Vitamin C Modulates the PI3K/AKT Pathway via Glutamate and Nitric Oxide in Developing Avian Retina Cells in Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
2.3. Retinal Culture
2.4. Treatment
2.5. Western Blotting
2.6. Cell Transfection
2.7. Extracellular Glutamate and Calcium Imaging
2.8. Immunocytochemistry
2.9. Statistical Analysis
3. Results
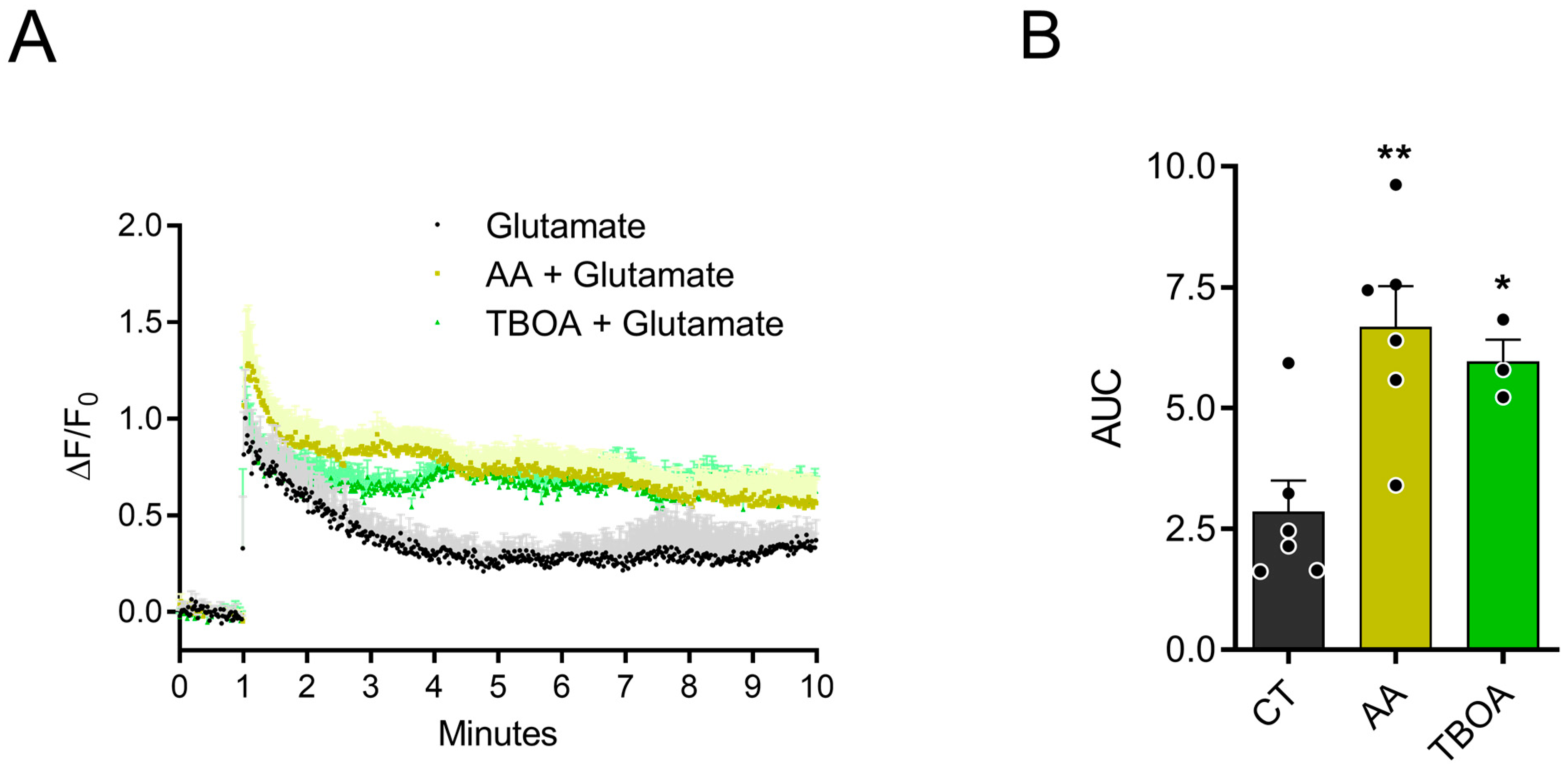
3.1. AA Promotes Accumulation of Extracellular Glutamate
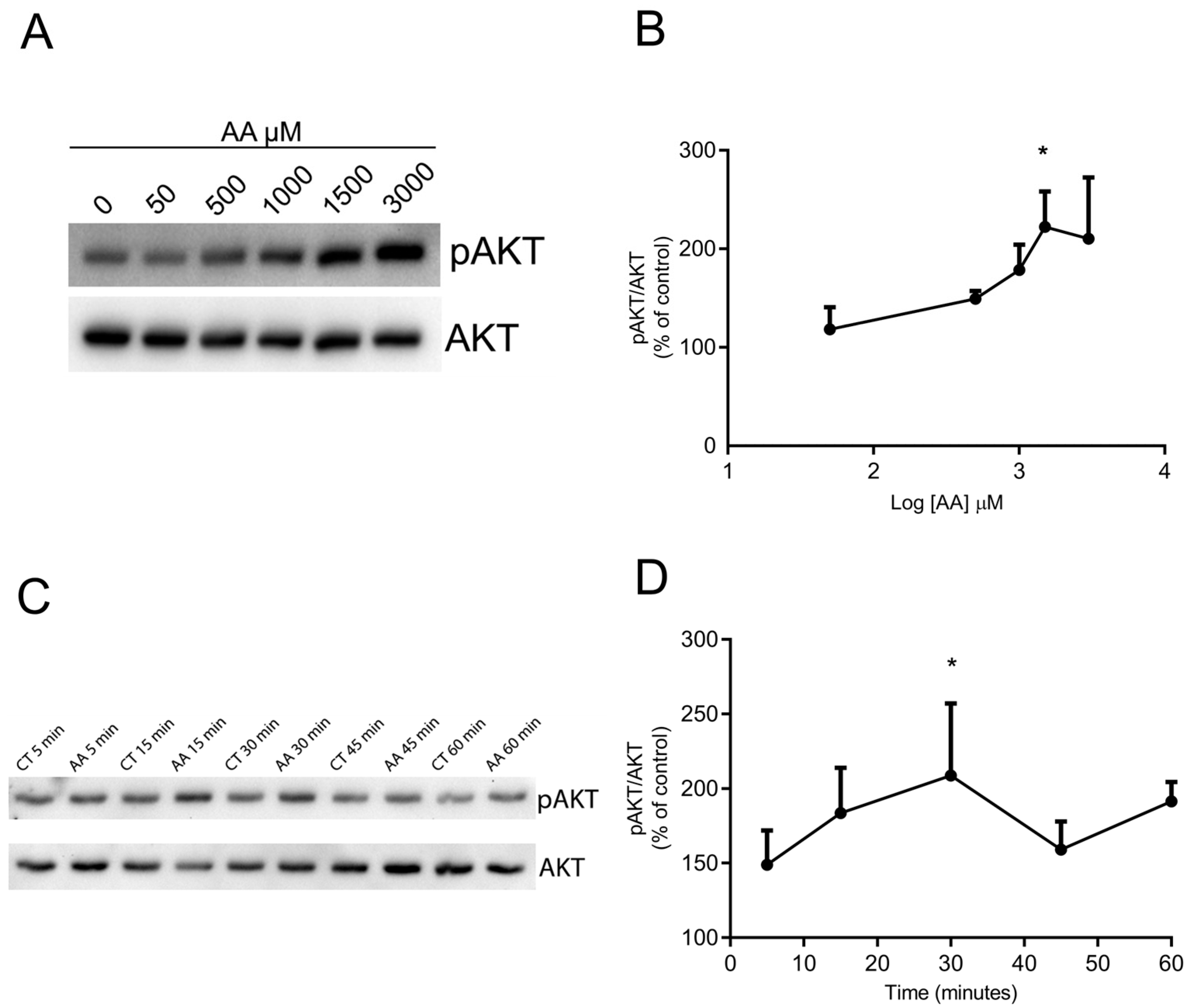
3.2. AA Promotes AKT Phosphorylation in a Concentration- and Time-Dependent Way
3.3. AA Induces Increased pAKT in Glial and Neuronal Cells
3.4. Activation of Ionotropic Glutamate Receptors Participate in AKT Phosphorylation Induced by AA
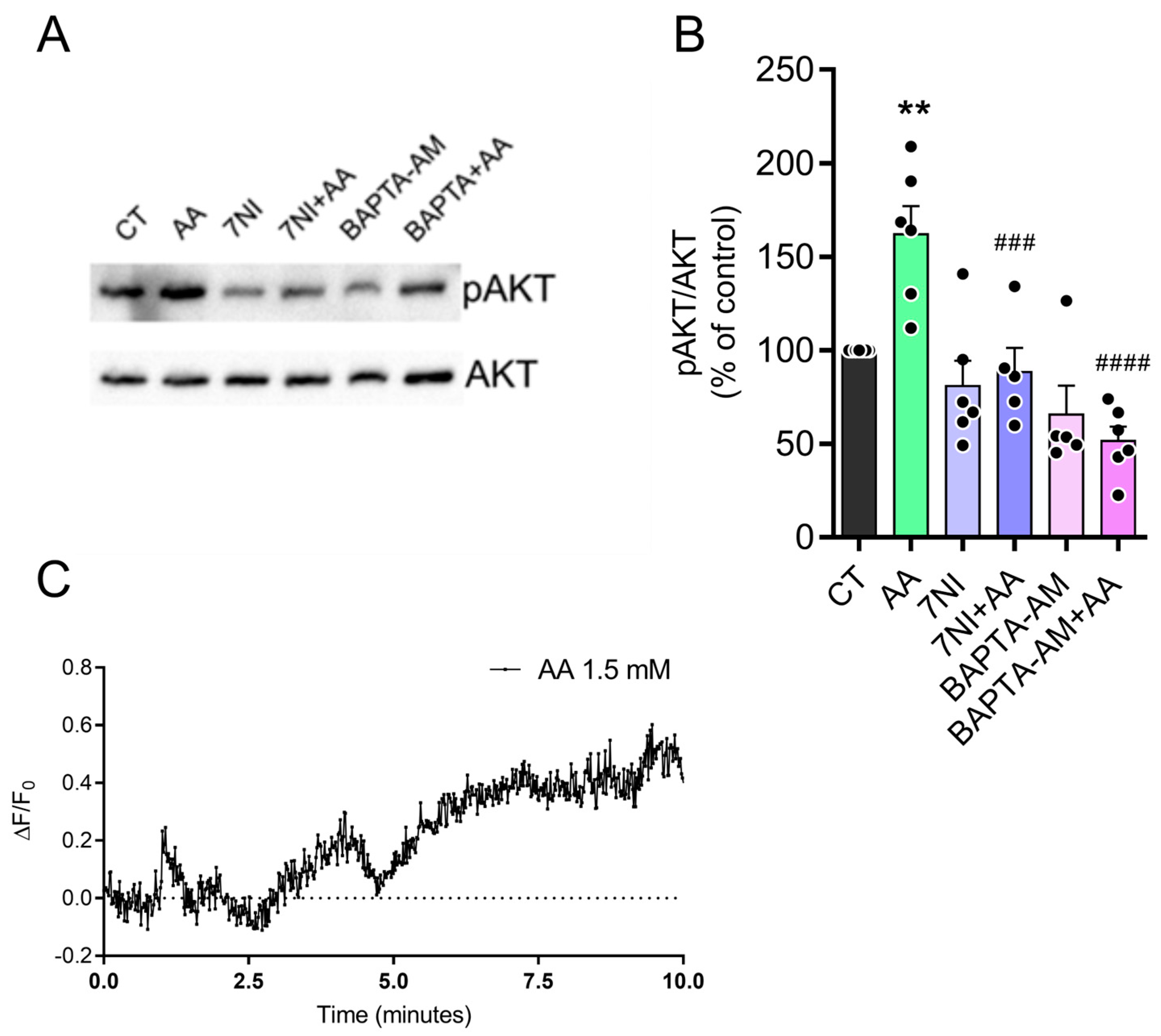
3.5. The Effect of AA on pAKT Levels Is Dependent on Calcium and Nitric Oxide (NO)
3.6. AA Induced AKT Phosphorylation via PI3K
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic Acid: Chemistry, Biology and the Treatment of Cancer. Biochim. Biophys. Acta-Rev. Cancer 2012, 1826, 443–457. [Google Scholar]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.N.; Yuan, Y.; Liu, F.; Han, J.G.; Sheng, L. A Computational Investigation on the Geometries, Stabilities, Antioxidant Activity, and the Substituent Effects of the L-Ascorbic Acid and Their Derivatives. Int. J. Quantum Chem. 2013, 113, 2220–2227. [Google Scholar] [CrossRef]
- Spoelstra-De Man, A.M.E.; Elbers, P.W.G.; Oudemans-Van Straaten, H.M. Vitamin C: Should We Supplement? Curr. Opin. Crit. Care 2018, 24, 248–255. [Google Scholar]
- Bürzle, M.; Hediger, M.A. Functional and Physiological Role of Vitamin C Transporters. In Current Topics in Membranes; Academic Press: Cambridge, MA, USA, 2012; Volume 70, pp. 357–375. [Google Scholar]
- Mun, G.H.; Kim, M.J.; Lee, J.H.; Kim, H.J.; Chung, Y.H.; Chung, Y.B.; Kang, J.S.; Hwang, Y.I.; Oh, S.H.; Kim, J.-G.; et al. Immunohistochemical Study of the Distribution of Sodium-Dependent Vitamin C Transporters in Adult Rat Brain. J. Neurosci. Res. 2006, 83, 919–928. [Google Scholar] [CrossRef]
- Portugal, C.C.; Miya, V.S.; Calaza, K.d.C.; Santos, R.A.M.; Paes-de-Carvalho, R. Glutamate Receptors Modulate Sodium-Dependent and Calcium-Independent Vitamin C Bidirectional Transport in Cultured Avian Retinal Cells. J. Neurochem. 2009, 108, 507–520. [Google Scholar] [CrossRef]
- Nualart, F.; Mack, L.; García, A.; Cisternas, P.; Bongarzone, E.R.; Heitzer, M.; Jara, N.; Martínez, F.; Ferrada, L.; Espinoza, F.; et al. Vitamin C Transporters, Recycling and the Bystander Effect in the Nervous System: SVCT2 versus Gluts. J. Stem Cell Res. Ther. 2014, 4, 209. [Google Scholar] [CrossRef]
- Portugal, C.C.; Da Encarnação, T.G.; Socodato, R.; Moreira, S.R.; Brudzewsky, D.; Ambrósio, A.F.; Paes-de-Carvalho, R. Nitric Oxide Modulates Sodium Vitamin C Transporter 2 (SVCT-2) Protein Expression via Protein Kinase G (PKG) and Nuclear Factor-ΚB (NF-ΚB). J. Biol. Chem. 2012, 287, 3860–3872. [Google Scholar] [CrossRef]
- da Encarnação, T.G.; Portugal, C.C.; Nogueira, C.E.; Santiago, F.N.; Socodato, R.; Paes-de-Carvalho, R. Dopamine Promotes Ascorbate Release from Retinal Neurons: Role of D1 Receptors and the Exchange Protein Directly Activated by CAMP Type 2 (EPAC2). Mol. Neurobiol. 2018, 55, 7858–7871. [Google Scholar] [CrossRef]
- Domith, I.; Socodato, R.; Portugal, C.C.; Munis, A.F.; Duarte-Silva, A.T.; Paes-de-Carvalho, R. Vitamin C Modulates Glutamate Transport and NMDA Receptor Function in the Retina. J. Neurochem. 2018, 144, 408–420. [Google Scholar] [CrossRef]
- Attwell, D.; Laughlin, S.B. An Energy Budget for Signaling in the Grey Matter of the Brain. J. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Lester, R.A. Excitatory Amino Acid Receptors in the Vertebrate Central Nervous System. Pharmacol. Rev. 1989, 41, 143–210. [Google Scholar] [CrossRef]
- Headley, P.M.; Grillner, S. Excitatory Amino Acids and Synaptic Transmission: The Evidence for a Physiological Function. Trends Pharmacol. Sci. 1990, 11, 205–211. [Google Scholar] [CrossRef]
- Moroz, L.L.; Nikitin, M.A.; Poličar, P.G.; Kohn, A.B.; Romanova, D.Y. Evolution of Glutamatergic Signaling and Synapses. Neuropharmacology 2021, 199, 108740. [Google Scholar] [CrossRef]
- Quinlan, E.M.; Philpot, B.D.; Huganir, R.L.; Bear, M.F. Rapid, Experience-Dependent Expression of Synaptic NMDA Receptors in Visual Cortex in Vivo. Nat. Neurosci. 1999, 2, 352–357. [Google Scholar] [CrossRef]
- Leibowitz, A.; Boyko, M.; Shapira, Y.; Zlotnik, A. Blood Glutamate Scavenging: Insight into Neuro Protection. Int. J. Mol. Sci. 2012, 13, 10041–10066. [Google Scholar]
- Willard, S.S.; Koochekpour, S. Glutamate, Glutamate Receptors, and Downstream Signaling Pathways. Int. J. Biol. Sci. 2013, 9, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Colbran, R.J.; Brown, A.M. Calcium/Calmodulin-Dependent Protein Kinase II and Synaptic Plasticity. Curr. Opin. Neurobiol. 2004, 14, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 1469–1658. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric Oxide Synthases: Structure, Function and Inhibition. Biochem. J. 2001, 357 Pt 3, 593–615. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric Oxide in the Central Nervous System: Neuroprotection versus Neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [PubMed]
- Thomas, D.D.; Heinecke, J.L.; Ridnour, L.A.; Cheng, R.Y.; Kesarwala, A.H.; Switzer, C.H.; McVicar, D.W.; Roberts, D.D.; Glynn, S.; Fukuto, J.M.; et al. Signaling and Stress: The Redox Landscape in NOS2 Biology. Free Radic. Biol. Med. 2015, 87, 204–225. [Google Scholar]
- Xue, G.; Hemmings, B.A. PKB/Akt-Dependent Regulation of Cell Motility. JNCI J. Natl. Cancer Inst. 2013, 105, 393–404. [Google Scholar] [CrossRef]
- Mora, A.; Komander, D.; van Aalten, D.M.F.; Alessi, D.R. PDK1, the Master Regulator of AGC Kinase Signal Transduction. Semin. Cell Dev. Biol. 2004, 15, 161–170. [Google Scholar]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and Regulation of Akt/PKB by the Rictor-MTOR Complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E.; Downward, J.; Greenberg, M.E. Akt Phosphorylation of BAD Couples Survival Signals to the Cell-Intrinsic Death Machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Meusburger, S.; Hawkins, C.J.; Riglar, D.T.; Lee, E.F.; Fairlie, W.D.; Huang, D.C.S.; Adams, J.M. Apoptosis Is Triggered When Prosurvival Bcl-2 Proteins Cannot Restrain Bax. Proc. Natl. Acad. Sci. USA 2008, 105, 18081–18087. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Kempf, R.C.; Long, J.; Laidler, P.; Mijatovic, S.; Maksimovic-Ivanic, D.; Stivala, F.; Mazzarino, M.C.; et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/MTOR Pathways in Controlling Growth and Sensitivity to Therapy-Implications for Cancer and Aging. Aging 2011, 3, 192–222. [Google Scholar] [CrossRef]
- Webb, A.E.; Brunet, A. FOXO Transcription Factors: Key Regulators of Cellular Quality Control. Trends Biochem. Sci. 2014, 39, 159–169. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.-L. TSC2 Is Phosphorylated and Inhibited by Akt and Suppresses MTOR Signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Mejía-García, T.A.; Portugal, C.C.; Encarnação, T.G.; Prado, M.A.M.; Paes-de-Carvalho, R. Nitric Oxide Regulates AKT Phosphorylation and Nuclear Translocation in Cultured Retinal Cells. Cell. Signal. 2013, 25, 2424–2439. [Google Scholar] [CrossRef] [PubMed]
- Saggioro, D. Anti-Apoptotic Effect of Tax: An NF-ΚB Path or a CREB Way? Viruses 2011, 3, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Bernard, L.P.; Dibona, V.L.; Wu, Q.; Zhang, H. Calcium Phosphate Transfection of Primary Hippocampal Neurons. J. Vis. Exp. 2013, 81, e50808. [Google Scholar] [CrossRef]
- Simon, B.; Huart, A.-S.; Wilmanns, M. Molecular Mechanisms of Protein Kinase Regulation by Calcium/Calmodulin. Bioorg. Med. Chem. 2015, 23, 2749–2760. [Google Scholar] [CrossRef]
- Knowles, R.G.; Palacios, M.; Palmer, R.M.; Moncada, S. Formation of Nitric Oxide from L-Arginine in the Central Nervous System: A Transduction Mechanism for Stimulation of the Soluble Guanylate Cyclase. Proc. Natl. Acad. Sci. USA 1989, 86, 5159–5162. [Google Scholar]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The Evolution of Phosphatidylinositol 3-Kinases as Regulators of Growth and Metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Portugal, C.C. Ascorbate and Its Transporter SVCT2: The Dynamic Duo’s Integrated Roles in CNS Neurobiology and Pathophysiology. Free Radic. Biol. Med. 2024, 212, 448–462. [Google Scholar] [CrossRef]
- Paterson, I.A.; Hertz, L. Sodium-independent Transport of Noradrenaline in Mouse and Rat Astrocytes in Primary Culture. J. Neurosci. Res. 1989, 23, 71–77. [Google Scholar] [CrossRef]
- Yoffe, J.R.; Borchardt, R.T. Effects of Ascorbic Acid on the Uptake of Serotonin in Differentiated Neuroblastoma Cells. Life Sci. 1982, 31, 489–493. [Google Scholar] [CrossRef]
- Tempone, M.H.; Borges-Martins, V.P.; César, F.; Alexandrino-Mattos, D.P.; de Figueiredo, C.S.; Raony, Í.; dos Santos, A.A.; Duarte-Silva, A.T.; Dias, M.S.; Freitas, H.R.; et al. The Healthy and Diseased Retina Seen through Neuron–Glia Interactions. Int. J. Mol. Sci. 2024, 25, 1120. [Google Scholar] [CrossRef]
- Duarte-Silva, A.T.; Ximenes, L.G.R.; Guimarães-Souza, M.; Domith, I.; Paes-de-Carvalho, R. Chemical Signaling in the Developing Avian Retina: Focus on Cyclic AMP and AKT-Dependent Pathways. Front. Cell Dev. Biol. 2022, 10, 1058925. [Google Scholar] [CrossRef] [PubMed]
- Calaza, K.d.C.; Gardino, P.F. Neurochemical Phenotype and Birthdating of Specific Cell Populations in the Chick Retina. An. Acad. Bras. Cienc. 2010, 82, 595–608. [Google Scholar] [CrossRef]
- Adler, R. A Model of Retinal Cell Differentiation in the Chick Embryo. Prog. Retin. Eye Res. 2000, 19, 529–557. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Wang, J.; Boyd, P.; Wang, F.; Santiago, C.; Jiang, L.; Yoo, S.; Lahne, M.; Todd, L.J.; Jia, M.; et al. Gene Regulatory Networks Controlling Vertebrate Retinal Regeneration. Science 2020, 370, eabb8598. [Google Scholar] [CrossRef]
- Lima, D.R.S.; Cossenza, M.; Garcia, C.G.; Portugal, C.C.; Marques, F.F.d.C.; Paes-de-Carvalho, R.; Pereira Netto, A.D. Determination of Ascorbic Acid in the Retina during Chicken Embryo Development Using High Performance Liquid Chromatography and UV Detection. Anal. Methods 2016, 8, 5441–5447. [Google Scholar] [CrossRef]
- Portugal, C.C.; Da Encarnacaõ, T.G.; Domith, I.; Dos Santos Rodrigues, A.; De Oliveira, N.A.; Socodato, R.; Paes-De-carvalho, R. Dopamine-Induced Ascorbate Release from Retinal Neurons Involves Glutamate Release, Activation of AMPA/Kainate Receptors and Downstream Signaling Pathways. Front. Neurosci. 2019, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Domith, I.; Duarte-Silva, A.T.; Garcia, C.G.; Calaza, K.D.C.; Paes-de-Carvalho, R.; Cossenza, M. Chlorogenic Acids Inhibit Glutamate Dehydrogenase and Decrease Intracellular ATP Levels in Cultures of Chick Embryo Retina Cells. Biochem. Pharmacol. 2018, 155, 393–402. [Google Scholar] [CrossRef]
- Socodato, R.; Santiago, F.N.; Portugal, C.C.; Domingues, A.F.; Santiago, A.R.; Relvas, J.B.; Ambróio, A.F.; Paes-de-Carvalho, R. Calcium-Permeable α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid Receptors Trigger Neuronal Nitric-Oxide Synthase Activation to Promote Nerve Cell Death in an Src Kinase-Dependent Fashion. J. Biol. Chem. 2012, 287, 38680–38694. [Google Scholar] [CrossRef]
- Majewska, M.D.; Bell, J.A.; London, E.D. Regulation of the NMDA Receptor by Redox Phenomena: Inhibitory Role of Ascorbate. Brain Res. 1990, 537, 328–332. [Google Scholar] [CrossRef]
- Mejía-García, T.A.; Paes-de-Carvalho, R. Nitric Oxide Regulates Cell Survival in Purified Cultures of Avian Retinal Neurons: Involvement of Multiple Transduction Pathways. J. Neurochem. 2007, 100, 382–394. [Google Scholar] [CrossRef]
- Mcmahon, D.G.; Ponomareva, L.V. Nitric Oxide and CGMP Modulate Retinal Glutamate Receptors. J. Neurophysiol. 1996, 76, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Boccuni, I.; Fairless, R. Retinal Glutamate Neurotransmission: From Physiology to Pathophysiological Mechanisms of Retinal Ganglion Cell Degeneration. Life 2022, 12, 638. [Google Scholar] [CrossRef]
- Cossenza, M.; Paes de Carvalho, R. L-Arginine Uptake and Release by Cultured Avian Retinal Cells: Differential Cellular Localization in Relation to Nitric Oxide Synthase. J. Neurochem. 2000, 74, 1885–1894. [Google Scholar] [CrossRef]
- Cossenza, M.; Socodato, R.; Portugal, C.C.; Domith, I.C.L.; Gladulich, L.F.H.; Encarnação, T.G.; Calaza, K.C.; Mendonça, H.R.; Campello-Costa, P.; Paes-de-Carvalho, R. Nitric Oxide in the Nervous System. In Vitamins and Hormones; Academic Press: Cambridge, MA, USA, 2014; Volume 96, pp. 79–125. [Google Scholar]
- Socodato, R.E.d.S.; Magalhães, C.R.; Paes-de-Carvalho, R. Glutamate and Nitric Oxide Modulate ERK and CREB Phosphorylation in the Avian Retina: Evidence for Direct Signaling from Neurons to Müller Glial Cells. J. Neurochem. 2009, 108, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef]
- Moretti, M.; Budni, J.; Freitas, A.E.; Rosa, P.B.; Rodrigues, A.L.S. Antidepressant-like Effect of Ascorbic Acid Is Associated with the Modulation of Mammalian Target of Rapamycin Pathway. J. Psychiatr. Res. 2014, 48, 16–24. [Google Scholar] [CrossRef]
- Divolis, G.; Mavroeidi, P.; Mavrofrydi, O.; Papazafiri, P. Differential Effects of Calcium on PI3K-Akt and HIF-1α Survival Pathways. Cell Biol. Toxicol. 2016, 32, 437–449. [Google Scholar] [CrossRef]
- Zylinska, L.; Lisek, M.; Guo, F.; Boczek, T. Vitamin C Modes of Action in Calcium-Involved Signaling in the Brain. Antioxidants 2023, 12, 231. [Google Scholar] [CrossRef]
- Kook, S.Y.; Lee, K.M.; Kim, Y.; Cha, M.Y.; Kang, S.; Baik, S.H.; Lee, H.; Park, R.; Mook-Jung, I. High-Dose of Vitamin C Supplementation Reduces Amyloid Plaque Burden and Ameliorates Pathological Changes in the Brain of 5XFAD Mice. Cell Death Dis. 2014, 5, e1083. [Google Scholar] [CrossRef]
- Badshah, H.; Ali, T.; Ahmad, A.; Kim, M.; Abid, N.; Shah, S.; Yoon, G.; Lee, H.; Kim, M. Co-Treatment with Anthocyanins and Vitamin C Ameliorates Ethanol- Induced Neurodegeneration via Modulation of GABAB Receptor Signaling in the Adult Rat Brain. CNS Neurol. Disord. Drug Targets 2015, 14, 791–803. [Google Scholar] [CrossRef]
- Semwal, B.C.; Singh, B.; Murti, Y.; Singh, S. Therapeutic Potential of Ascorbic Acid in the Management of Alzheimer’s: An Update. Curr. Pharm. Biotechnol. 2023, 25, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Supp, A.D.; Avila, S.; Mastella, G.A.; Damásio, L.; de Oliveira, I.H.; Godoi, A.K.; Michels, A.; Schuck, P.F.; Zugno, A.I. Ascorbic Acid Supplementation Attenuates Schizophrenia-like Symptoms in an Animal Model Induced by Ketamine. Int. J. Dev. Neurosci. 2021, 81, 26–36. [Google Scholar] [CrossRef]
- Covarrubias-Pinto, A.; Acuña, A.; Beltrán, F.; Torres-Díaz, L.; Castro, M. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef]
- Zor, R.K.; Erşan, S.; Küçük, E.; Yıldırım, G.; Sarı, İ. Serum Malondialdehyde, Monocyte Chemoattractant Protein-1, and Vitamin C Levels in Wet Type Age-Related Macular Degeneration Patients. Ther. Adv. Ophthalmol. 2020, 12, 2515841420951682. [Google Scholar] [CrossRef]
- Kocot, J.; Luchowska-Kocot, D.; Kiełczykowska, M.; Musik, I.; Kurzepa, J. Does Vitamin C Influence Neurodegenerative Diseases and Psychiatric Disorders? Nutrients 2017, 9, 659. [Google Scholar] [CrossRef]
- Moretti, M.; Fraga, D.B.; Rodrigues, A.L.S. Preventive and Therapeutic Potential of Ascorbic Acid in Neurodegenerative Diseases. CNS Neurosci. Ther. 2017, 23, 921–929. [Google Scholar] [PubMed]
- Espinoza, F.; Magdalena, R.; Saldivia, N.; Jara, N.; Martínez, F.; Ferrada, L.; Salazar, K.; Ávila, F.; Nualart, F. Vitamin C Recycling Regulates Neurite Growth in Neurospheres Differentiated In Vitro. Antioxidants 2020, 9, 1276. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Suo, N.; Cui, X.; Yuan, Q.; Xie, X. Vitamin C Promotes Oligodendrocytes Generation and Remyelination. Glia 2018, 66, 1302–1316. [Google Scholar] [CrossRef]
- Salazar, K.; Jara, N.; Ramírez, E.; de Lima, I.; Smith-Ghigliotto, J.; Muñoz, V.; Ferrada, L.; Nualart, F. Role of Vitamin C and SVCT2 in Neurogenesis. Front. Neurosci. 2023, 17, 1155758. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte-Silva, A.T.; Domith, I.; Gonçalves-da-Silva, I.; Paes-de-Carvalho, R. Vitamin C Modulates the PI3K/AKT Pathway via Glutamate and Nitric Oxide in Developing Avian Retina Cells in Culture. Brain Sci. 2025, 15, 369. https://doi.org/10.3390/brainsci15040369
Duarte-Silva AT, Domith I, Gonçalves-da-Silva I, Paes-de-Carvalho R. Vitamin C Modulates the PI3K/AKT Pathway via Glutamate and Nitric Oxide in Developing Avian Retina Cells in Culture. Brain Sciences. 2025; 15(4):369. https://doi.org/10.3390/brainsci15040369
Chicago/Turabian StyleDuarte-Silva, Aline T., Ivan Domith, Isabele Gonçalves-da-Silva, and Roberto Paes-de-Carvalho. 2025. "Vitamin C Modulates the PI3K/AKT Pathway via Glutamate and Nitric Oxide in Developing Avian Retina Cells in Culture" Brain Sciences 15, no. 4: 369. https://doi.org/10.3390/brainsci15040369
APA StyleDuarte-Silva, A. T., Domith, I., Gonçalves-da-Silva, I., & Paes-de-Carvalho, R. (2025). Vitamin C Modulates the PI3K/AKT Pathway via Glutamate and Nitric Oxide in Developing Avian Retina Cells in Culture. Brain Sciences, 15(4), 369. https://doi.org/10.3390/brainsci15040369







