Utilization of Medicinal Plants in Mental Disorders: Neuroplasticity and Neuroprotection in Biomodels
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.1.1. Source of Information
2.1.2. Review Question
- -
- Population: Biomodels used in the study of mental disorders.
- -
- Intervention: Administration of medicinal plants.
- -
- Comparison: Not applicable.
- -
- Outcomes: Measures of neuroplasticity and neuroprotection.
2.1.3. Search Terms
2.1.4. Eligibility and Exclusion Criteria
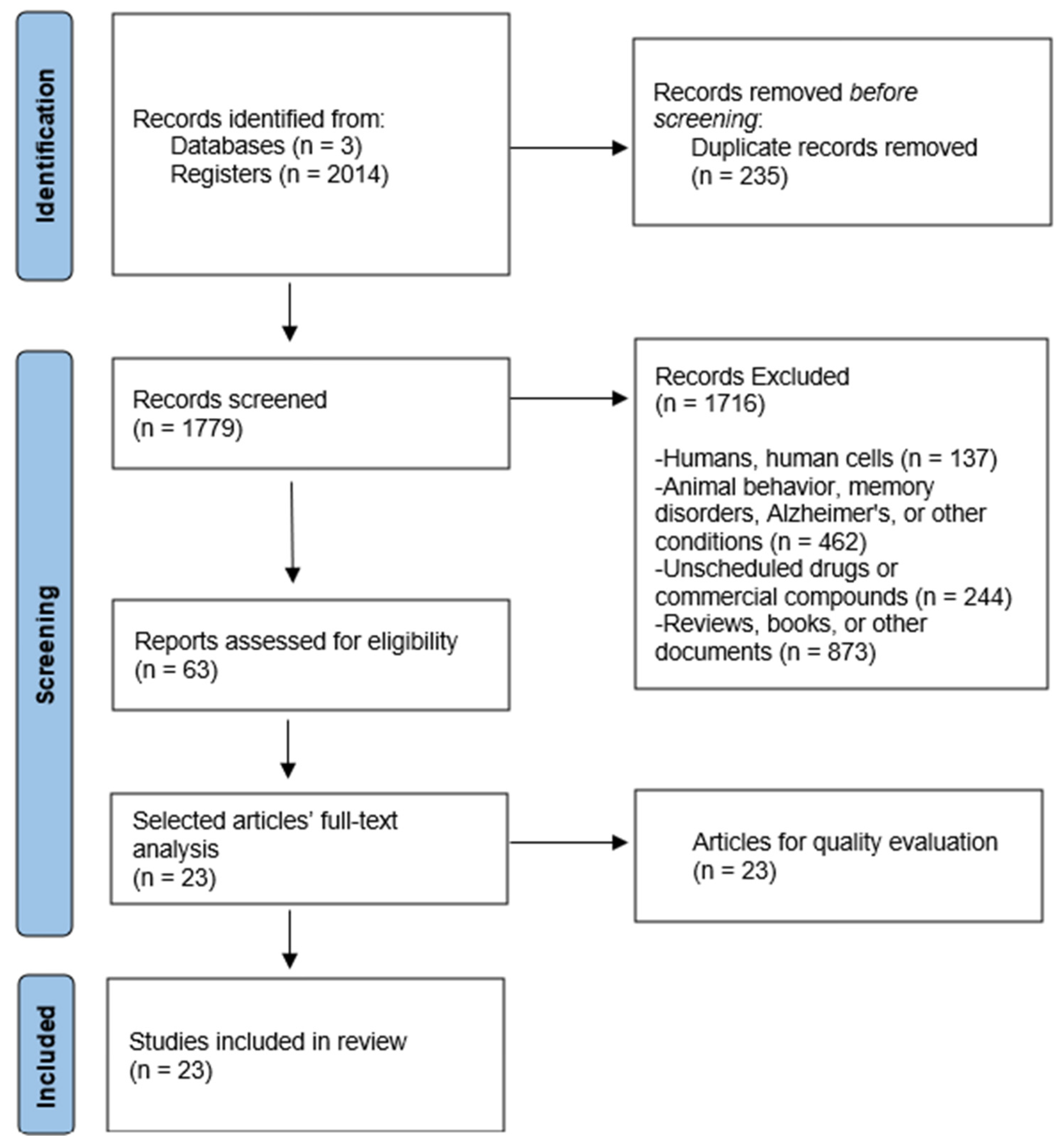
2.2. Study Selection
2.3. Quality Evaluation
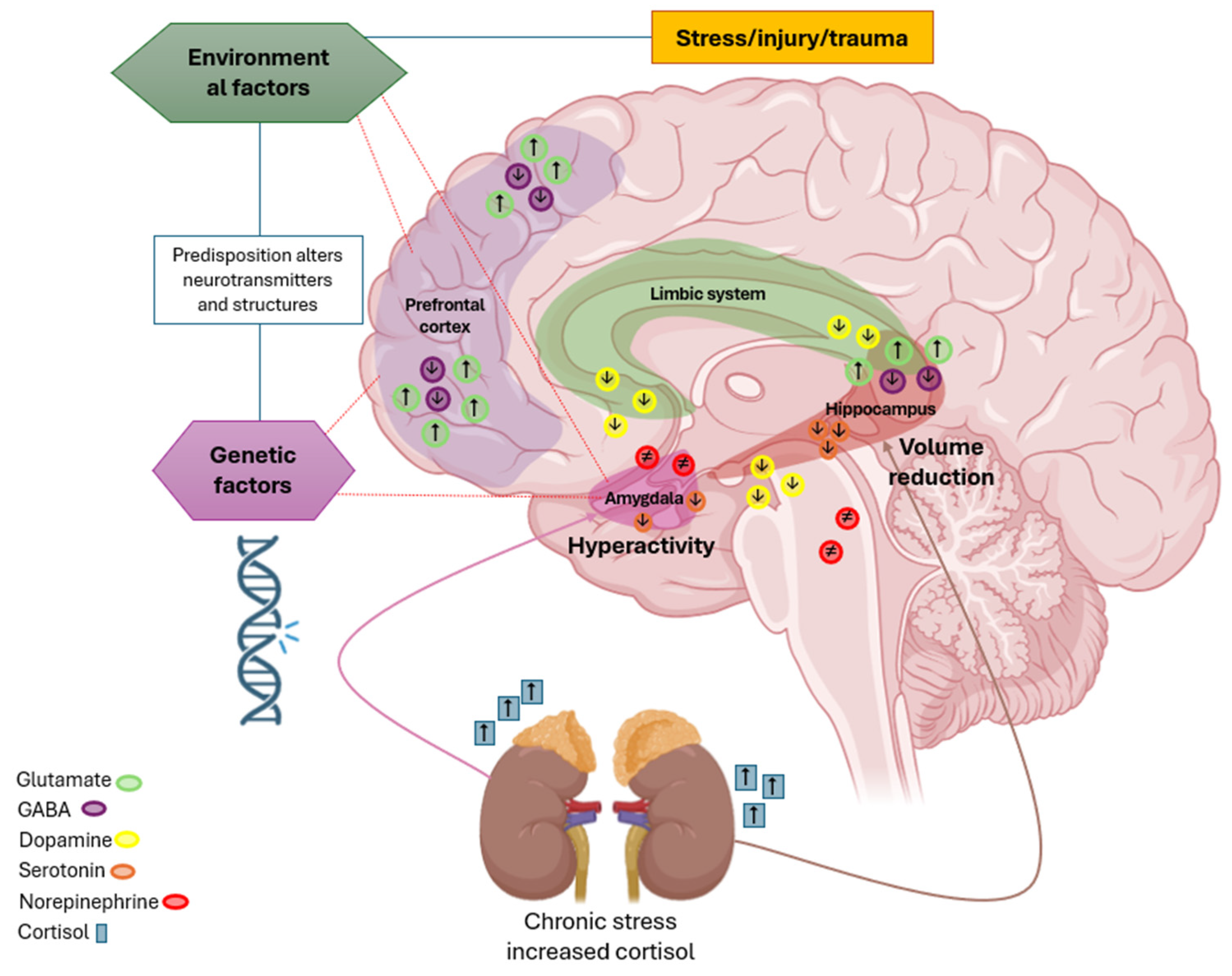
3. Results
3.1. Methodological Quality
3.2. Data Extraction and Synthesis
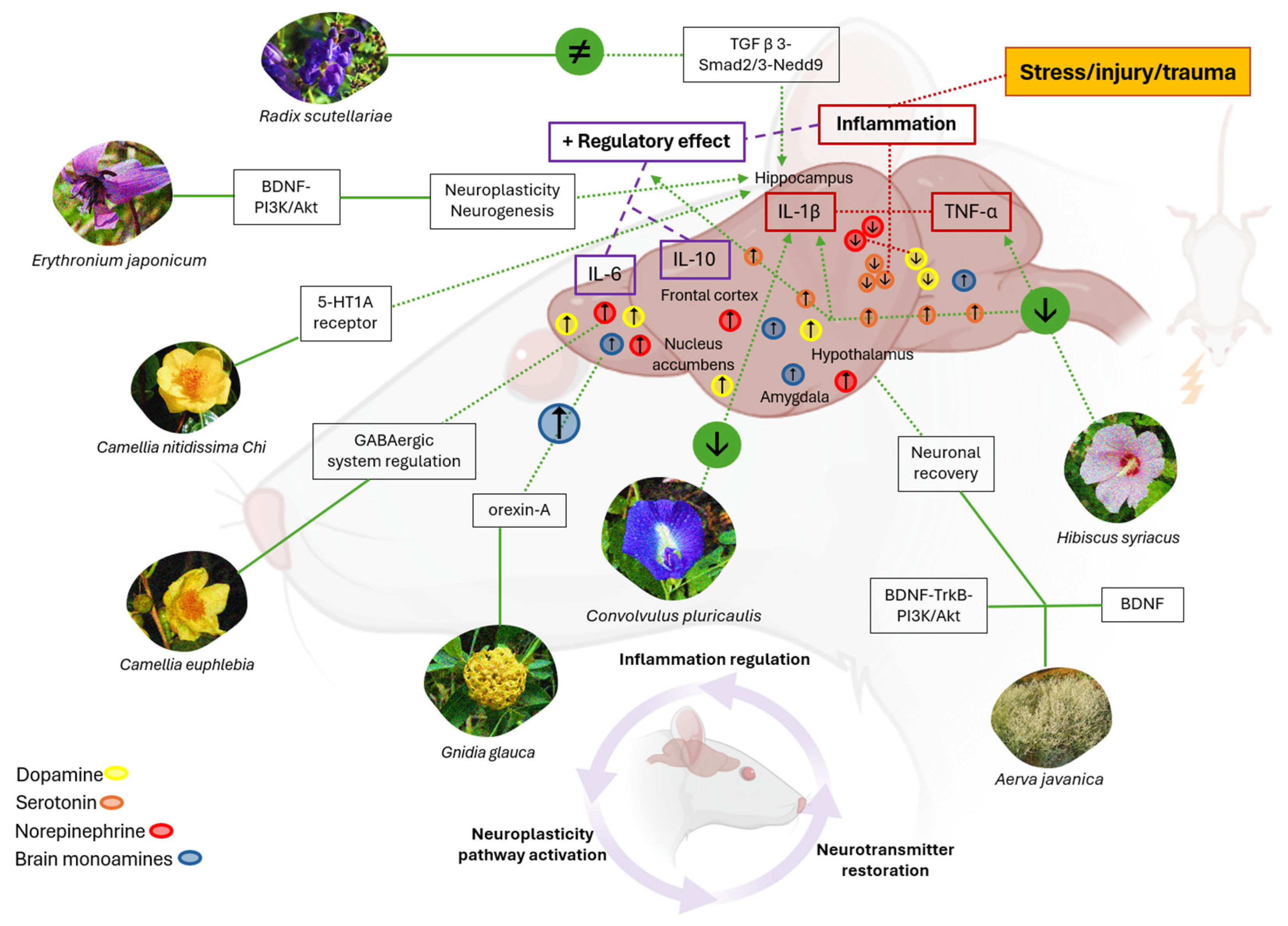
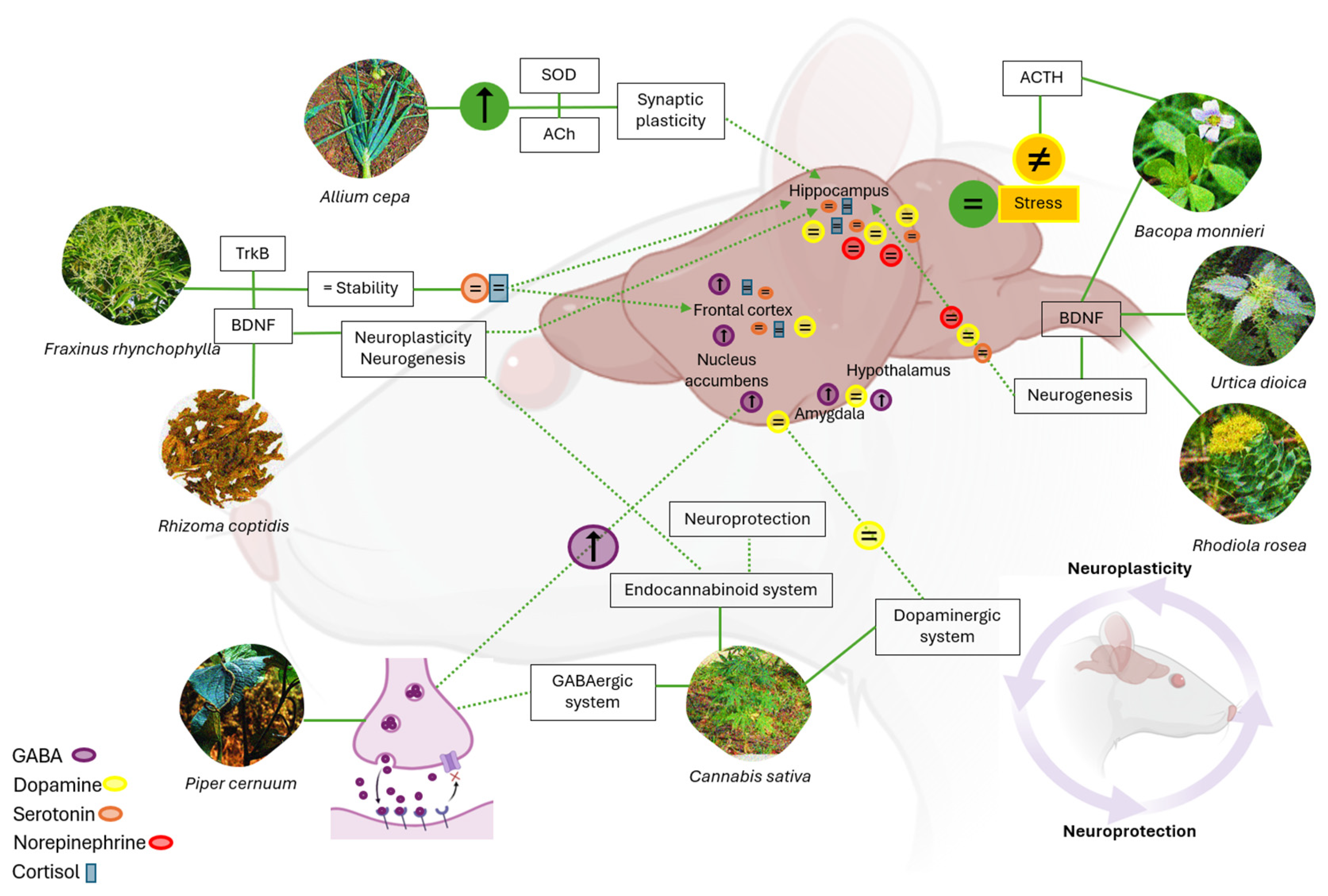
4. Discussion
4.1. Phytomedicine Impact on Cytokines, Neurotransmitters, and Neuroplasticity
4.2. Role of Neurotransmitters in Neuroplasticity and Neuroprotection
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| MDs | mental disorders |
| HPA | hypothalamic–pituitary–adrenal |
| WHO | World Health Organization |
| GABA | gamma-aminobutyric acid |
| CBD | cannabidiol |
| BDNF | brain-derived neurotrophic factor |
| THC | tetrahydrocannabinol |
| TNF-α | tumor necrosis factor alpha |
| IL-1β | interleukin-1 beta |
| IL-6 | interleukin-6 |
| IL-10 | interleukin-10 |
| AChE | acetylcholinesterase |
Appendix A
| Database | Search Date | Search Equation | Articles Found |
|---|---|---|---|
| Scopus | 1 October 2025 | (((((((Plants) OR (Medicinal plants)) OR (Phytochemicals)) OR (Traditional Medicine)) OR (Phytotherapy)) AND (Neuronal Plasticity)) AND (Neuroprotective Agents)) AND (Mental Disorder) | 1950 |
| PubMed | 1 November 2025 | (((((((Plants) OR (Medicinal plants)) OR (Phytochemicals)) OR (Traditional Medicine)) OR (Phytotherapy)) AND (Neuronal Plasticity)) AND (Neuroprotective Agents)) AND (Mental Disorder) | 46 |
| Dimensions | 1 May 2025 | (((((((Plants) OR (Medicinal plants)) OR (Phytochemicals)) OR (Traditional Medicine)) OR (Phytotherapy)) AND (Neuronal Plasticity)) AND (Neuroprotective Agents)) AND (Mental Disorder) | 18 |
References
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: New approaches to hippocampal neurogenesis. J. Neuroinflammation 2023, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Duman, R. Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Mol. Psychiatry 2020, 25, 530–543. [Google Scholar] [CrossRef]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as Neuroprotective Agent: From Bench to Bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Petković, A.; Chaudhury, D. Encore: Behavioural animal models of stress, depression and mood disorders. Front. Behav. Neurosci. 2022, 16, 931964. [Google Scholar] [CrossRef]
- Lashgari, N.A.; Roudsari, N.M.; Shamsnia, H.S.; Shayan, M.; Momtaz, S.; Abdolghaffari, A.H. TLR/mTOR inflammatory signaling pathway: Novel insight for the treatment of schizophrenia. Can. J. Physiol. Pharmacol. 2023, 102, 150–160. [Google Scholar] [CrossRef]
- Fathinezhad, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Depression and Treatment with Effective Herbs. Curr. Pharm. Des. 2019, 25, 738–745. [Google Scholar] [CrossRef]
- World Health Organization. The WHO Special Initiative for Mental Health (2019–2023): Universal Health Coverage for Mental Health. World Health Organization. 2019. Licencia: CC BY-NC-SA 3.0 IGO. Available online: https://iris.who.int/handle/10665/310981 (accessed on 7 December 2023).
- Arias, D.; Saxena, S.; Verguet, S. Quantifying the global burden of mental disorders and their economic value. eClinicalMedicine 2022, 54, 101675. [Google Scholar] [CrossRef]
- Moitra, M.; Owens, S.; Hailemariam, M.; Wilson, K.S.; Mensa-Kwao, A.; Gonese, G.; Kamamia, C.K.; White, B.; Young, D.M.; Collins, P.Y. Global Mental Health: Where We Are and Where We Are Going. Curr. Psychiatry Rep. 2023, 25, 301–311. [Google Scholar] [CrossRef]
- Asher, G.N.; Gerkin, J.; Gaynes, B.N. Complementary Therapies for Mental Health Disorders. Med. Clin. N. Am. 2017, 101, 847–864. [Google Scholar] [CrossRef] [PubMed]
- Rosson, S.; de Filippis, R.; Croatto, G.; Collantoni, E.; Pallottino, S.; Guinart, D.; Brunoni, A.R.; Dell’osso, B.; Pigato, G.; Hyde, J.; et al. Brain stimulation and other biological non-pharmacological interventions in mental disorders: An umbrella review. Neurosci. Biobehav. Rev. 2022, 139, 104743. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Stein, D.J. Generalised Anxiety Disorder and Depression: Contemporary Treatment Approaches. Adv. Ther. 2021, 38 (Suppl. 2), 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother. Res. 2018, 32, 1147–1162. [Google Scholar] [CrossRef]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef]
- Ota, M.; Ni, H.; Maki, Y.; Kato, D.; Moriguchi, S.; Nakayama, S.; Oiwa, Y.; Ishiuchi, K.; Makino, T. Binding activity of Valeriana fauriei root extract on GABAA receptor flunitrazepam sites and distribution of its active ingredients in the brain of mice—A comparison with that of V. officinalis root. J. Ethnopharmacol. 2021, 278, 114262. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D. Saffron (Crocus sativus) for depression: A systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Hum. Psychopharmacol. 2014, 29, 517–527. [Google Scholar] [CrossRef]
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Khallouki, F.; El Hessni, A.; Ibrahimi, S.O.; Latruffe, N.; et al. Saffron (Crocus sativus L.): A Source of Nutrients for Health and for the Treatment of Neuropsychiatric and Age-Related Diseases. Nutrients 2022, 14, 597. [Google Scholar] [CrossRef]
- Hirshler, Y.; Doron, R. Neuroplasticity-related mechanisms underlying the antidepressant-like effects of traditional herbal medicines. Eur. Neuropsychopharmacol. 2017, 27, 945–958. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Brivio, P.; Dell’Agli, M.; Calabrese, F. Botanicals as Modulators of Neuroplasticity: Focus on BDNF. Neural Plast. 2017, 2017, 5965371. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaça, M.V.; Scarante, F.F.; Joca, S.R.L.; Sales, A.J.; Gomes, F.V.; Sonego, A.B.; Rodrigues, N.S.; Galve-Roperh, I.; Guimarães, F.S. Plastic and Neuroprotective Mechanisms Involved in the Therapeutic Effects of Cannabidiol in Psychiatric Disorders. Front. Pharmacol. 2017, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Gupta, G.L.; Fernandes, J. Protective effect of Convolvulus pluricaulis against neuroinflammation associated depressive behavior induced by chronic unpredictable mild stress in rat. Biomed. Pharmacother. 2019, 109, 1698–1708. [Google Scholar] [CrossRef]
- Samad, N.; Saleem, A. Administration of Allium cepa L. bulb attenuates stress-produced anxiety and depression and improves memory in male mice. Metab. Brain Dis. 2018, 33, 271–281. [Google Scholar] [CrossRef]
- Kim, Y.H.; Im, A.R.; Park, B.K.; Paek, S.H.; Choi, G.; Kim, Y.R.; Whang, W.K.; Lee, K.H.; Oh, S.-E.; Lee, M.Y. Antidepressant-Like and Neuroprotective Effects of Ethanol Extract from the Root Bark of Hibiscus syriacus L. BioMed Res. Int. 2018, 2018, 7383869. [Google Scholar] [CrossRef]
- Kim, Y.R.; Park, B.K.; Kim, Y.H.; Shim, I.; Kang, I.C.; Lee, M.Y. Antidepressant Effect of Fraxinus rhynchophylla Hance Extract in a Mouse Model of Chronic Stress-Induced Depression. BioMed Res. Int. 2018, 2018, 8249563. [Google Scholar] [CrossRef]
- Kumar, S.; Mondal, A.C. Neuroprotective, Neurotrophic and Anti-oxidative Role of Bacopa monnieri on CUS Induced Model of Depression in Rat. Neurochem. Res. 2016, 41, 3083–3094. [Google Scholar] [CrossRef]
- Chen, W.C.; Lai, Y.S.; Lin, S.H.; Lu, K.H.; Lin, Y.E.; Panyod, S.; Ho, C.-T.; Sheen, L.-Y. Anti-depressant effects of Gastrodia elata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling. J. Ethnopharmacol. 2016, 182, 190–199. [Google Scholar] [CrossRef]
- He, D.; Wang, X.; Zhang, P.; Luo, X.; Li, X.; Wang, L.; Li, S.; Xu, Y. Evaluation of the Anxiolytic and Antidepressant Activities of the Aqueous Extract from Camellia euphlebia Merr. ex Sealy in Mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 618409. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Udayabanu, M. Urtica dioica extract attenuates depressive like behavior and associative memory dysfunction in dexamethasone induced diabetic mice. Metab. Brain Dis. 2014, 29, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.A.; Jurcevic, J.D.; Malheiros, A.; Cazarin, C.A.; Dalmagro, A.P.; do Espírito Santo, C.; da Silva, L.M.; de Souza, M.M. Neuropharmacology Potential of the Hydroalcoholic Extract from the Leaves of Piper cernuum: Anxiolytic, Hypnotic, and Antidepressant-Like Effects. Evid.-Based Complement. Altern. Med. 2023, 2023, 1183809. [Google Scholar] [CrossRef]
- Arshad, H.M.; Ahmad, F.U.; Lodhi, A.H. Methanolic Extract of Aerva javanica Leaves Prevents LPS-Induced Depressive Like Behavior in Experimental Mice. Drug Des. Devel. Ther. 2022, 16, 4179–4204. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, B.; Gao, C.; Yan, S.; Du, Q.; Yu, H.; Li, P. Camellia nitidissima Chi extract promotes adult hippocampal neurogenesis and attenuates chronic corticosterone-induced depressive behaviours through regulating Akt/GSK3β/CREB signaling pathway. J. Funct. Foods 2022, 95, 105199. [Google Scholar] [CrossRef]
- Arika, W.M.; Kibiti, C.M.; Njagi, J.M.; Ngugi, M.P. Effects of DCM Leaf Extract of Gnidia glauca (Fresen) on Locomotor Activity, Anxiety, and Exploration-Like Behaviors in High-Fat Diet-Induced Obese Rats. Behav. Neurol. 2019, 2019, 7359235. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, C.; Xiao, D.; Zhang, W.; Zhou, L.; Gu, S.; Qu, R. Radix Scutellariae Ameliorates Stress-Induced Depressive-Like Behaviors via Protecting Neurons through the TGFβ3-Smad2/3-Nedd9 Signaling Pathway. Neural Plast. 2020, 2020, 8886715. [Google Scholar] [CrossRef]
- Qi, Y.; Ni, S.; Heng, X.; Qu, S.; Ge, P.; Zhao, X.; Yao, Z.; Guo, R.; Yang, N.; Zhang, Q.; et al. Uncovering the Potential Mechanisms of Coptis chinensis Franch. for Serious Mental Illness by Network Pharmacology and Pharmacology-Based Analysis. Drug Des. Devel. Ther. 2022, 16, 325–342. [Google Scholar] [CrossRef]
- Lim, D.W.; Park, J.; Han, D.; Lee, J.; Kim, Y.T.; Lee, C. Anti-Inflammatory Effects of Asian Fawn Lily (Erythronium japonicum) Extract on Lipopolysaccharide-Induced Depressive-Like Behavior in Mice. Nutrients 2020, 12, 3809. [Google Scholar] [CrossRef]
- Yu, H.; Shao, S.; Xu, J.; Guo, H.; Zhong, Z.; Xu, J. Persimmon leaf extract alleviates chronic social defeat stress-induced depressive-like behaviors by preventing dendritic spine loss via inhibition of serotonin reuptake in mice. Chin. Med. 2022, 17, 65. [Google Scholar] [CrossRef]
- Liu, E.Y.; Yang, C.L.; Tsai, J.C.; Cheng, H.Y.; Peng, W.H. Antidepressive mechanisms of rhynchophylline in mice with chronic unpredictable stress-induced depression. J. Ethnopharmacol. 2023, 309, 116302. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, J.; Hamedeyazdan, S.; Torbati, M.; Farajdokht, F.; Fakhari, A.; Mahmoudi, J.; Araj-Khodaei, M.; Sadigh-Eteghad, S. Melissa officinalis L. hydro-alcoholic extract inhibits anxiety and depression through prevention of central oxidative stress and apoptosis. Exp. Physiol. 2020, 105, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Pei, W.J.; Liu, L.; Chen, K.; Cheng, Y.; Piao, X.L. Neuroprotective effects of gypenosides on LPS-induced anxiety and depression-like behaviors. Int. Immunopharmacol. 2024, 143 Pt 1, 113367. [Google Scholar] [CrossRef] [PubMed]
- Fernandes E Mendonça, L.M.; Joshi, A.B.; Bhandarkar, A.; Shaikh, S.; Fernandes, S.; Joshi, H.; Joshi, S. Potential anxiolytic therapeutics from Hybanthus enneaspermus (L.) F. Muell.—Mitigate anxiety by plausibly modulating the GABAA-Cl− channel. Neurochem. Int. 2024, 178, 105804. [Google Scholar] [CrossRef]
- Tao, X.; Zhou, Y.; Wang, Z.; Wang, L.; Xia, T.; Yan, M.; Chang, Q. Cajaninstilbene acid ameliorates depression-like behaviors in mice by suppressing TLR4/NF-κB mediated neuroinflammation and promoting autophagy. Behav. Brain Res. 2024, 471, 115142. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Pei, Y.; Yang, Q.; Zheng, L.; Wang, G.; Sun, Y.; Yang, W.; Liu, L. The total alkaloids of Sophora alopecuroides L. improve depression-like behavior in mice via BDNF-mediated AKT/mTOR signaling pathway. J. Ethnopharmacol. 2023, 316, 116723. [Google Scholar] [CrossRef]
- Estela-Zape, J.L.; Libreros-Chica, D.C.; Noreña-Buitrón, L.D.; Sierra-Olea, J.M. Efectos terapéuticos de las plantas medicinales en los trastornos de ansiedad: Revisión exploratoria. Psiquiatr. Biológica 2024, 31, 100495. [Google Scholar] [CrossRef]
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gupta, G. et al. (2019) [27] |  |  |  |  |  |  |  |  |  |  | 8 |
| Samad, N. et al. (2017) [28] |  |  |  |  |  |  |  |  |  |  | 8 |
| Kim, Y. et al. (2018) [29] |  |  |  |  |  |  |  |  |  |  | 8 |
| Kim, Y. et al. (2018) [30] |  |  |  |  |  |  |  |  |  |  | 8 |
| Kumar, S. et al. (2016) [31] |  |  |  |  |  |  |  |  |  |  | 8 |
| Chen, W. et al. (2016) [32] |  |  |  |  |  |  |  |  |  |  | 8 |
| He, D. et al. (2015) [33] |  |  |  |  |  |  |  |  |  |  | 8 |
| Patel, S. et al. (2014) [34] |  |  |  |  |  |  |  |  |  |  | 8 |
| Maia, M. et al. (2023) [35] |  |  |  |  |  |  |  |  |  |  | 7 |
| Arshad, H. et al. (2022) [36] |  |  |  |  |  |  |  |  |  |  | 6 |
| Tsoi, B. (2022) [37] |  |  |  |  |  |  |  |  |  |  | 9 |
| Arika, W. et al. (2019) [38] |  |  |  |  |  |  |  |  |  |  | 8 |
| Zhao, F. et al. (2020) [39] |  |  |  |  |  |  |  |  |  |  | 6 |
| Qi, Y. et al. (2022) [40] |  |  |  |  |  |  |  |  |  |  | 8 |
| Lim, D. et al. (2020) [41] |  |  |  |  |  |  |  |  |  |  | 6 |
| Yu, H. et al. (2022) [42] |  |  |  |  |  |  |  |  |  |  | 8 |
| Liu, E.Y. et al. (2023) [43] |  |  |  |  |  |  |  |  |  |  | 6 |
| Ghazizadeh, J. et al. (2020) [44] |  |  |  |  |  |  |  |  |  |  | 8 |
| Zhao, F. et al. (2020) [39] |  |  |  |  |  |  |  |  |  |  | 4 |
| Guo, M. et al. (2024) [45] |  |  |  |  |  |  |  |  |  |  | 6 |
| Fernandes, L.M. et al. (2024) [46] |  |  |  |  |  |  |  |  |  |  | 4 |
| Tao, X. et al. (2024) [47] |  |  |  |  |  |  |  |  |  |  | 6 |
| Li, J. et al. (2023) [48] |  |  |  |  |  |  |  |  |  |  | 9 |
| Author and Year | Plants | Family | Objective | Sample Size | Study Design | Control Group | Instruments | Results |
|---|---|---|---|---|---|---|---|---|
| Gupta, G. et al., 2019 [27] | Convolvulus pluricaulis | Convolvulaceae | Effects on neuroinflammation and monoamines in depression | 36 male Wistar rats | In vivo, in vitro | 6 groups: no stress + control, CUMS + control, 3 CPE groups, and CUMS + fluoxetine | Chromatography, acute toxicity, and blood and brain extraction | CPE reduced cytokines and enhanced neurotransmitter levels |
| Samad, N. et al., 2017 [28] | Allium cepa | Amarilidáceas | Impact on biochemical and behavioral changes | 24 male albino Wistar rats | In vivo, in vitro | Onion extract vs. control | Brain tissue extraction | Improved anxiety, depression, and memory |
| Kim, Y. et al., 2018 [29] | Hibiscus syriacus | Malvaceae | Effects on depressive behaviors and neurotrophic factors | 36 male C57/BL6 mice | In vivo, in vitro | 6 groups: saline and stress + treatments | Cell culture and neuroblastoma cells | Reduced corticosterone levels |
| Kim, Y. et al., 2018 [30] | Fraxinus rhynchophylla | Oleaceae | Prevention of depressive behavior post-stress | Male C57BL/6 mice | In vivo, in vitro | PBS controls and FX treatments | Brain tissue samples | FX reduced depressive behaviors via serotonin modulation |
| Kumar, S. et al., 2016 [31] | Bacopa monnieri | Plantaginaceae | Neuroprotective effects on stress-induced depression | 32 male Sprague-Dawley rats | In vivo and in vitro | 4 groups: stress, BME, and IMI treatments | Biochemical assays and brain sectioning | BME reversed depressive effects by enhancing antioxidant levels |
| Chen, W. et al., 2016 [32] | Gastrodia elata | Orchidaceae | Antidepressant compounds and neurogenesis | 40 male Sprague-Dawley rats | In vivo and in vitro | 4 groups: WGE, GAS, and HBA treatments | HPLC-UV | WGE modulated monoamine metabolism |
| He, D. et al., 2015 [33] | Camellia euphlebia | Theaceae | Anxiolytic and antidepressant activities | 30 male Kunming mice | In vivo and in vitro | 5 groups: NaCl, diazepam, fluoxetine, and CEE | Brain homogenization | Increased neurotransmitters and dopamine release |
| Patel, S. et al., 2014 [34] | Urtica dioica | Urticaceae | Effects on diabetes-induced cognitive impairment | Adult Swiss albino mice | In vivo and in vitro | 5 groups: dexamethasone and UD treatments | HPLC-UV | Reversed depressive behaviors by reducing oxidative stress |
| Maia, M. et al., 2023 [35] | Piper cernuum | Piperaceae | Neuropharmacological effects | Female Swiss mice | In vivo and in vitro | GABA estimation via spectrophotometry | GABA levels | Exhibited antidepressant and anxiolytic properties |
| Arshad, H. et al., 2022 [36] | Aer Aerva javanica | Amaranthaceae | Pharmacological activities in LPS-induced depression | 60 male Swiss albino mice | In vivo, in vitro, and in silico | 6 groups: saline, imipramine, and Aj Cr treatments | Molecular docking | Aj Cr showed antidepressant effects |
| Tsoi, B., 2022 [37] | Camellia nitidissima | Theaceae | Hippocampal neurogenesis and corticosterone-induced depression | 72 male C57BL/6 N mice | In vivo and in vitro | 6 groups: control, CORT, and CNC treatments | Plasma analysis and hippocampal neuron culture | CNC improved behavior through Akt/GSK3β/CREB signaling |
| Arika, W. et al., 2019 [38] | Gnidia glauca | Thymelaeaceae | Effects on locomotor and anxiety-like behaviors | 30 female rats | In vivo | 6 groups: diet + control and treatments | GC-MS | Increased locomotor and exploratory behavior |
| Zhao, F. et al., 2020 [39] | Radix Scutellariae | Lamiaceae | Antidepressant effects in CUMS model | 50 male adult ICR mice | In vivo and in vitro | CUMS, fluoxetine, and RS treatments | Hippocampus extraction | Improved behaviors via TGF β pathway |
| Qi, Y. et al., 2022 [40] | Coptis chinensis | Ranunculaceae | Therapeutic mechanism in severe mental disorders | 60 male SPF C57BL/6 mice | In vivo, in vitro, and in silico | 6 groups: DZP and RC treatments | Blood and brain tissue extraction and molecular docking | Demonstrated anxiolytic effects |
| Lim, D. et al., 2020 [41] | Erythronium japonicum | Liliáceas | Anti-inflammatory effects in LPS-induced depression | 50 male ICR mice | In vivo and in vitro | 5 groups: sham, control, and treatments | Hippocampus extraction dose of EJE | Reduced neuroinflammation and depressive behaviors |
| Yu H; et al., 2022 [42] | Diospyros kaki Thunb | Ebenaceae | Activity on neurotransmitters in depression | CD-1 male mice | In vivo and in vitro | 4 groups: low or high doses of PLE or fluoxetine | Golgi staining and immunofluorescence | It relieved depressive behaviors by inhibiting serotonin reuptake |
| Liu E; et al., 2023 [43] | Uncaria rhynchophylla | Rubiáceas | Antidepressant effects of RH | C57BL/6 male mice | In vivo and in vitro | 6 groups with different doses of RH or fluoxetine | Western blot test | Increased 5-HT levels in the cortex and hippocampus |
| Ghazizadeh J et al., 2020 [44] | Melissa officinalis | Lamiaceae | Antidepressant effects of MO | 60 male albino BALB/c mice | In vivo and in vitro | 5 randomized groups with different stress techniques | Homogenization and TBAR assay | It attenuated stress-induced anxious and depressive behaviors |
| Zhao F. et al., 2020 [39] | Radix Scutellariae | Lamiaceae | Antidepressant effects and action on the TGF β signaling pathway | Adult male ICR mice | In vivo | 2 groups | Immunohistochemistry and Nissl staining | Reversed the decrease in TGF β 3 protein |
| Guo, M; et al., 2024 [45] | Gynostemma pentaphyllum | Cucurbitaceae. G. pentaphyllum | Neuroprotective effects of Gyp on anxiety and depression | Mice | In vivo | Gyp and fluoxetine hydrochloride | Ultrasonic sonication and resin chromatography | Improved anxiety and depression |
| Fernandes, LM; et al., 2024 [46] | Hybanthus enneaspermus | Violaceae | Anxiolytic activity of ethanolic extract of Hybanthus enneaspermus | Mice | In vivo and in silico | Hybanthus enneaspermus and diazepam hydrochloride | Extraction, fractionation, and biofraction | Significantly mitigated anxiety |
| Tao, X; et al., 2024 [47] | Ácido cajaninstilbeno | Fabaceae | Effects of CSA on depressive behavior | Male C57BL/6 J and BALB/ mice | In vivo and in vitro | 2 groups: CSA | Molecular analysis | It exerted antidepressant effects |
| Li, J; et al., 2023 [48] | Sophora alopecuroides L. | Fabáceas | Ameliorative effect of Sophora alopecuroides L. on depressive behavior | Mice | In vivo and in vitro | ALK from Sophora alopecuroides L. | Molecular biology and incubation | It showed antidepressant effects |
| Author and Year | Plants/Segment | Mental Disorders | Administration Duration and Dosage | Neurotransmitters | Effects on the Nervous System |
|---|---|---|---|---|---|
| Gupta, G. et al., 2019 [27] | Convolvulus pluricaulis (dried leaves) | Depression | 50–100 mg/kg CPE or 10 mg/kg fluoxetine, once daily for 7 days | Serotonin and norepinephrine | Restored serotonin and norepinephrine levels in the hippocampus and prefrontal cortex |
| Samad, N. et al., 2017 [28] | Allium cepa (stem) | Anxiety and depression | 200 mg/kg/day for 14 days | Acetylcholine | Increased brain acetylcholine, enhancing memory processes through neuroplasticity |
| Kim, Y. et al., 2018 [29] | Hibiscus syriacus (root) | Depression and stress | 200 mg/kg for 22 days | Serotonin | Reduced depressive behavior via CREB/BDNF signaling, enhancing cognitive function |
| Kim, Y. et al., 2018 [30] | Fraxinus rhynchophyl (stem) | Depression | 100–400 mg/kg for 2 weeks | Serotonin | Increased serotonin, decreased cortisol, and elevated BDNF in the hippocampus |
| Kumar, S. et al., 2016 [31] | Bacopa monnieri (leaves) | Depression | 80 mg/kg | BDNF | Improved behavioral anomalies and increased ACTH, corticosterone, BDNF, and hippocampal neurogenesis |
| Chen, W. et al., 2016 [32] | Gastrodia elata (stem) | Depression | 500 mg/kg WGE, 100 mg/kg GAS, and HBA for 2 weeks | Serotonin and monoamines | Decreased monoamine turnover and influenced the dopaminergic system |
| El, D. et al., 2015 [33] | Camellia euphlebia (leaves) | Anxiety and depression | 100–400 mg/kg/day for 7 days | GABA, norepinephrine, and dopamine | Increased 5-HT and DA levels, providing anxiolytic and antidepressant effects |
| Patel, S. et al., 2014 [34] | Urtica dioica (leaves) | Depression | 50–100 mg/kg/day | Acetylcholine | Modulated acetylcholine release, improving memory and depressive symptoms |
| Maia, M. et al., 2023 [35] | Piper cernuum (leaves) | Depression and anxiety | 50–150 mg/kg for 15 days | GABA and serotonin | Increased GABA levels, optimizing neurotransmission |
| Arshad, H. et al., 2022 [36] | Aerva javanic (leaves) | Depression | 100–500 mg/kg for 14 days | Norepinephrine, dopamine, catecholamines, and BDNF | Normalized BDNF levels, reduced oxidative stress, and mitigated depressive behavior |
| Tsoi, B; 2022 [37] | Camellia nitidissima (dried leaves) | Depression and anxiety | 10–50 mg/kg for 40 days | Serotonin | Increased serotonin levels and promoted neurogenesis |
| Arika, W. et al., 2019 [38] | Gnidia glauca (fresh leaves) | Anxiety | 200–300 mg/kg for 12 weeks | GABA and dopamine | Anxiolytic effects through dopamine release and GABAergic activation |
| Zhao, F. et al., 2020 [39] | Radix Scutellariae (dried leaves) | Depression | 1.5 g/kg for 4 weeks | FGVβ3 and Nedd9 | Modulated neuroprotection, anxiolytic effects, and TGF β 3–Smad2/3–Nedd9 pathway |
| Qi, Y. et al., 2022 [40] | Coptis chinensis (fresh leaves) | Anxiety | 146–584 mg/kg/day for 6 days | Dopamine and serotonin | Provided neuroprotection by regulating inflammatory factors |
| Lim, D. et al., 2020 [41] | Erythronium japonicum (leaves) | Depression | 100–300 mg/kg for 7 days | BDNF | Reduced inflammatory cytokines and improved depressive behavior by activating BDNF–PI3K/Akt pathway |
| Yu, H. et al., 2022 [42] | Diospyros kaki Thunb (leaves) | Depression | 30–60 mg/kg or fluoxetine 10.0 mg/kg for 10 days | Serotonin | Inhibits 5HT reuptake and regulates the BDNF signaling pathway in the cortex |
| Liu, E. et al., 2023 [43] | Uncaria rhynchophylla (leaves) | Depression | RH at 25 mg/kg or fluoxetine 10 mg/kg for 28 days | Serotonin | Significantly increased 5-HT levels in the cortex |
| Ghazizadeh, J; et al., 2020 [44] | Melissa officinalis (leaves) | Depression and anxiety | MO at 50, 75, and 150 mg kg, for 14 days | Serotonin | Anti-inflammatory, antimicrobial, antioxidant, sedative, and neuroprotective effects |
| Zhao, F. et al., 2020 [39] | Radix Scutellariae (root) | Depression | RS at 0.75 g/kg and fluoxetine at 1.5 g/kg for 4 weeks | Serotonin and GABA | Mediated the TGF β 3–Smad2/3–Nedd9 signaling pathway, potential mechanism of the neuroprotective effect |
| Guo, M. et al., 2024 [45] | Gynostemma pentaphyllum (leaves) | Anxiety and depression | Gyp at 50, 100, or 200 mg/kg with fluoxetine hydrochloride | NLRP3/Caspase-1/ASC in PFC | Optimization in cytokine expression in the hippocampus and PFC, with IL-1β showing the most pronounced regulation |
| Fernandes, LM. et al., 2024 [46] | Hybanthus enneaspermus (leaves) | Anxiety | 400 mg/kg Hybanthus enneaspermus | GABA, 5-HT, NA, and DA | Improved GABA levels, attenuated glutamate, and enhanced levels of NA, 5-HT, DA, and antioxidant enzymes |
| Tao, X. et al., 2024 [47] | Ácid cajaninstilbeno: Cajanus cajan (legume) | Depression | Group 1: CSA (7.5, 15, and 30 mg/kg) Group 2: CSA (7.5–30 mg/kg) | TLR4/NF-κB | It counteracted the activation of the TLR4/NF-κB pathway and the reduction in autophagy levels |
| Li, J. et al., 2023 [48] | Sophora alopecuroides L. (leaves) | Depression | ALK from Sophora alopecuroides L. | BDNF–AKT–mTOR | Antidepressant effect of ALKs from Sophora alopecuroides L. based on the BDNF–AKT–mTOR signaling pathway of the prefrontal cortex |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estela-Zape, J.L.; Sanclemente-Cardoza, V.; Noreña-Buitrón, L.D.; Ordoñez-Mora, L.T. Utilization of Medicinal Plants in Mental Disorders: Neuroplasticity and Neuroprotection in Biomodels. Brain Sci. 2025, 15, 366. https://doi.org/10.3390/brainsci15040366
Estela-Zape JL, Sanclemente-Cardoza V, Noreña-Buitrón LD, Ordoñez-Mora LT. Utilization of Medicinal Plants in Mental Disorders: Neuroplasticity and Neuroprotection in Biomodels. Brain Sciences. 2025; 15(4):366. https://doi.org/10.3390/brainsci15040366
Chicago/Turabian StyleEstela-Zape, Jose Luis, Valeria Sanclemente-Cardoza, Lizeth Dayana Noreña-Buitrón, and Leidy Tatiana Ordoñez-Mora. 2025. "Utilization of Medicinal Plants in Mental Disorders: Neuroplasticity and Neuroprotection in Biomodels" Brain Sciences 15, no. 4: 366. https://doi.org/10.3390/brainsci15040366
APA StyleEstela-Zape, J. L., Sanclemente-Cardoza, V., Noreña-Buitrón, L. D., & Ordoñez-Mora, L. T. (2025). Utilization of Medicinal Plants in Mental Disorders: Neuroplasticity and Neuroprotection in Biomodels. Brain Sciences, 15(4), 366. https://doi.org/10.3390/brainsci15040366







