged and Older Adults: The Role of Sarcopenia and Depressive Symptoms. Brain Sciences. 2025; 15(4):408. https://doi.org/10.3390/brainsci15040408
Chicago/Turabian StyleJin, Shengxuan, Jianqian Chao, Qian Jin, Beibei Yang, Gangrui Tan, Leixia Wang, and Yanqian Wu. 2025. "Longitudinal Trajectories of Cognitive Function Among Chinese Middle-Aged and Older Adults: The Role of Sarcopenia and Depressive Symptoms" Brain Sciences 15, no. 4: 408. https://doi.org/10.3390/brainsci15040408
APA StyleJin, S., Chao, J., Jin, Q., Yang, B., Tan, G., Wang, L., & Wu, Y. (2025). Longitudinal Trajectories of Cognitive Function Among Chinese Middle-Aged and Older Adults: The Role of Sarcopenia and Depressive Symptoms. Brain Sciences, 15(4), 408. https://doi.org/10.3390/brainsci15040408





