Stroke in Young Adults: An Overview and Non-Pharmacological Preventive Strategies
Abstract
1. Introduction
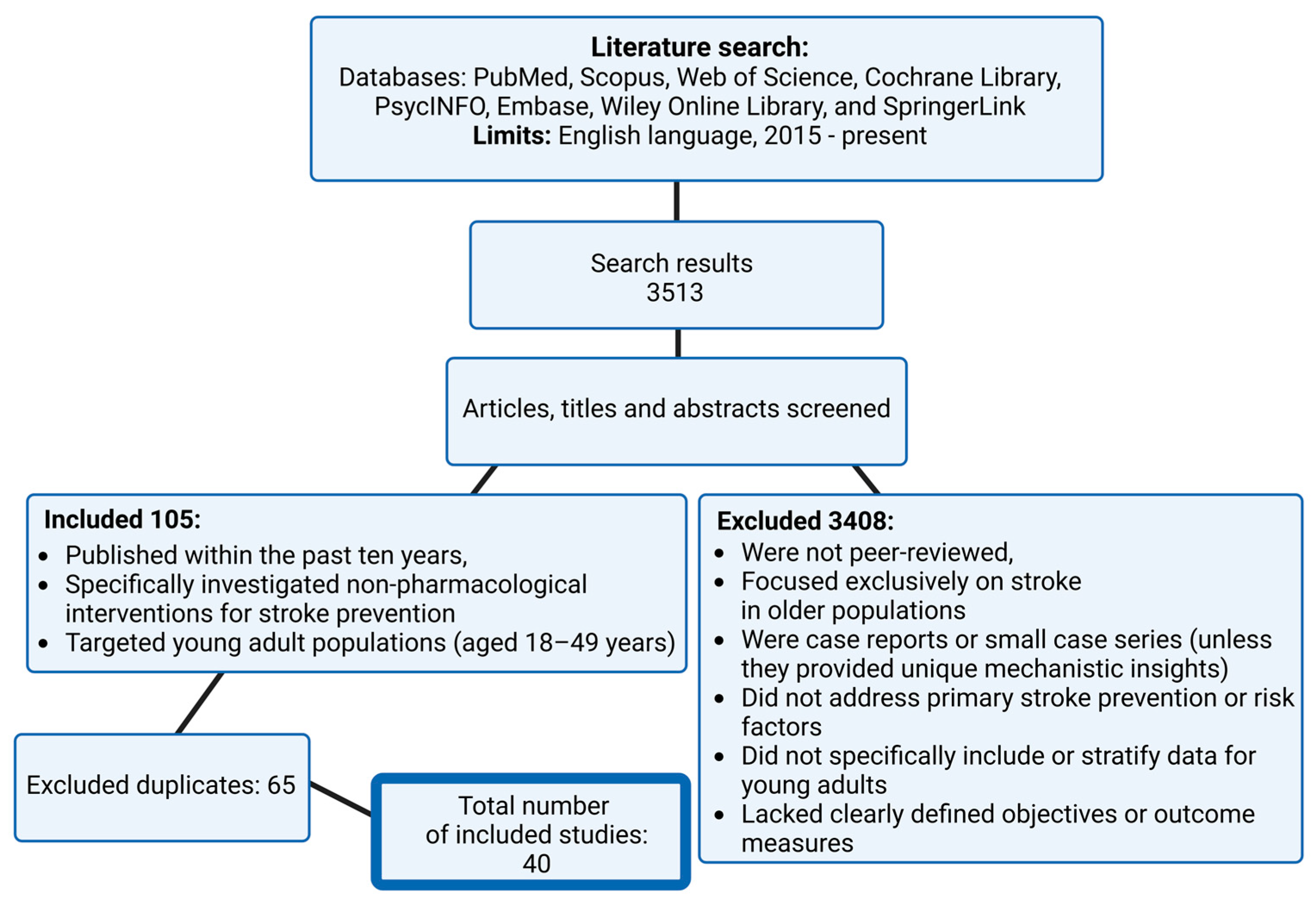
2. Materials and Methods
3. Overview of Strokes
3.1. Definition and Classification
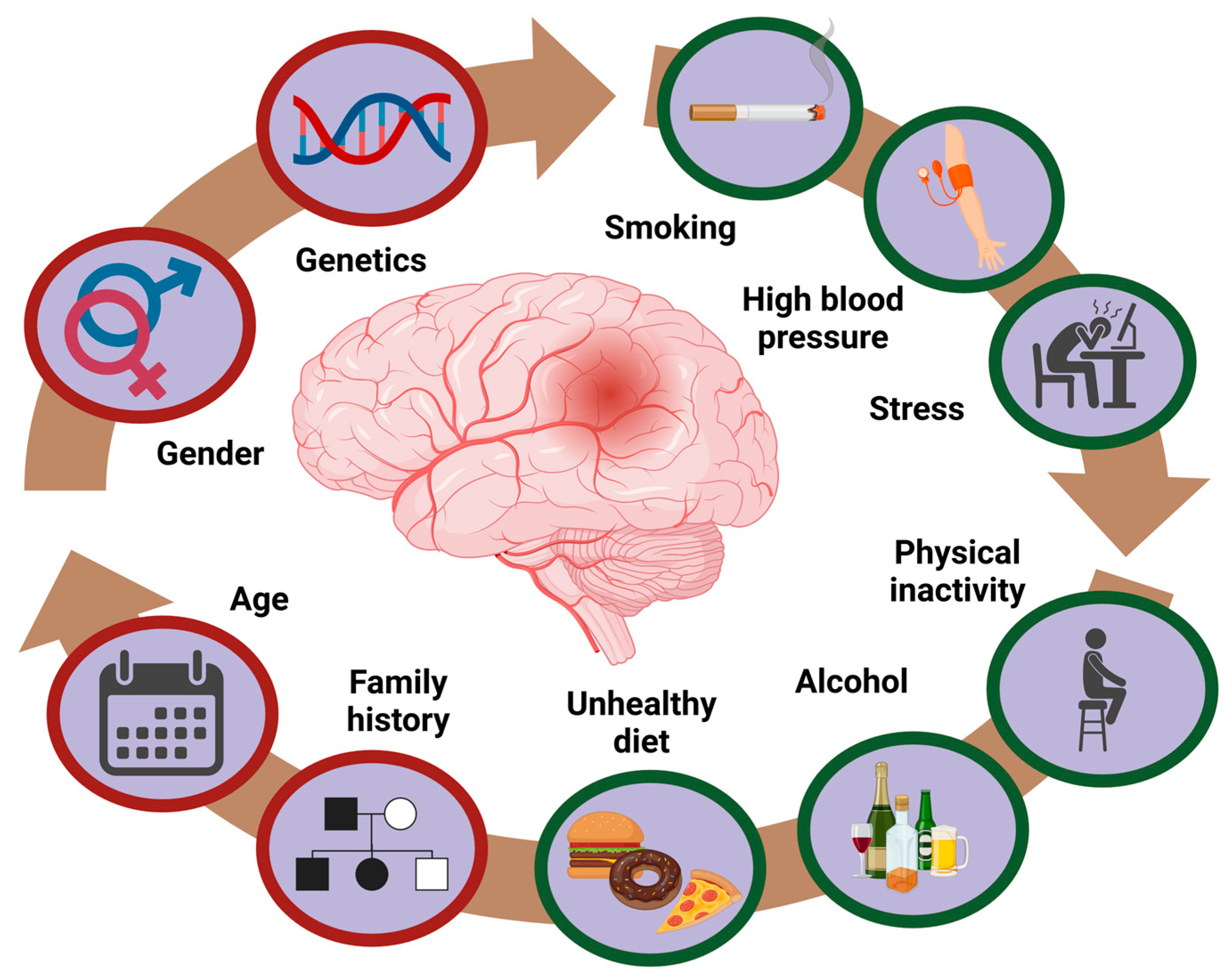
3.2. Epidemiology and Risk Factors
Autosomal Dominant Polycystic Kidney Disease (ADPKD) as a Significant Stroke Risk in Young Adults
3.3. Screening and Early Detection
4. Clinical Studies on Stroke in Young Adults: Treatment and Outcomes
4.1. Comparative Insights: Stroke in Younger vs. Older Adults
4.2. Comparative Summary of Non-Pharmacological Strategies in Young Adults
5. Dietary and Lifestyle Modifications
6. Physical Activity Increase
7. Stress Reduction
8. Cessation of Smoking
9. Mental Health Improvement
10. Public Health Initiatives and Education
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, Regional, and National Burden of Stroke, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar]
- Bukhari, S.; Yaghi, S.; Bashir, Z. Stroke in Young Adults. J. Clin. Med. 2023, 12, 4999. [Google Scholar] [CrossRef] [PubMed]
- Smajlović, D. Strokes in Young Adults: Epidemiology and Prevention. Vasc. Health Risk Manag. 2015, 11, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Yahya, T.; Jilani, M.H.; Khan, S.U.; Mszar, R.; Hassan, S.Z.; Blaha, M.J.; Blankstein, R.; Virani, S.S.; Johansen, M.C.; Vahidy, F.; et al. Stroke in Young Adults: Current Trends, Opportunities for Prevention, and Pathways Forward. Am. J. Prev. Cardiol. 2020, 3, 100085. [Google Scholar] [CrossRef]
- Rutten-Jacobs, L.C.; Arntz, R.M.; Maaijwee, N.A.; Schoonderwaldt, H.C.; Dorresteijn, L.D.; van Dijk, E.J.; de Leeuw, F.E. Long-Term Mortality after Stroke among Adults Aged 18 to 50 Years. JAMA 2013, 309, 1136–1144. [Google Scholar] [CrossRef]
- Dopler, B. Stroke Prevention. Del. J. Public Health 2023, 9, 6–10. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.B.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef]
- Murphy, S.J.; Werring, D.J. Stroke: Causes and Clinical Features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef]
- Tadi, P.; Lui, F. Acute Stroke. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lioutas, V.; Ivan, C.S.; Himali, J.J.; Aparicio, H.J.; Leveille, T.; Romero, J.R.; Beiser, A.S.; Seshadri, S. Incidence of Transient Ischemic Attack and Association with Long-Term Risk of Stroke. JAMA 2021, 325, 373–381. [Google Scholar] [CrossRef]
- Nassif, M.; Annink, M.E.; Yang, H.; Rettig, T.; Roos, Y.; van den Brink, R.; Tijssen, J.; Mulder, B.; de Winter, R.J.; Bouma, B.J. Long-Term (>10-Year) Clinical Follow-up after Young Embolic Stroke/TIA of Undetermined Source. Int. J. Stroke 2021, 16, 7–11. [Google Scholar] [CrossRef]
- Gore, M.; Bansal, K.; Khan, S.M.Z.; Lui, F.; Asuncion, R.M.D. Lacunar Stroke. In StatPearls; StatPearls Publishing: Treasure Island FL, USA, 2024; Available online: https://www.statpearls.com (accessed on 20 February 2025).
- Palacio, S.; McClure, L.A.; Benavente, O.R.; Bazan, C.; Pergola, P.; Hart, R.G. Lacunar Strokes in Patients with Diabetes Mellitus: Risk Factors, Infarct Location, and Prognosis. Stroke 2014, 45, 2689–2694. [Google Scholar] [CrossRef]
- Katsnelson, M.J.; Della-Morte, D.; Rundek, T. Stroke in young. Period. Biol. 2012, 114, 347–353. [Google Scholar]
- Yew, K.S.; Cheng, E.M. Diagnosis of Acute Stroke. Am. Fam. Physician 2015, 91, 528–536. [Google Scholar] [PubMed]
- Andersson, J.; Rejnö, Å.; Jakobsson, S.; Hansson, P.-O.; Nielsen, S.J. Symptoms at Stroke Onset as Described by Patients: A Qualitative Study. BMC Neurol. 2024, 24, 150. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of Subtype of Acute Ischemic Stroke: Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Drozdowska, B.A.; Singh, S.; Quinn, T.J. Thinking About the Future: A Review of Prognostic Scales Used in Acute Stroke. Front. Neurol. 2019, 10, 274. [Google Scholar] [CrossRef]
- Pandian, J.D.; Gall, S.L.; Kate, M.P.; Silva, G.S.; Akinyemi, R.O.; Ovbiagele, B.I.; Lavados, P.M.; Gandhi, D.B.C.; Thrift, A.G. Prevention of Stroke: A Global Perspective. Lancet 2018, 392, 1269–1278. [Google Scholar] [CrossRef]
- Guzik, A.; Bushnell, C. Stroke Epidemiology and Risk Factor Management. Continuum 2017, 23, 15–39. [Google Scholar] [CrossRef]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97 (Suppl. S2), S6–S16. [Google Scholar] [CrossRef]
- Stefanovic Budimkic, M.; Pekmezovic, T.; Beslac-Bumbasirevic, L.; Ercegovac, M.; Berisavac, I.; Stanarcevic, P.; Padjen, V.; Jovanović, D.R. Long-Term Prognosis in Ischemic Stroke Patients Treated with Intravenous Thrombolytic Therapy. J. Stroke Cerebrovasc. Dis. 2017, 26, 196–203. [Google Scholar] [CrossRef]
- Pu, L.; Wang, L.; Zhang, R.; Zhao, T.; Jiang, Y.; Han, L. Projected Global Trends in Ischemic Stroke Incidence, Deaths and Disability-Adjusted Life Years from 2020 to 2030. Stroke 2023, 54, 1330–1339, Erratum in: Stroke 2024, 55, e23. [Google Scholar] [CrossRef] [PubMed]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef] [PubMed]
- Aigner, A.; Grittner, U.; Rolfs, A.; Norrving, B.; Siegerink, B.; Busch, M.A. Contribution of Established Stroke Risk Factors to the Burden of Stroke in Young Adults. Stroke 2017, 48, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Aradine, E.M.; Ryan, K.A.; Cronin, C.A.; Wozniak, M.A.; Cole, J.W.; Chaturvedi, S.; Dutta, T.L.M.; Hou, Y.; Mehndiratta, P.; Motta, M.; et al. Black-White Differences in Ischemic Stroke Risk Factor Burden in Young Adults. Stroke 2022, 53, e66–e69. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Whelton, P.K.; Sorond, F.; Carey, R.M. Blood Pressure Management in Stroke. Hypertension 2020, 76, 1688–1695. [Google Scholar] [CrossRef]
- Li, A.; Ji, Y.; Zhu, S.; Hu, Z.-H.; Xu, X.-J.; Wang, Y.-W.; Jian, X.-Z. Risk Probability and Influencing Factors of Stroke in Followed-up Hypertension Patients. BMC Cardiovasc. Disord. 2022, 22, 328. [Google Scholar] [CrossRef]
- Smyth, A.; O’Donnell, M.; Rangarajan, S.; Hankey, G.J.; Oveisgharan, S.; Canavan, M.; McDermott, C.; Xavier, D.; Zhang, H.; Damasceno, A.; et al. Alcohol Intake as a Risk Factor for Acute Stroke: The INTERSTROKE Study. Neurology 2023, 100, e142–e153. [Google Scholar] [CrossRef]
- Berger, K.; Ajani, U.A.; Kase, C.S.; Gaziano, J.M.; Buring, J.E.; Glynn, R.J.; Hennekens, C.H. Light-to-Moderate Alcohol Consumption and the Risk of Stroke Among U.S. Male Physicians. N. Engl. J. Med. 1999, 341, 1557–1564. [Google Scholar] [CrossRef]
- Iso, H.; Baba, S.; Mannami, T.; Sasaki, S.; Okada, K.; Konishi, M.; Tsugane, S.; JPHC Study Group. Alcohol Consumption and Risk of Stroke Among Middle-Aged Men: The JPHC Study Cohort I. Stroke 2004, 35, 1124–1129. [Google Scholar] [CrossRef]
- George, M.G. Risk Factors for Ischemic Stroke in Younger Adults: A Focused Update. Stroke 2020, 51, 729–735. [Google Scholar] [CrossRef]
- Khan, M.; Wasay, M.; O’Donnell, M.J.; Iqbal, R.; Langhorne, P.; Rosengren, A.; Damasceno, A.; Oguz, A.; Lanas, F.; Pogosova, N.; et al. Risk Factors for Stroke in the Young (18–45 Years): A Case-Control Analysis of INTERSTROKE Data from 32 Countries. Neuroepidemiology 2023, 57, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.W.; Yu, T.M.; Huang, S.T.; Sun, K.T.; Lo, Y.C.; Fu, P.K.; Lee, B.J.; Chen, C.H.; Lin, C.L.; Kao, C.H. Young-Adult Polycystic Kidney Disease is Associated with Major Cardiovascular Complications. Int. J. Environ. Res. Public Health 2018, 15, 903. [Google Scholar] [CrossRef] [PubMed]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of Unruptured Intracranial Aneurysms, with Emphasis on Sex, Age, Comorbidity, Country, and Time Period: A Systematic Review and Meta-Analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Chebib, F.T.; Tawk, R.G. All Patients with ADPKD Should Undergo Screening for Intracranial Aneurysms: CON. Kidney360 2024, 5, 495–498. [Google Scholar] [CrossRef]
- Hung, P.H.; Lin, C.H.; Hung, K.Y.; Muo, C.H.; Chung, M.C.; Chang, C.H.; Chung, C.J. Clinical Burden of Autosomal Dominant Polycystic Kidney Disease. Aging 2020, 12, 3899–3910. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) ADPKD Work Group. KDIGO 2025 Clinical Practice Guideline for the Evaluation, Management, and Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD). Kidney Int. 2025, 107, S1–S239. [Google Scholar] [CrossRef]
- Liao, C.-H.; Lin, Y.-T.; Tsai, Y.-H.; Chien, H.-Y.; Chen, C.-C. The Impact of Autosomal Dominant Polycystic Kidney Disease on the Presence of Cerebral Microbleeds: A Case-Control Matched Study. J. Stroke Cerebrovasc. Dis. 2025, 34, 107663. [Google Scholar] [CrossRef]
- Kelleher, C.L.; McFann, K.K.; Johnson, A.M.; Schrier, R.W. Characteristics of Hypertension in Young Adults with Autosomal Dominant Polycystic Kidney Disease Compared with the General U.S. Population. Am. J. Hypertens. 2004, 17, 1029–1034. [Google Scholar] [CrossRef]
- Martínez, V.; Furlano, M.; Sans, L.; Pulido, L.; García, R.; Pérez-Gómez, M.V.; Sánchez-Rodríguez, J.; Blasco, M.; Castro-Alonso, C.; Fernández-Fresnedo, G.; et al. Autosomal Dominant Polycystic Kidney Disease in Young Adults. Clin. Kidney J. 2022, 16, 985–995. [Google Scholar] [CrossRef]
- Zhang, Y.; He, D.; Zhang, W.; Xing, Y.; Guo, Y.; Wang, F.; Jia, J.; Yan, T.; Liu, Y.; Lin, S. ACE Inhibitor Benefit to Kidney and Cardiovascular Outcomes for Patients with Non-Dialysis Chronic Kidney Disease Stages 3–5: A Network Meta-Analysis of Randomised Clinical Trials. Drugs 2020, 80, 797–811. [Google Scholar] [CrossRef]
- Kataoka, H.; Akagawa, H.; Ushio, Y.; Sato, M.; Manabe, S.; Makabe, S.; Kawachi, K.; Akihisa, T.; Iwasa, N.; Yoshida, R.; et al. Mutation Type and Intracranial Aneurysm Formation in Autosomal Dominant Polycystic Kidney Disease. Stroke Vasc. Interv. Neurol. 2022, 2, e000203. [Google Scholar] [CrossRef]
- Zuurbier, C.; Greving, J.P.; Rinkel, G.; Ruigrok, Y.M. Higher Risk of Intracranial Aneurysms and Subarachnoid Haemorrhage in Siblings of Families with Intracranial Aneurysms. Eur. Stroke J. 2020, 5, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Mensing, L.A.; van Tuijl, R.J.; de Kort, G.A.; van der Schaaf, I.C.; Visseren, F.L.; Rinkel, G.J.; Velthuis, B.K.; Ruigrok, Y.M.; UCC-SMART Study Group. Screening for Intracranial Aneurysms in Persons ≥35 Years with Hypertension and Atherosclerotic Disease Who Smoke(d). Eur. Stroke J. 2023, 8, 1071–1078. [Google Scholar] [CrossRef]
- Catania, M.; De Rosa, L.I.; Kola, K.; Brambilla Pisoni, M.; Manunta, P.; Vezzoli, G.; Sciarrone Alibrandi, M.T. Early Detection Matters: Bridging Evidence and Practice, a Call for Enhanced Cardiovascular Screening in ADPKD. Kidney Int. Rep. 2024, 10, 622. [Google Scholar] [CrossRef]
- Boot, E.; Ekker, M.S.; Putaala, J.; Kittner, S.; De Leeuw, F.-E.; Tuladhar, A.M. Ischaemic Stroke in Young Adults: A Global Perspective. J. Neurol. Neurosurg. Psychiatry 2020, 91, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Stack, C.A.; Cole, J.W. Stroke in Young Adults. In Stroke; Dehkharghani, S., Ed.; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572004/ (accessed on 25 March 2025).
- Singhal, A.B.; Biller, J.; Elkind, M.S.; Fullerton, H.J.; Jauch, E.C.; Kittner, S.J.; Levine, D.A.; Levine, S.R. Recognition and Management of Stroke in Young Adults and Adolescents. Neurology 2013, 81, 1089–1097. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Xu, F.; Guo, M.; Yang, Y.; Zhong, L.; Weng, X.; Liu, X. A Systematic Review and Meta-Analysis Comparing FAST and BEFAST in Acute Stroke Patients. Front. Neurol. 2022, 12, 765069. [Google Scholar] [CrossRef]
- Lin, S.F.; Chen, C.I.; Hu, H.H.; Bai, C.-H. Predicting Functional Outcomes of Posterior Circulation Acute Ischemic Stroke in First 36 h of Stroke Onset. J. Neurol. 2018, 265, 926–932. [Google Scholar] [CrossRef]
- Brouwer, J.; Smaal, J.A.; Emmer, B.J.; de Ridder, I.R.; van den Wijngaard, I.R.; de Leeuw, F.E.; Hofmeijer, J.; van Zwam, W.H.; Martens, J.M.; Roos, Y.B.W.E.M.; et al. Endovascular Thrombectomy in Young Patients With Stroke: A MR CLEAN Registry Study. Stroke 2022, 53, 34–42. [Google Scholar] [CrossRef]
- Putaala, J.; Metso, T.M.; Metso, A.J.; Mäkelä, E.; Haapaniemi, E.; Salonen, O.; Kaste, M.; Tatlisumak, T. Thrombolysis in Young Adults With Ischemic Stroke. Stroke 2009, 40, 2085–2091. [Google Scholar] [CrossRef]
- Dutta, T.; Ryan, K.A.; Thompson, O.; Lopez, H.; Fecteau, N.; Sparks, M.J.; Chaturvedi, S.; Cronin, C.; Mehndiratta, P.; Nunez Gonzalez, J.R.; et al. Marijuana Use and the Risk of Early Ischemic Stroke: The Stroke Prevention in Young Adults Study. Stroke 2021, 52, 3184–3190. [Google Scholar] [CrossRef] [PubMed]
- Aycock, D.M.; Clark, P.C.; Hayat, M.J.; Salazar, L.F.; Eriksen, M.P. Stroke Counseling Intervention for Young Adult African Americans: A Randomized Controlled Trial. Nurs. Res. 2023, 72, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.H.S.; Chau, J.P.C.; Choi, K.C.; Shum, E.W.C.; Yeung, J.H.M.; Li, S.H. Promoting Community Reintegration Using Narratives and Skills Building for Young Adults with Stroke: A Protocol for a Randomised Controlled Trial. BMC Neurol. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.P.; Lo, T.L.T.; Wan, A.H.Y.; Leung, P.P.Y.; Pang, M.Y.C. A Randomised Controlled Trial of Expressive Arts-Based Intervention for Young Stroke Survivors. BMC Complement. Med. Ther. 2021, 21, 7. [Google Scholar] [CrossRef]
- Li, L.; Scott, C.A.; Rothwell, P.M. Association of Younger vs. Older Ages with Changes in Incidence of Stroke and Other Vascular Events, 2002–2018. JAMA 2022, 328, 563–574. [Google Scholar] [CrossRef]
- Huggins, H.E.; Brady, M.; Emma, J.P.; Thaler, D.E.; Leung, L.Y. Differences in Presenting Symptoms of Acute Stroke among Young and Older Adults. J. Stroke Cerebrovasc. Dis. 2020, 29, 104871. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Reeves, M.J.; Zhao, X.; Olson, D.M.; Smith, E.E.; Saver, J.L.; Schwamm, L.H.; Get With the Guidelines-Stroke Steering Committee and Investigators. Age-Related Differences in Characteristics, Performance Measures, Treatment Trends, and Outcomes in Patients with Ischemic Stroke. Circulation 2010, 121, 879–891. [Google Scholar] [CrossRef]
- Lutski, M.; Zucker, I.; Shohat, T.; Tanne, D. Characteristics and Outcomes of Young Patients with First-Ever Ischemic Stroke Compared to Older Patients: The National Acute Stroke ISraeli Registry. Front. Neurol. 2017, 8, 421. [Google Scholar] [CrossRef]
- Patel, U.K.; Dave, M.; Lekshminarayanan, A.; Malik, P.; DeMasi, M.; Chandramohan, S.; Pillai, S.; Tirupathi, R.; Shah, S.; Jani, V.B.; et al. Risk Factors and Incidence of Acute Ischemic Stroke: A Comparative Study Between Young Adults and Older Adults. Cureus 2021, 13, e14670. [Google Scholar] [CrossRef]
- Matuja, S.S.; Munseri, P.; Khanbhai, K. The Burden and Outcomes of Stroke in Young Adults at a Tertiary Hospital in Tanzania: A Comparison with Older Adults. BMC Neurol. 2020, 20, 206. [Google Scholar] [CrossRef]
- Moosa, A.; Osama, D.; Alnidawi, F.; Algillidary, S.; Hussein, A.; Das, P. Risk Factors, Incidence, and Outcome of Stroke: A Retrospective Cross-Sectional Hospital-Based Study Comparing Young Adults and Elderly. Cureus 2023, 15, e40614. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D. Diet for Stroke Prevention. Stroke Vasc. Neurol. 2018, 3, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Reddin, C.; Murphy, R.; Hankey, G.J.; Judge, C.; Xavier, D.; Rosengren, A.; Ferguson, J.; Alvarez-Iglesias, A.; Oveisgharan, S.; Iversen, H.K.; et al. Association of Psychosocial Stress with Risk of Acute Stroke. JAMA Netw. Open 2022, 5, e2244836. [Google Scholar] [CrossRef] [PubMed]
- Sobalska, A.; Tomczyk, K.; Furman, J.; Łabuz-Roszak, B. Assessment of Adult Eating Habits in the Nutritional Prevention of Stroke. Wiad. Lek. 2020, 73, 1904–1908. [Google Scholar] [CrossRef]
- Mälstam, E.; Asaba, E.; Åkesson, E.; Guidetti, S.; Patomella, A.-H. The Feasibility of Make My Day—A Randomized Controlled Pilot Trial of a Stroke Prevention Program in Primary Healthcare. Int. J. Environ. Res. Public Health 2023, 20, 6828. [Google Scholar] [CrossRef]
- Von Sarnowski, B.; Putaala, J.; Grittner, U.; Gaertner, B.; Schminke, U.; Curtze, S.; Huber, R.; Tanislav, C.; Hölscher, T.; Engelhorn, T.; et al. Lifestyle Risk Factors for Ischemic Stroke and Transient Ischemic Attack in Young Adults in the Stroke in Young Fabry Patients Study. Stroke 2013, 44, 119–125. [Google Scholar] [CrossRef]
- Loucks, E.B.; Schuman-Olivier, Z.; Britton, W.B.; Fresco, D.M.; Desbordes, G.; Brewer, J.A.; Fulwiler, C. Mindfulness and Cardiovascular Disease Risk: State of the Evidence, Plausible Mechanisms, and Theoretical Framework. Curr. Cardiol. Rep. 2015, 17, 112. [Google Scholar] [CrossRef]
- Calderone, A.; Latella, D.; Impellizzeri, F.; de Pasquale, P.; Famà, F.; Quartarone, A.; Calabrò, R.S. Neurobiological Changes Induced by Mindfulness and Meditation: A Systematic Review. Biomedicines 2024, 12, 2613. [Google Scholar] [CrossRef]
- Markidan, J.; Cole, J.W.; Cronin, C.A.; Merino, J.G.; Phipps, M.S.; Wozniak, M.A.; Kittner, S.J. Smoking and Risk of Ischemic Stroke in Young Men. Stroke 2018, 49, 1276–1278. [Google Scholar] [CrossRef]
- Fisher, M.; Lees, K.; Spence, J.D. Nutrition and Stroke Prevention. Stroke 2006, 37, 2430–2435. [Google Scholar]
- Amoah, D.; Schmidt, M.; Mather, C.; Prior, S.; Herath, M.P.; Bird, M.-L. An International Perspective on Young Stroke Incidence and Risk Factors: A Scoping Review. BMC Public Health 2024, 24, 1627. [Google Scholar] [CrossRef]
- Dearborn, J.L.; Urrutia, V.C.; Kernan, W.N. The Case for Diet: A Safe and Efficacious Strategy for Secondary Stroke Prevention. Front. Neurol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Michalakis, K.; Miras, A.; Hatzitolios, A.; Savopoulos, C. Nutrition in the Primary and Secondary Prevention of Stroke. Maturitas 2012, 72, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Si Larbi, M.; Lahmidi, S.; Boukari, A.K.; Boukeloua, A. Ischemic and Non-ischemic Stroke in Young Adults—A Look at Risk Factors and Outcome in a Developing Country. Cureus 2021, 13, e17079. [Google Scholar] [CrossRef]
- Martens, L.G.; Luo, J.; Willems van Dijk, K.; Jukema, J.W.; Noordam, R.; van Heemst, D. Diet-Derived Antioxidants Do Not Decrease Risk of Ischemic Stroke: A Mendelian Randomization Study in 1 Million People. J. Am. Heart Assoc. 2021, 10, e022567. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Hernán, M.A.; Giovannucci, E.; Kawachi, I.; Stampfer, M.J.; Willett, W.C. Relation of Consumption of Vitamin E, Vitamin C, and Carotenoids to Risk for Stroke Among Men in the United States. Ann. Intern. Med. 1999, 130, 963–970. [Google Scholar]
- Boccardi, V.; Tagliafico, L.; Persia, A.; Page, E.; Ottaviani, S.; Cremonini, A.L.; Borgarelli, C.; Pisciotta, L.; Mecocci, P.; Nencioni, A.; et al. The Potential Effects of Red Wine and Its Components on Neurocognitive Disorders: A Narrative Review. Nutrients 2024, 16, 3431. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt Intake, Stroke, and Cardiovascular Disease: Meta-Analysis of Prospective Studies. BMJ 2009, 339, b4567. [Google Scholar]
- Sarikaya, H.; Ferro, J.; Arnold, M. Stroke Prevention—Medical and Lifestyle Measures. Eur. Neurol. 2015, 73, 150–157. [Google Scholar]
- Prior, P.L.; Suskin, N. Exercise for Stroke Prevention. Stroke Vasc. Neurol. 2018, 3, 2. [Google Scholar]
- Lee, C.D.; Folsom, A.R.; Blair, S.N. Physical Activity and Stroke Risk: A Meta-Analysis. Stroke 2003, 34, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Howard, V.J.; McDonnell, N.M. Physical Activity in Primary Stroke Prevention: Just Do It! Stroke 2015, 46, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Viktorisson, A.; Palstam, A.; Nyberg, F.; Berg, C.; Lissner, L.; Sunnerhagen, K.S. Domain-Specific Physical Activity and Stroke in Sweden. JAMA Netw. Open 2024, 7, e2413453. [Google Scholar] [CrossRef] [PubMed]
- De Santis, F.; Romoli, M.; Foschi, M.; Sciancalepore, F.D.; D’Anna, L.; Barba, L.; Abu-Rumeileh, S.; Sacco, S.; Ornello, R. Risk of Stroke with Different Levels of Leisure-Time Physical Activity: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Neurol. Neurosurg. Psychiatry 2024, 95, 504–514. [Google Scholar] [CrossRef]
- Grau, A.J.; Barth, C.; Geletneky, B.; Ling, P.; Palm, F.; Lichy, C.; Becher, H.; Buggle, F. Association between Recent Sports Activity, Sports Activity in Young Adulthood, and Stroke. Stroke 2009, 40, 426–431. [Google Scholar] [CrossRef]
- Reinholdsson, M.; Palstam, A.; Sunnerhagen, K.S. Prestroke Physical Activity Could Influence Acute Stroke Severity (Part of PAPSIGOT). Neurology 2018, 91, e1461–e1467. [Google Scholar] [CrossRef]
- Ohlsson, C.; Bygdell, M.; Sondén, A.; Jern, C.; Rosengren, A.; Kindblom, J.M. BMI Increase through Puberty and Adolescence Is Associated with Risk of Adult Stroke. Neurology 2017, 89, 363–369. [Google Scholar] [CrossRef]
- Booth, J.; Connelly, L.; Lawrence, M.; Chalmers, C.; Joice, S.; Becker, C.; Dougall, N. Evidence of Perceived Psychosocial Stress as a Risk Factor for Stroke in Adults: A Meta-Analysis. BMC Neurol. 2015, 15, 233. [Google Scholar] [CrossRef]
- Wake, A.; O’Donnell, A.W. Longitudinal Relationships Between Financial Stress, Career-Related Optimism, and Psychological Distress During Emerging Adulthood in Australia. Youth Soc. 2024, 56, 1336–1363. [Google Scholar] [CrossRef]
- Kim, B.J.; Cho, I.S.; Cho, K.I. Impact of Mindfulness-Based Stress Reduction Therapy on Myocardial Function and Endothelial Dysfunction in Female Patients with Microvascular Angina. J. Cardiovasc. Ultrasound 2017, 25, 118–123. [Google Scholar] [CrossRef]
- Liu, D.; Yang, L.; Liu, P.; Wang, Y.; Gao, L. Impact of Cannabis Abuse on the Occurrence of Stroke in Young People: A Systematic Review and Meta-Analysis. Front. Neurol. 2024, 15, 1426023. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Smoking and Cardiovascular Disease. Available online: https://www.cdc.gov/tobacco/about/cigarettes-and-cardiovascular-disease.html (accessed on 23 February 2025).
- Shinton, R.; Beevers, G. Meta-Analysis of Relation Between Cigarette Smoking and Stroke. BMJ 1989, 298, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Fitzgerald, J.L.; Gallagher, C.; Thomas, G.; Middeldorp, M.E.; Sanders, P. Rates, Predictors, and Impact of Smoking Cessation after Stroke or Transient Ischemic Attack: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2021, 30, 106012. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, P.M.; Liberman, A.L.; Restifo, D.; Abramson, E.L.; Navi, B.B.; Kamel, H.; Parikh, N.S. Cost-Effectiveness of Smoking Cessation Interventions in Patients with Ischemic Stroke and Transient Ischemic Attack. Stroke 2023, 54, 992–1000. [Google Scholar] [CrossRef]
- Lambiase, M.J.; Kubzansky, L.D.; Thurston, R.C. Positive Psychological Health and Stroke Risk: The Benefits of Emotional Vitality. Health Psychol. 2015, 34, 1043–1046. [Google Scholar] [CrossRef]
- Park, C.S.; Choi, E.K.; Han, K.D.; Ahn, H.J.; Kwon, S.; Lee, S.R.; Oh, S.; Lip, G.Y.H. Increased cardiovascular events in young patients with mental disorders: A nationwide cohort study. Eur. J. Prev. Cardiol. 2023, 30, 1582–1592. [Google Scholar] [CrossRef]
- Ramasubbu, R. Therapy for prevention of post-stroke depression. Expert Opin. Pharmacother. 2011, 12, 2177–2187. [Google Scholar] [CrossRef]
- Greenlund, K.J.; Giles, W.H.; Keenan, N.L.; Croft, J.B.; Mensah, G.A. Physician Advice, Patient Actions, and Health-Related Quality of Life in Secondary Prevention of Stroke through Diet and Exercise. Stroke 2002, 33, 565–570. [Google Scholar] [CrossRef]
- Rizk, H.I.; Magdy, R.; Emam, K.; Mohammed, M.S.; Aboulfotooh, A.M. Substance use disorder in young adults with stroke: Clinical characteristics and outcome. Acta Neurol. Belg. 2024, 124, 65–72. [Google Scholar] [CrossRef]
- George, M.G.; Matters, M.D.; McGruder, H.F.; Valderrama, A.L.; Xie, J. The Role of Public Health in Promoting Quality Improvement in Care for Stroke and Heart Disease. Prev. Chronic Dis. 2008, 5, A62. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC2396967/pdf/PCD52A62.pdf (accessed on 3 March 2025).
- Centers for Disease Control and Prevention. Preventing Stroke: Tips for Prevention. MMWR Morb Mortal Wkly Rep. Available online: https://www.cdc.gov/mmwr/volumes/66/wr/mm6618a5.htm (accessed on 23 February 2025).
- Lambert, C.; Chang, W.; Parker, R.; Allen, K.; Stevens, L.; Blood, J.; Nystrom, K.; Forman, R. Enhancing Stroke Knowledge Among Youth: Insights from Stroke Busters. J. Stroke Cerebrovasc. Dis. 2024, 33, 108078. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.A.; Ha, L.; Levine, S.R.; Pratt, C.B. Stroke Knowledge and Barriers to Stroke Prevention among African Americans: Implications for Health Communication. J. Health Commun. 2003, 8, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Rahme, D.; Salameh, P.; Haddad, C.; Sacre, H.; Bahlol, M.; Darwish, R.M.; El Khatib, S.; Safwan, J.; Sakr, F.; et al. Evaluating the Influence of a 3-Min Online Video on the Community Knowledge of Stroke in Four Arab Countries. Front. Public Health 2024, 12, 1342490. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, I.; O’Connell, E.; O’Brien, S.; Weathers, E.; Cornally, N.; Kilonzo, B.; McCarthy, G. The Irish National Stroke Awareness Campaign: A Stroke of Success? Appl. Nurs. Res. 2014, 27, e13–e19. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Leira, E.C.; Maas, M.B.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
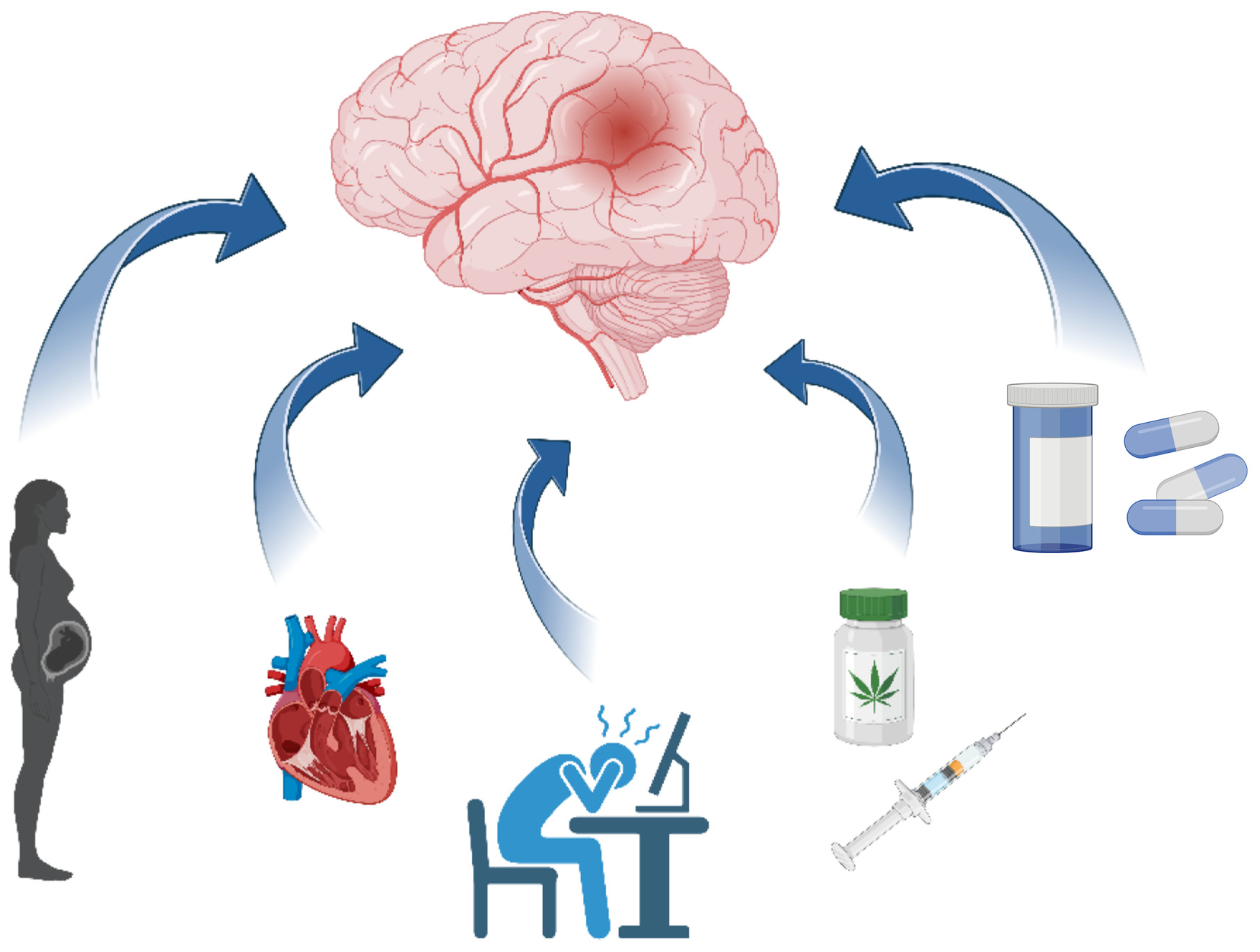
| Study (Author, Year) | Study Design | Sample Size | Key Findings | Limitations |
|---|---|---|---|---|
| Brouwer et al. (2022) [52] | Prospective registry (MR CLEAN) | 3256 (310 aged 18–49) | Endovascular thrombectomy: 61% of young patients achieved mRS 0–2 at 90 days; mortality was lower than older group (7% vs. 32%) | Observational design; no control group |
| Putaala et al. (2009) [53] | Retrospective cohort | 48 young adults | IV thrombolysis yielded excellent 3-month outcomes; no deaths reported | Small sample size; retrospective bias |
| Dutta et al. (2021) [54] | Population-based case-control | 2242 total (1090 cases, 1152 controls) | No significant association between marijuana use and early ischemic stroke | Self-reported exposure; residual confounding |
| Aycock et al. (2023) [55] | Feasibility RCT | 30 African American young adults | Improved stroke risk perception and health behaviors via SCORRE intervention | Pilot nature; short duration and small cohort |
| Lo et al. (2021) [56] | RCT protocol | Target: 208 | Narrative-based skills-building aims to improve reintegration and self-efficacy | Results pending; study ongoing |
| Chan et al. (2021) [57] | RCT | Target: 154 | Expressive arts therapy improved mood, stress, and QoL in young survivors | Generalizability limited; long-term outcomes not yet assessed |
| Singhal et al. (2013) [49] | Expert consensus | N/A | 10–15% of ischemic strokes occur in young adults; highlighted diagnostic delays | No original data; relies on expert opinion |
| Study (Author, Year) | Population | Comparison Focus | Younger Adults | Older Adults |
|---|---|---|---|---|
| Li et al., 2022 [58] | National registry data, USA (2002–2018) | Incidence trend | ↑ 67% ischemic stroke incidence under 55 | ↓ 15% incidence in ≥55 |
| Huggins et al., 2020 [59] | 432 stroke inpatients (18–50 vs. >50) | Symptom presentation | 43% had fluctuating/ progressive symptoms | 27% had fluctuating/ progressive symptoms |
| Fonarow et al., 2010 [60] | >500,000 ischemic stroke admissions | Comorbidities and short-term outcomes | Fewer comorbidities; lower in-hospital mortality; better functional recovery | More AF, prior stroke, HTN; higher mortality |
| Lutski et al., 2017 [61] | Ischemic stroke registry | Functional outcome and etiology | 70% mRS ≤ 2; more lacunar/cryptogenic strokes | Lower favorable outcome; more atherosclerosis/cardio-embolism |
| Patel et al., 2021 [62] | 4.2M AIS admissions (USA, 2003–2014) | Risk factors | More obesity, drug use, HIV, congenital heart disease, autoimmune disorders | More traditional risk factors (HTN, AF, CA) |
| Matuja et al., 2020 [63] | 369 stroke patients in Tanzania | Risk profile and mortality | 42.3% hemorrhagic stroke; HTN, HIV, contraception, sickle cell, LDL ↑; 49.1% 30-day mortality | 27.2% hemorrhagic stroke; 67.2% 30-day mortality |
| Moosa et al., 2021 [64] | 513 stroke patients in Bahrain | Stroke type and comorbidities | 21.5% hemorrhagic stroke; HTN (43%), DM (24.7%), DL (16.1%) | 11.6% hemorrhagic; HTN (74%), DM (63%), DL (32.2%) |
| Study (Author, Year) | Intervention | Population | Key Findings | Notes |
|---|---|---|---|---|
| Aycock et al., 2023 [55] | Nurse-led Health Education | Young African American adults (18–45) | Improved stroke risk perception, increased health behavior adherence | RCT; population-specific |
| Chan et al., 2021 [57] | Expressive Arts Therapy | Young stroke survivors (Chinese cohort) | Improved mental health, emotional expression and quality of life | RCT; post-stroke focus |
| Spence, 2018 [65] | Mediterranean-style Diet | General population; data cited for young adults | Associated with significantly reduced stroke incidence in observational studies | Not age-stratified; observational data |
| Reddin et al., 2022 [66] | Stress Reduction/Perceived Control | INTERSTROKE population (includes young adults) | Higher locus of control associated with lower stroke risk | Large-scale observational study |
| Sobalska et al., 2020 [67] | Dietary Pattern Assessment | Polish young adults (20–45) | Moderate adherence to preventive diets | Cross-sectional; context-dependent |
| Mälstam et al., 2023 [68] | “Make My Day” Lifestyle Program | Adults aged 45–75 (includes younger subgroup) | Improved physical activity and reduced sedentary behavior | Pilot RCT; not powered to assess stroke incidence |
| von Sarnowski et al., 2013 [69] | Physical Activity (Leisure-Time) | Young adults (<55), n = 5023 | Physical inactivity PAR = 59.7%; most prevalent in women <35 | Case-control; specific to young adults |
| Loucks et al., 2015 [70] | Mindfulness-Based Stress Reduction (MBSR) | Young and middle-aged adults with CV risk | Reduced inflammatory markers; improved autonomic function | Mixed-methods; theoretical and empirical basis |
| Calderone et al., 2024 [71] | Cognitive–Behavioral Therapy (CBT) | Young adults (pilot study) | Reduced cortisol levels, improved cardiovascular health indicators | Pilot RCT; small sample size |
| Markidan et al., 2018 [72] | Smoking Cessation Counseling | Young men | Dose-dependent reduction in ischemic stroke risk after quitting | Large population-based study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sič, A.; Andrejić, N.; Ivanović, J.; Karadžić Ristanović, V.; Gajić, S.; Bjelić, D.; Baralić, M.; Stojanovic, N. Stroke in Young Adults: An Overview and Non-Pharmacological Preventive Strategies. Brain Sci. 2025, 15, 375. https://doi.org/10.3390/brainsci15040375
Sič A, Andrejić N, Ivanović J, Karadžić Ristanović V, Gajić S, Bjelić D, Baralić M, Stojanovic N. Stroke in Young Adults: An Overview and Non-Pharmacological Preventive Strategies. Brain Sciences. 2025; 15(4):375. https://doi.org/10.3390/brainsci15040375
Chicago/Turabian StyleSič, Aleksandar, Nikola Andrejić, Jovana Ivanović, Vidna Karadžić Ristanović, Selena Gajić, Danka Bjelić, Marko Baralić, and Nikola Stojanovic. 2025. "Stroke in Young Adults: An Overview and Non-Pharmacological Preventive Strategies" Brain Sciences 15, no. 4: 375. https://doi.org/10.3390/brainsci15040375
APA StyleSič, A., Andrejić, N., Ivanović, J., Karadžić Ristanović, V., Gajić, S., Bjelić, D., Baralić, M., & Stojanovic, N. (2025). Stroke in Young Adults: An Overview and Non-Pharmacological Preventive Strategies. Brain Sciences, 15(4), 375. https://doi.org/10.3390/brainsci15040375









