The Neural Correlates of Consciousness: A Spectral Exponent Approach to Diagnosing Disorders of Consciousness
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Clinical Assessment
2.3. CRS-R Index Calculation
2.4. EEG Acquisition
2.5. EEG Preprocessing
2.6. SE Estimation
2.7. Statistical Analysis
2.8. Follow-Up Assessments
3. Results
3.1. Demographic Characteristics
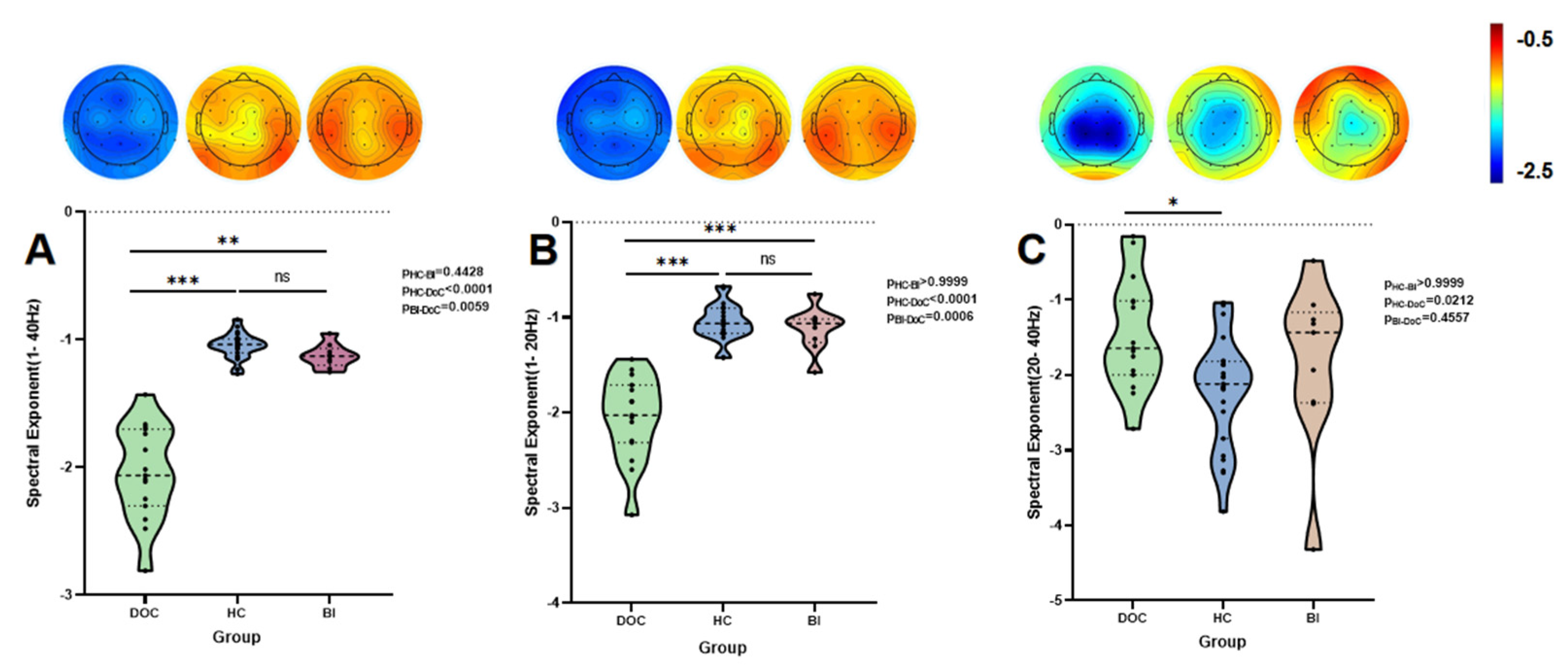
3.2. Group-Level Analysis
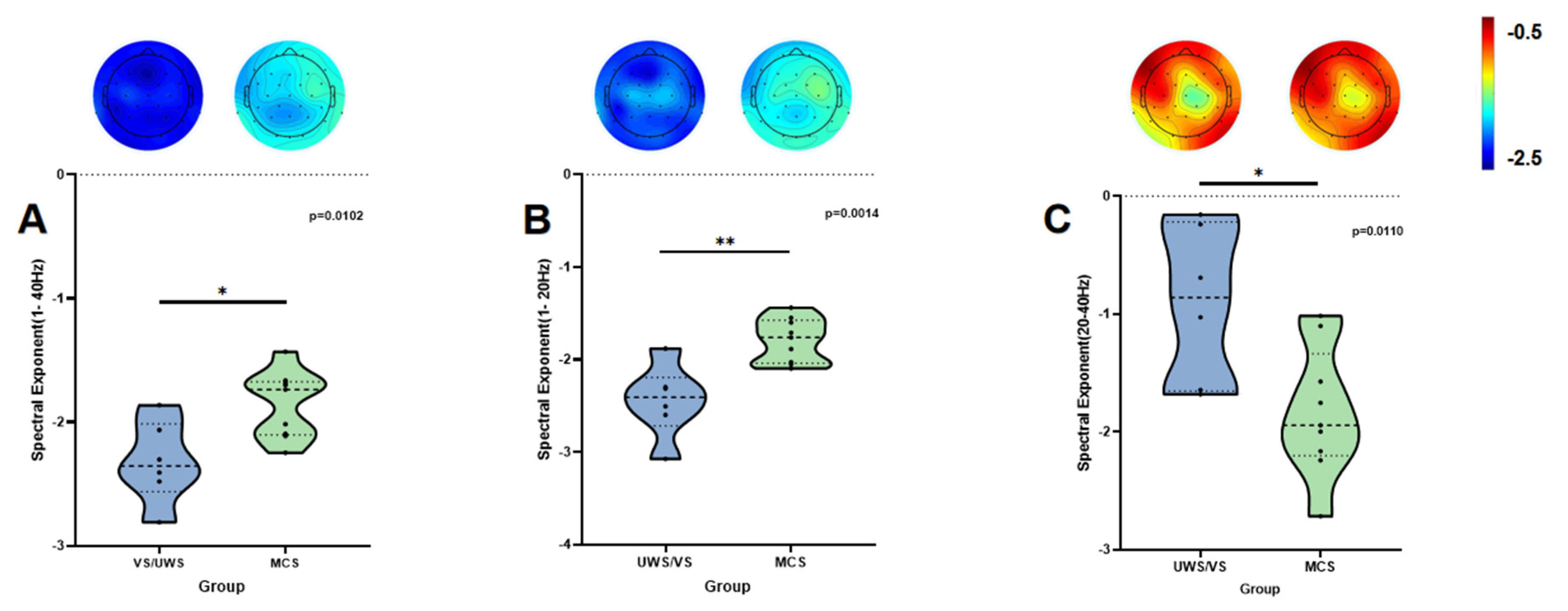
3.3. Results of Subgroup Analysis
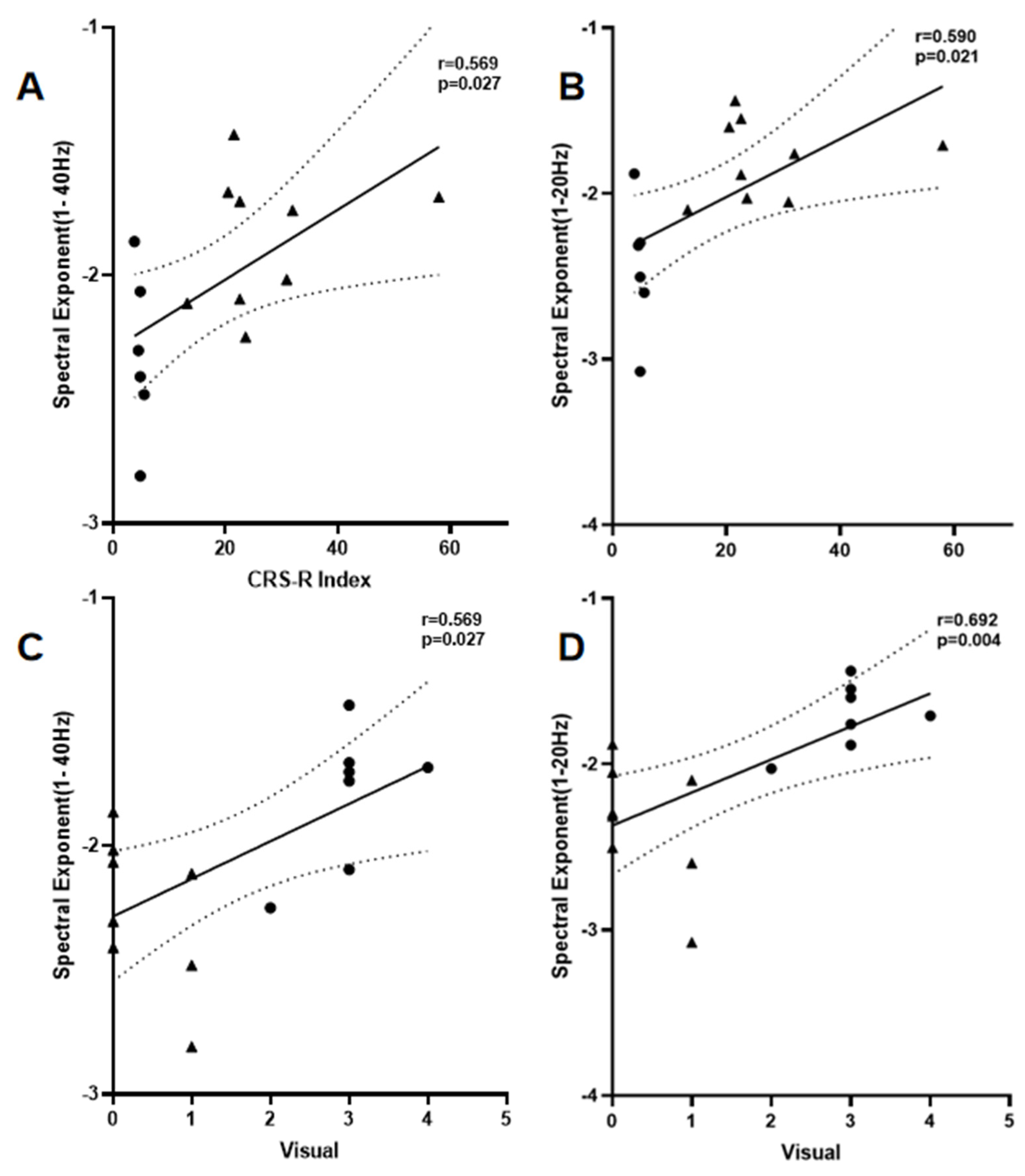
3.4. Correlation Analysis
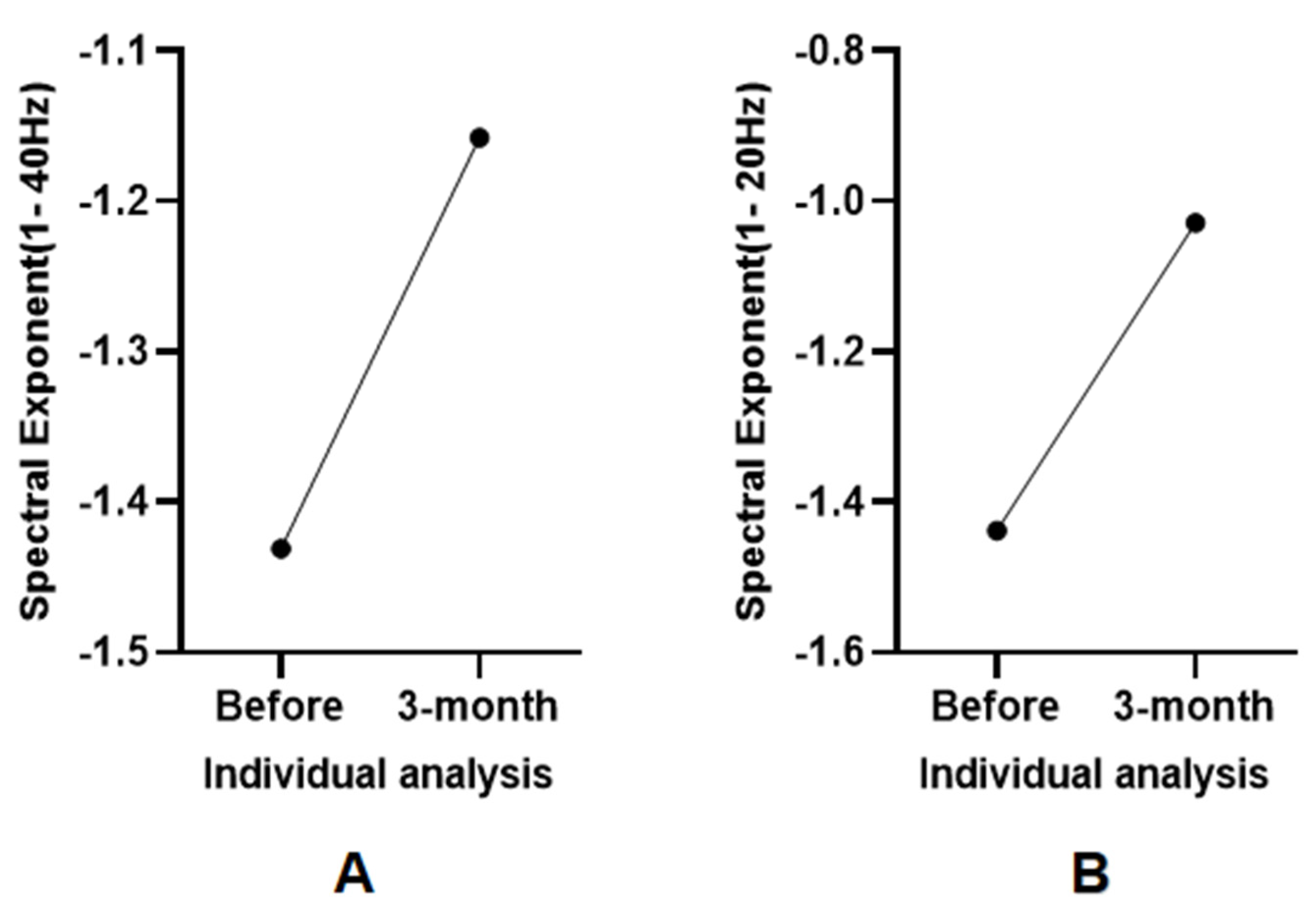
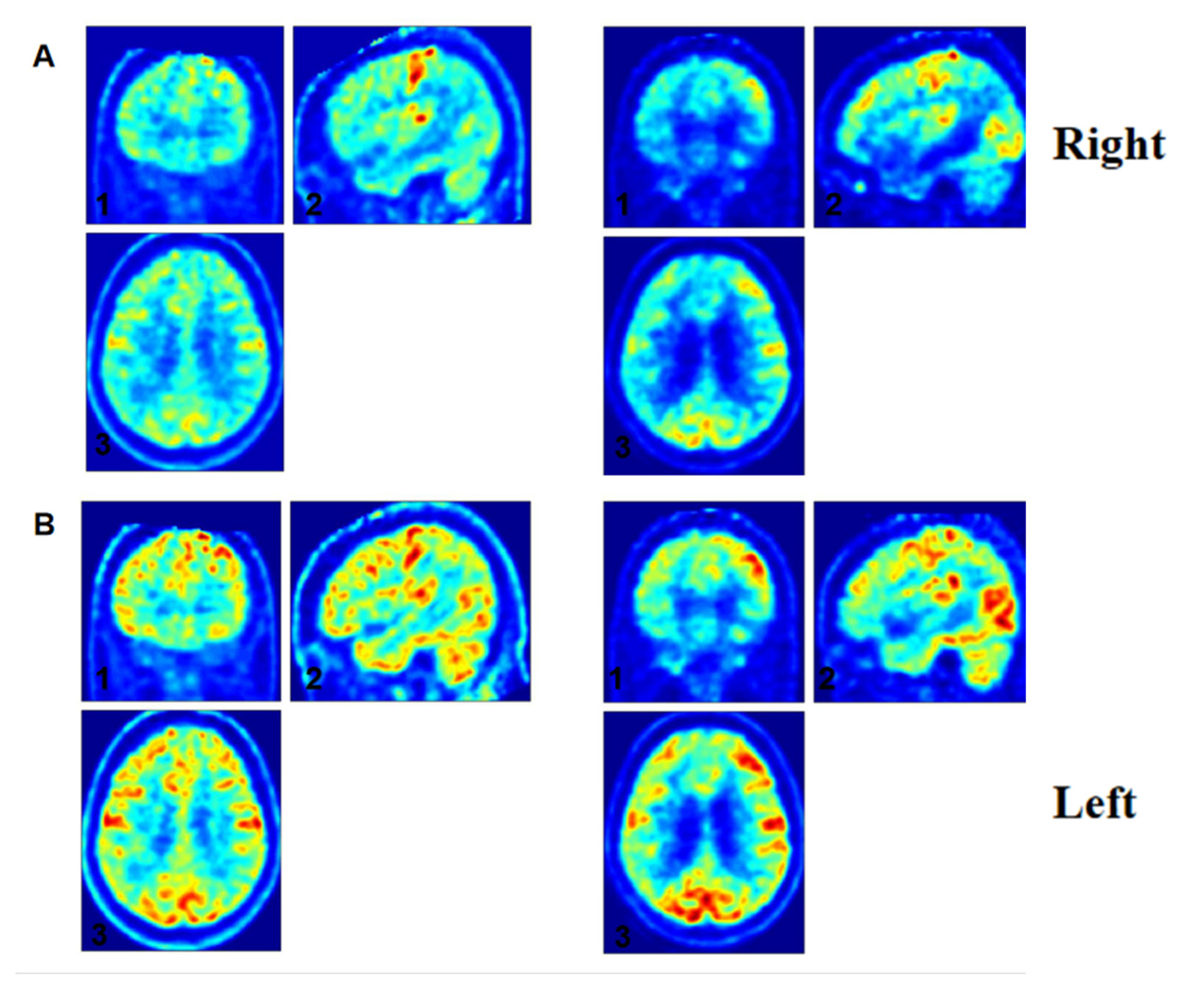
3.5. Individual Analysis
3.6. Individual Electrophysiological-Metabolic Coupling Analysis
4. Discussion
4.1. Neural Mechanisms and Frequency-Dependent Dynamics
4.2. Behavioral Correlates and Alpha Oscillation Mediation
4.3. Prognostic Potential and Neuro-Behavioral Dissociation
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frith, C.D. The neural basis of consciousness. Psychol. Med. 2021, 51, 550–562. [Google Scholar]
- Scammell, T.E.; Arrigoni, E.; Lipton, J. Neural Circuitry of Wakefulness and Sleep. Neuron 2017, 93, 747–765. [Google Scholar] [PubMed]
- Adamantidis, A.R.; Gutierrez Herrera, C.; Gent, T.C. Oscillating circuitries in the sleeping brain. Nat. Rev. Neurosci. 2019, 20, 746–762. [Google Scholar] [PubMed]
- Bodien, Y.G.; Allanson, J.; Cardone, P.; Bonhomme, A.; Carmona, J.; Chatelle, C.; Chennu, S.; Conte, M.; Dehaene, S.; Finoia, P.; et al. Cognitive Motor Dissociation in Disorders of Consciousness. N. Engl. J. Med. 2024, 391, 598–608. [Google Scholar]
- Schnakers, C. Assessing consciousness and cognition in disorders of consciousness. NeuroRehabilitation 2024, 54, 11–21. [Google Scholar]
- Golden, K.; Bodien, Y.G.; Giacino, J.T. Disorders of Consciousness Classification and Taxonomy. Phys. Med. Rehabil. Clin. N. Am. 2024, 35, 15–33. [Google Scholar]
- Brown, E.N.; Lydic, R.; Schiff, N.D. General Anesthesia, Sleep, and Coma. N. Engl. J. Med. 2010, 363, 2638–2650. [Google Scholar]
- Kondziella, D.; Bender, A.; Diserens, K.; van Erp, W.; Estraneo, A.; Formisano, R.; Laureys, S.; Naccache, L.; Ozturk, S.; Rohaut, B.; et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol. 2020, 27, 741–756. [Google Scholar]
- Molteni, E.; Canas, L.D.S.; Briand, M.M.; Estraneo, A.; Font, C.C.; Formisano, R.; Fufaeva, E.; Gosseries, O.; Howarth, R.A.; Lanteri, P.; et al. Scoping Review on the Diagnosis, Prognosis, and Treatment of Pediatric Disorders of Consciousness. Neurology 2023, 101, e581–e593. [Google Scholar] [CrossRef]
- Thibaut, A.; Panda, R.; Annen, J.; Sanz, L.R.; Naccache, L.; Martial, C.; Chatelle, C.; Aubinet, C.; Bonin, E.A.; Barra, A.; et al. Preservation of Brain Activity in Unresponsive Patients Identifies MCS Star. Ann. Neurol. 2021, 90, 89–100. [Google Scholar]
- Young, M.J.; Edlow, B.L.; Bodien, Y.G. Covert consciousness. NeuroRehabilitation 2024, 54, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.; et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018, 91, 450–460. [Google Scholar] [PubMed]
- Casali, A.G.; Gosseries, O.; Rosanova, M.; Boly, M.; Sarasso, S.; Casali, K.R.; Casarotto, S.; Bruno, M.A.; Laureys, S.; Tononi, G.; et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 2013, 5, 198ra105. [Google Scholar] [CrossRef]
- Tremblay, S.; Rogasch, N.C.; Premoli, I.; Blumberger, D.M.; Casarotto, S.; Chen, R.; Di Lazzaro, V.; Farzan, F.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Clinical utility and prospective of TMS–EEG. Clin. Neurophysiol. 2019, 130, 802–844. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, P.B.; Frey, H.P.; Velazquez, A.; Thompson, S.; Brodie, D.; Moitra, V.; Rabani, L.; Park, S.; Agarwal, S.; Falo, M.C.; et al. Dynamic regimes of neocortical activity linked to corticothalamic integrity correlate with outcomes in acute anoxic brain injury after cardiac arrest. Ann. Clin. Transl. Neurol. 2017, 4, 119–129. [Google Scholar] [CrossRef]
- Chennu, S.; Finoia, P.; Kamau, E.; Allanson, J.; Williams, G.B.; Monti, M.M.; Noreika, V.; Arnatkeviciute, A.; Canales-Johnson, A.; Olivares, F.; et al. Spectral Signatures of Reorganised Brain Networks in Disorders of Consciousness. PLoS Comput. Biol. 2014, 10, e1003887. [Google Scholar] [CrossRef]
- Sitt, J.D.; King, J.R.; El Karoui, I.; Rohaut, B.; Faugeras, F.; Gramfort, A.; Cohen, L.; Sigman, M.; Dehaene, S.; Naccache, L. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014, 137 Pt 8, 2258–2270. [Google Scholar] [CrossRef]
- Pani, S.M.; Saba, L.; Fraschini, M. Clinical applications of EEG power spectra aperiodic component analysis: A mini-review. Clin. Neurophysiol. 2022, 143, 1–13. [Google Scholar] [CrossRef]
- Colombo, M.A.; Napolitani, M.; Boly, M.; Gosseries, O.; Casarotto, S.; Rosanova, M.; Brichant, J.F.; Boveroux, P.; Rex, S.; Laureys, S.; et al. The spectral exponent of the resting EEG indexes the presence of consciousness during unresponsiveness induced by propofol, xenon, and ketamine. NeuroImage 2019, 189, 631–644. [Google Scholar] [CrossRef]
- Miskovic, V.; MacDonald, K.J.; Rhodes, L.J.; Cote, K.A. Changes in EEG multiscale entropy and power-law frequency scaling during the human sleep cycle. Hum. Brain Mapp. 2019, 40, 538–551. [Google Scholar] [CrossRef]
- Alnes, S.L.; De Lucia, M.; Rossetti, A.O.; Tzovara, A. Complementary roles of neural synchrony and complexity for indexing consciousness and chances of surviving in acute coma. NeuroImage 2021, 245, 118638. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Peterson, E.J.; Voytek, B. Inferring synaptic excitation/inhibition balance from field potentials. NeuroImage 2017, 158, 70–78. [Google Scholar]
- Lombardi, F.; Herrmann, H.J.; de Arcangelis, L. Balance of excitation and inhibition determines 1/f power spectrum in neuronal networks. Chaos 2017, 27, 047402. [Google Scholar] [PubMed]
- Robinson, P.A.; Rennie, C.J.; Wright, J.J.; Bahramali, H.; Gordon, E.; Rowe, D.L. Prediction of electroencephalographic spectra from neurophysiology. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2001, 63 Pt 1, 021903. [Google Scholar]
- Wannez, S.; Heine, L.; Thonnard, M.; Gosseries, O.; Laureys, S.; Coma Science Group Collaborators. The repetition of behavioral assessments in diagnosis of disorders of consciousness: Repeated CRS-R Assessments for Diagnosis in DOC. Ann. Neurol. 2017, 81, 883–889. [Google Scholar] [CrossRef]
- Annen, J.; Filippini, M.M.; Bonin, E.; Cassol, H.; Aubinet, C.; Carrière, M.; Gosseries, O.; Thibaut, A.; Barra, A.; Wolff, A.; et al. Diagnostic accuracy of the CRS-R index in patients with disorders of consciousness. Brain Inj. 2019, 33, 1409–1412. [Google Scholar] [CrossRef]
- Tononi, G.; Boly, M.; Massimini, M.; Koch, C. Integrated information theory: From consciousness to its physical substrate. Nat. Rev. Neurosci. 2016, 17, 450–461. [Google Scholar]
- Mashour, G.A.; Roelfsema, P.; Changeux, J.P.; Dehaene, S. Conscious Processing and the Global Neuronal Workspace Hypothesis. Neuron 2020, 105, 776–798. [Google Scholar]
- Witkin, J.M.; Shafique, H.; Smith, J.L.; Marini, A.M.; Lipsky, R.H.; Delery, E. Mechanistic and therapeutic relationships of traumatic brain injury and γ-amino-butyric acid (GABA). Pharmacol. Ther. 2024, 256, 108609. [Google Scholar]
- Podvalny, E.; Noy, N.; Harel, M.; Bickel, S.; Chechik, G.; Schroeder, C.E.; Mehta, A.D.; Tsodyks, M.; Malach, R. A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J. Neurophysiol. 2015, 114, 505–519. [Google Scholar]
- Li, Y.; Chen, L.; Zhu, D.; Chen, Y.; Qin, W.; Cui, J. Propofol downregulates the activity of glutamatergic neurons in the basal forebrain via affecting intrinsic membrane properties and postsynaptic GABAARs. Neuroreport 2020, 31, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.A.; Comanducci, A.; Casarotto, S.; Derchi, C.C.; Annen, J.; Viganò, A.; Mazza, A.; Trimarchi, P.D.; Boly, M.; Fecchio, M.; et al. Beyond alpha power: EEG spatial and spectral gradients robustly stratify disorders of consciousness. Cereb. Cortex 2023, 33, 7193–7210. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Kaiser, D.; Cichy, R.M. Visual Imagery and Perception Share Neural Representations in the Alpha Frequency Band. Curr. Biol. 2020, 30, 2621–2627.e5. [Google Scholar] [CrossRef] [PubMed]
- Peylo, C.; Hilla, Y.; Sauseng, P. Cause or consequence? Alpha oscillations in visuospatial attention. Trends Neurosci. 2021, 44, 705–713. [Google Scholar] [CrossRef]
- Barttfeld, P.; Uhrig, L.; Sitt, J.D.; Sigman, M.; Jarraya, B.; Dehaene, S. Signature of consciousness in the dynamics of resting-state brain activity. Proc. Natl. Acad. Sci. USA 2015, 112, 887–892. [Google Scholar] [CrossRef]

| Patient ID | Age | Sex | Etiology | Time Since Injury (Days) | CRS-R Subscale Scores | CRS-R Index | Diagnosis | ABCD EEG Typology |
|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | NTBI | 490 | 0-2-2-1-0-2 | 13.17667 | MCS | B |
| 2 | 55 | M | NTBI | 174 | 1-0-2-1-0-1 | 4.50333 | VS | B |
| 3 | 51 | M | TBI | 419 | 2-4-5-2-0-2 | 57.98667 | MCS | B |
| 4 | 34 | M | NTBI | 62 | 1-0-2-1-0-2 | 4.83667 | VS | B |
| 5 | 16 | M | NTBI | 41 | 1-3-1-1-0-2 | 21.50667 | MCS | B |
| 6 | 49 | M | TBI | 419 | 1-3-2-1-0-2 | 22.54667 | MCS | B |
| 7 | 60 | M | NTBI | 214 | 1-3-2-1-0-1 | 22.21333 | MCS | C |
| 8 | 36 | M | NTBI | 171 | 1-0-2-1-0-1 | 4.50333 | VS | B |
| 9 | 72 | M | TBI | 307 | 1-1-5-1-0-2 | 30.88667 | MCS | B |
| 10 | 50 | F | TBI | 218 | 1-3-2-1-0-1 | 22.21333 | MCS | C |
| 11 | 53 | F | NTBI | 40 | 0-3-2-0-0-2 | 20.46667 | MCS | B |
| 12 | 54 | M | NTBI | 95 | 0-0-2-1-0-2 | 3.79667 | VS | A |
| 13 | 65 | M | TBI | 95 | 1-1-2-1-0-1 | 5.54333 | VS | A |
| 14 | 71 | M | NTBI | 102 | 0-1-2-1-0-2 | 4.83667 | VS | A |
| 15 | 65 | F | TBI | 476 | 1-2-1-1-0-2 | 13.17667 | MCS | B |
| Patient ID | Age | Sex | Etiology | Lesion | Injury Type (Cortical/Subcortical/Combined) |
|---|---|---|---|---|---|
| 1 | 41 | M | TBI | Right frontal lobe and left basal ganglia hematoma | Combined |
| 2 | 37 | F | TBI | Subarachnoid hemorrhage, left hemispheric cerebral hematoma | Combined |
| 3 | 50 | M | NTBI | Right frontal lobe hemorrhage | Cortical |
| 4 | 42 | M | NTBI | Right basal ganglia hemorrhage | Subcortical |
| 5 | 58 | M | NTBI | Left frontal lobe hemorrhage | Cortical |
| 6 | 16 | M | TBI | Left cerebellar hemisphere abnormalities and basilar artery stenosis | Combined |
| 7 | 70 | M | NTBI | Subarachnoid hemorrhage | Subcortical |
| 8 | 49 | M | NTBI | Left basal ganglia hemorrhage | Subcortical |
| 9 | 60 | M | TBI | Bilateral frontal lobe hematomas and subarachnoid hemorrhage | Combined |
| Characteristic | HC Group (N = 23) | BI Group (N = 9) | DoC Group (N = 15) | Statistical Analysis | |
|---|---|---|---|---|---|
| Age (years) mean ± std | 51.2 ± 12.2 | 47.0 ± 15.6 | 53.3 ± 15.4 | F(2) = 0.580 p = 0.564 | |
| Sex (%) | Male | 6 (26.1%) | 8 (88.9%) | 12 (80.0%) | χ2(2) = 15.752 p < 0.001 *** |
| Female | 17 (73.9%) | 1 (11.1%) | 3 (20.0%) | ||
| Etiology (%) | TBI | 4 (44.4%) | 6 (40%) | χ2(1) = 0.667 p = 0.541 | |
| NTBI | 5 (55.6%) | 9 (60%) | |||
| Time since injury (days) mean ± SD | 109.33 ± 79.07 | 221.53 ± 161.31 | U = 37.00 p = 0.069 |
| Frequency | HC Group | BI Group | DoC Group | H | p | Post Hoc Comparisons (Bonferroni-Adjusted) | BF10 | η² |
|---|---|---|---|---|---|---|---|---|
| β1–40 Hz | −1.038 (0.109) | −1.129 (0.131) | −2.064 (0.601) | 32.097 | 1.072 × 10−7 | HC > DoC (p < 0.001) ***, BI > DoC (p = 0.0059) * | 5.632 × 1013 | 0.81 |
| β1–20 Hz | −1.063 (0.266) | −1.063 (0.246) | −2.028 (0.604) | 29.738 | 3.487 × 10−7 | HC > DoC (p < 0.001) ***, BI > DoC (p = 0.0006) *** | 5.996 × 109 | 0.71 |
| β20–40 Hz | −2.118 (1.026) | −1.435 (1.203) | −1.643 (0.982) | 7.579 | 0.023 | DoC < HC (p = 0.0212) * | 2.834 | 0.15 |
| MCS | VS/UWS | t | p | BF10 | Cohen’s d | |
|---|---|---|---|---|---|---|
| β1–40 Hz | −1.854 ± 0.271 | −2.321 ± 0.331 | −3.000 | 0.0102 | 5.096 | 1.580 |
| β1–20 Hz | −1.791 ± 0.238 | −2.445 ± 0.395 | −4.025 | 0.0014 | 21.612 | 2.121 |
| β20–40 Hz | −1.832 ± 0.545 | −0.906 ± 0.665 | 2.961 | 0.0110 | 4.839 | −1.561 |
| Parameter | CRS-R Index | Visual Subscale | ||
|---|---|---|---|---|
| r | p | r | p | |
| β1–40 Hz | 0.569 * | 0.027 | 0.566 * | 0.027 |
| β1–20 Hz | 0.590 * | 0.021 | 0.684 ** | 0.004 |
| β20–40 Hz | −0.459 | 0.085 | −0.631 * | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, A.; Zhao, W.; Hu, N.; Laureys, S.; Di, H. The Neural Correlates of Consciousness: A Spectral Exponent Approach to Diagnosing Disorders of Consciousness. Brain Sci. 2025, 15, 377. https://doi.org/10.3390/brainsci15040377
Zhao Y, Wang A, Zhao W, Hu N, Laureys S, Di H. The Neural Correlates of Consciousness: A Spectral Exponent Approach to Diagnosing Disorders of Consciousness. Brain Sciences. 2025; 15(4):377. https://doi.org/10.3390/brainsci15040377
Chicago/Turabian StyleZhao, Ying, Anqi Wang, Weiqiao Zhao, Nantu Hu, Steven Laureys, and Haibo Di. 2025. "The Neural Correlates of Consciousness: A Spectral Exponent Approach to Diagnosing Disorders of Consciousness" Brain Sciences 15, no. 4: 377. https://doi.org/10.3390/brainsci15040377
APA StyleZhao, Y., Wang, A., Zhao, W., Hu, N., Laureys, S., & Di, H. (2025). The Neural Correlates of Consciousness: A Spectral Exponent Approach to Diagnosing Disorders of Consciousness. Brain Sciences, 15(4), 377. https://doi.org/10.3390/brainsci15040377




