Lithium Treatment Increases FKBP5 Protein but Not mRNA Expression in the Pituitary Gland of Depressive-like Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat Model of Depression
2.2. Molecular Analysis
2.3. Protein Analysis
2.4. Corticosterone Analysis
2.5. Statistical Analysis
3. Results
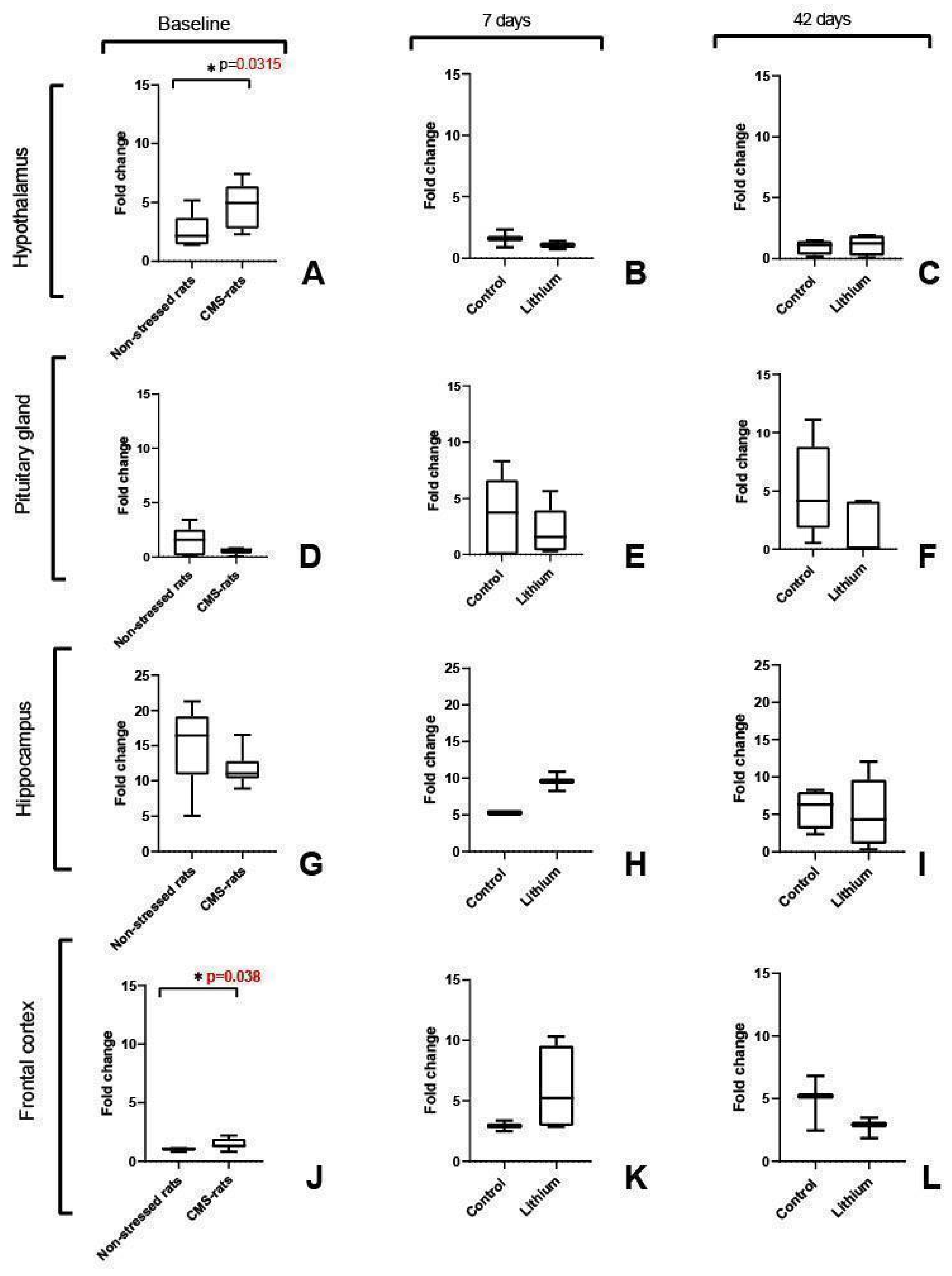
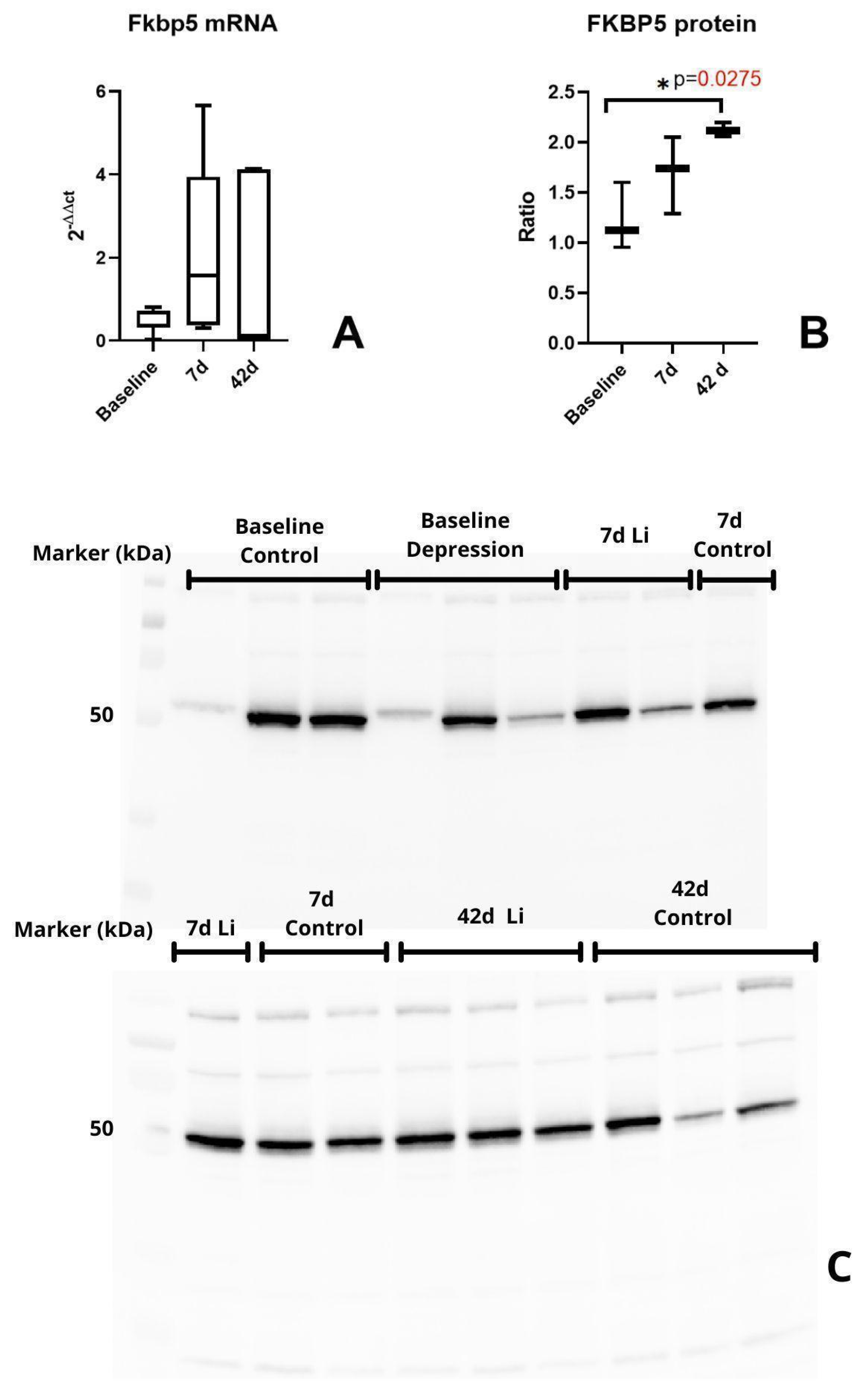
3.1. Fkbp5 Expression Is Significantly Increased in the Hypothalamus and Frontal Cortex of Stressed Rats
3.2. Mir-511-5p Expression Was Not Detected in the Rat Brain Regions
3.3. FKBP5 Protein Significantly Increased During Long-Term Lithium Treatment in the Pituitary
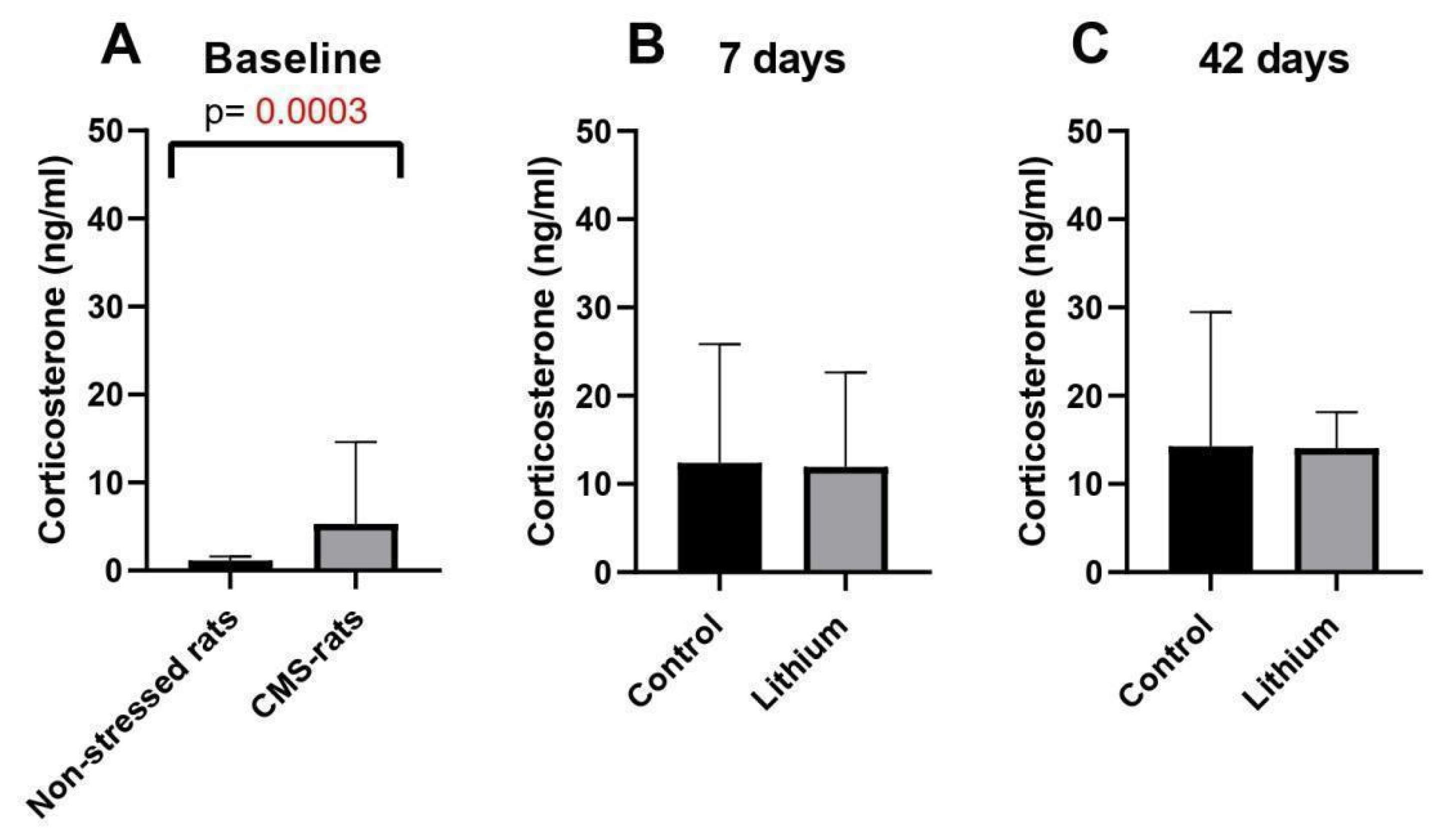
3.4. Chronic Mild Stress and Long-Term Lithium Treatment Affect Corticosterone Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FKBP5 | FK506-binding protein 5 |
| HPA axis | Hypothalamic–pituitary–adrenal axis |
| GR | Glucocorticoid receptor |
| GWA | Genome-wide association |
| CMS | Chronic mild stress |
| PVN | Paraventricular nucleus |
| CRH | Corticotropin-releasing hormone |
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Kupcova, I.; Danisovic, L.; Klein, M.; Harsanyi, S. Effects of the COVID-19 Pandemic on Mental Health, Anxiety, and Depression. BMC Psychol. 2023, 11, 108. [Google Scholar] [CrossRef]
- Wang, Q.; Shelton, R.C.; Dwivedi, Y. Interaction between Early-Life Stress and FKBP5 Gene Variants in Major Depressive Disorder and Post-Traumatic Stress Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2018, 225, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of Stress throughout the Lifespan on the Brain, Behaviour and Cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Liu, C.; Marioni, R.E.; Hedman, Å.K.; Pfeiffer, L.; Tsai, P.-C.; Reynolds, L.M.; Just, A.C.; Duan, Q.; Boer, C.G.; Tanaka, T.; et al. A DNA Methylation Biomarker of Alcohol Consumption. Mol. Psychiatry 2018, 23, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Miller, A.H. Glucocorticoid Receptors in Major Depression: Relevance to Pathophysiology and Treatment. Biol. Psychiatry 2001, 49, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Grad, I.; Picard, D. The Glucocorticoid Responses Are Shaped by Molecular Chaperones. Mol. Cell Endocrinol. 2007, 275, 2–12. [Google Scholar] [CrossRef]
- Wochnik, G.M.; Rüegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-Binding Proteins 51 and 52 Differentially Regulate Dynein Interaction and Nuclear Translocation of the Glucocorticoid Receptor in Mammalian Cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef]
- Zhang, X.; Clark, A.F.; Yorio, T. FK506-Binding Protein 51 Regulates Nuclear Transport of the Glucocorticoid Receptor β and Glucocorticoid Responsiveness. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1037–1047. [Google Scholar] [CrossRef]
- Binder, E.B. The Role of FKBP5, a Co-Chaperone of the Glucocorticoid Receptor in the Pathogenesis and Therapy of Affective and Anxiety Disorders. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S186–S195. [Google Scholar] [CrossRef]
- Häusl, A.S.; Brix, L.M.; Hartmann, J.; Pöhlmann, M.L.; Lopez, J.-P.; Menegaz, D.; Brivio, E.; Engelhardt, C.; Roeh, S.; Bajaj, T.; et al. The Co-Chaperone Fkbp5 Shapes the Acute Stress Response in the Paraventricular Nucleus of the Hypothalamus of Male Mice. Mol. Psychiatry 2021, 26, 3060–3076. [Google Scholar] [CrossRef] [PubMed]
- Criado-Marrero, M.; Smith, T.M.; Gould, L.A.; Kim, S.; Penny, H.J.; Sun, Z.; Gulick, D.; Dickey, C.A.; Blair, L.J. FKBP5 and Early Life Stress Affect the Hippocampus by an Age-Dependent Mechanism. Brain Behav. Immun. Health 2020, 9, 100143. [Google Scholar] [CrossRef] [PubMed]
- Füchsl, A.M.; Langgartner, D.; Reber, S.O. Mechanisms Underlying the Increased Plasma ACTH Levels in Chronic Psychosocially Stressed Male Mice. PLoS ONE 2013, 8, e84161. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Sabbagh, J.J.; Blair, L.J.; Darling, A.L.; Wen, X.; Dickey, C.A. MicroRNA-511 Binds to FKBP5 mRNA, Which Encodes a Chaperone Protein, and Regulates Neuronal Differentiation. J. Biol. Chem. 2016, 291, 17897–17906. [Google Scholar] [CrossRef]
- Ising, M.; Maccarrone, G.; Brückl, T.; Scheuer, S.; Hennings, J.; Holsboer, F.; Turck, C.W.; Uhr, M.; Lucae, S. FKBP5 Gene Expression Predicts Antidepressant Treatment Outcome in Depression. Int. J. Mol. Sci. 2019, 20, 485. [Google Scholar] [CrossRef]
- Malekpour, M.; Shekouh, D.; Safavinia, M.E.; Shiralipour, S.; Jalouli, M.; Mortezanejad, S.; Azarpira, N.; Ebrahimi, N.D. Role of FKBP5 and Its Genetic Mutations in Stress-Induced Psychiatric Disorders: An Opportunity for Drug Discovery. Front. Psychiatry 2023, 14, 1182345. [Google Scholar] [CrossRef]
- Szczepankiewicz, A.; Narozna, B.; Rybakowski, J.K.; Kliwicki, S.; Czerski, P.; Dmitrzak-Węglarz, M.; Skibińska, M.; Twarowska-Hauser, J.; Pawlak, J. Genes Involved in Stress Response Influence Lithium Efficacy in Bipolar Patients. Bipolar Disord. 2018, 20, 753–760. [Google Scholar] [CrossRef]
- Bschor, T.; Lewitzka, U.; Sasse, J.; Adli, M.; Köberle, U.; Bauer, M. Lithium Augmentation in Treatment-Resistant Depression: Clinical Evidence, Serotonergic and Endocrine Mechanisms. Pharmacopsychiatry 2003, 36 (Suppl. S3), S230–S234. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.-K. An Oldie but Goodie: Lithium in the Treatment of Bipolar Disorder through Neuroprotective and Neurotrophic Mechanisms. Int. J. Mol. Sci. 2017, 18, 2679. [Google Scholar] [CrossRef]
- Willner, P.; Muscat, R.; Papp, M. Chronic Mild Stress-Induced Anhedonia: A Realistic Animal Model of Depression. Neurosci. Biobehav. Rev. 1992, 16, 525–534. [Google Scholar] [CrossRef]
- Papp, M.; Willner, P. Models of Affective Illness: Chronic Mild Stress in the Rat. Curr. Protoc. 2023, 3, e712. [Google Scholar] [CrossRef] [PubMed]
- Szczepankiewicz, D.; Celichowski, P.; Kołodziejski, P.A.; Pruszyńska-Oszmałek, E.; Sassek, M.; Zakowicz, P.; Banach, E.; Langwiński, W.; Sakrajda, K.; Nowakowska, J.; et al. Transcriptome Changes in Three Brain Regions during Chronic Lithium Administration in the Rat Models of Mania and Depression. Int. J. Mol. Sci. 2021, 22, 1148. [Google Scholar] [CrossRef]
- Langwiński, W.; Nowakowska, J.; Sakrajda, K.; Ziarniak, K.; Stachowiak, Z.; Kachel, M.; Narożna, B.; Szczepankiewicz, A. An Optimized QIAzol-Based Protocol for Simultaneous miRNA, RNA, and Protein Isolation from Precision-Cut Lung Slices (PCLS). Respir. Res. 2024, 25, 422. [Google Scholar] [CrossRef]
- Kachel, M.; Dola, A.; Kubiak, M.; Majewska, W.; Nowakowska, J.; Langwiński, W.; Hryhorowicz, S.; Szczepankiewicz, A. MicroRNA Expression Profile Is Altered by Short-Term and Chronic Lithium Treatment in a Rat Model of Depression. J. Mol. Neurosci. 2024, 74, 116. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Calabrese, F.; Anacker, C.; Racagni, G.; Pariante, C.M.; Riva, M.A. Glucocorticoid Receptor and FKBP5 Expression Is Altered Following Exposure to Chronic Stress: Modulation by Antidepressant Treatment. Neuropsychopharmacology 2013, 38, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Yurtsever, T.; Streit, F.; Foo, J.C.; Trifonova, S.; Kumsta, R.; Muller, C.P.; Turner, J.D.; Meyer, J.; Schote, A.B. Temporal Dynamics of Cortisol-Associated Changes in mRNA Expression of Glucocorticoid Responsive Genes FKBP5, GILZ, SDPR, PER1, PER2 and PER3 in Healthy Humans. Psychoneuroendocrinology 2019, 102, 63–67. [Google Scholar] [CrossRef]
- Miller, A.H.; Spencer, R.L.; Pulera, M.; Kang, S.; McEwen, B.S.; Stein, M. Adrenal Steroid Receptor Activation in Rat Brain and Pituitary Following Dexamethasone: Implications for the Dexamethasone Suppression Test. Biol. Psychiatry 1992, 32, 850–869. [Google Scholar] [CrossRef]
- Zimmer, C.; Hanson, H.E.; Martin, L.B. FKBP5 Expression Is Related to HPA Flexibility and the Capacity to Cope with Stressors in Female and Male House Sparrows. Horm. Behav. 2021, 135, 105038. [Google Scholar] [CrossRef]
- Cussotto, S.; Cryan, J.F.; O’Leary, O.F. The Hippocampus and Dorsal Raphe Nucleus Are Key Brain Areas Associated with the Antidepressant Effects of Lithium Augmentation of Desipramine. Neurosci. Lett. 2017, 648, 14–20. [Google Scholar] [CrossRef]
- Tatro, E.T.; Everall, I.P.; Masliah, E.; Hult, B.J.; Lucero, G.; Chana, G.; Soontornniyomkij, V.; Achim, C.L. HIV Neurobehavioral Research Center Differential Expression of Immunophilins FKBP51 and FKBP52 in the Frontal Cortex of HIV-Infected Patients with Major Depressive Disorder. J. Neuroimmune Pharmacol. 2009, 4, 218–226. [Google Scholar] [CrossRef]
- Smigan, L.; Perris, C. Cortisol Changes in Long-Term Lithium Therapy. Neuropsychobiology 2008, 11, 219–223. [Google Scholar] [CrossRef] [PubMed]


| Group | Number of Animals |
|---|---|
| Baseline—CMS (stress-exposed rats) | 8 |
| Baseline—control (non-stress-exposed rats) | 6 |
| 7 days—lithium (CMS + lithium) | 3 |
| 7 days—control (CMS + water) | 3 |
| 42 days—lithium (CMS + lithium) | 3 |
| 42 days—control (CMS + water) | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, M.; Majewska, W.; Kachel, M.; Dola, A.; Koga, W.; Nowakowska, J.; Langwiński, W.; Szczepankiewicz, A. Lithium Treatment Increases FKBP5 Protein but Not mRNA Expression in the Pituitary Gland of Depressive-like Rats. Brain Sci. 2025, 15, 389. https://doi.org/10.3390/brainsci15040389
Kubiak M, Majewska W, Kachel M, Dola A, Koga W, Nowakowska J, Langwiński W, Szczepankiewicz A. Lithium Treatment Increases FKBP5 Protein but Not mRNA Expression in the Pituitary Gland of Depressive-like Rats. Brain Sciences. 2025; 15(4):389. https://doi.org/10.3390/brainsci15040389
Chicago/Turabian StyleKubiak, Mikołaj, Wiktoria Majewska, Maria Kachel, Antonina Dola, Weronika Koga, Joanna Nowakowska, Wojciech Langwiński, and Aleksandra Szczepankiewicz. 2025. "Lithium Treatment Increases FKBP5 Protein but Not mRNA Expression in the Pituitary Gland of Depressive-like Rats" Brain Sciences 15, no. 4: 389. https://doi.org/10.3390/brainsci15040389
APA StyleKubiak, M., Majewska, W., Kachel, M., Dola, A., Koga, W., Nowakowska, J., Langwiński, W., & Szczepankiewicz, A. (2025). Lithium Treatment Increases FKBP5 Protein but Not mRNA Expression in the Pituitary Gland of Depressive-like Rats. Brain Sciences, 15(4), 389. https://doi.org/10.3390/brainsci15040389







