Abstract
(1) Background. Eye movement abnormalities are increasingly recognized as early biomarkers of Parkinson’s disease (PD), reflecting both motor and cognitive dysfunction. Advances in eye-tracking technology provide objective, quantifiable measures of saccadic impairments, fixation instability, smooth pursuit deficits, and pupillary changes. These advances offer new opportunities for early diagnosis, disease monitoring, and neurorehabilitation. (2) Objective. This narrative review explores the relationship between oculomotor dysfunction and PD pathophysiology, highlighting the potential applications of eye tracking in clinical and research settings. (3) Methods. A comprehensive literature review was conducted, focusing on peer-reviewed studies examining eye movement dysfunction in PD. Relevant publications were identified through PubMed, Scopus, and Web of Science, using key terms, such as “eye movements in Parkinson’s disease”, “saccadic control and neurodegeneration”, “fixation instability in PD”, and “eye-tracking for cognitive assessment”. Studies integrating machine learning (ML) models and VR-based interventions were also included. (4) Results. Patients with PD exhibit distinct saccadic abnormalities, including hypometric saccades, prolonged saccadic latency, and increased anti-saccade errors. These impairments correlate with executive dysfunction and disease progression. Fixation instability and altered pupillary responses further support the role of oculomotor metrics as non-invasive biomarkers. Emerging AI-driven eye-tracking models show promise for automated PD diagnosis and progression tracking. (5) Conclusions. Eye tracking provides a reliable, cost-effective tool for early PD detection, cognitive assessment, and rehabilitation. Future research should focus on standardizing clinical protocols, validating predictive AI models, and integrating eye tracking into multimodal treatment strategies.
1. Introduction
Parkinson’s disease (PD) is a chronic, progressive neurodegenerative disorder primarily affecting dopaminergic neurons in the substantia nigra. This degeneration leads to motor dysfunctions such as bradykinesia, rigidity, and resting tremor [1]. However, beyond these hallmark motor symptoms, non-motor impairments significantly impact patients’ quality of life. Among these, cognitive decline, executive dysfunction, visual disturbances, spatial memory, and language function have gained increasing attention in recent years [2,3].
Emerging evidence suggests that eye movement abnormalities are among the earliest detectable neurological deficits in PD, preceding some of the more commonly recognized motor impairments [4]. These deficits include alterations in saccadic eye movements, fixation stability, smooth pursuit tracking, and pupillary responses, all of which are linked to dysfunctions in cortico-basal ganglia loops and other neural pathways involved in oculomotor control [5]. Given that PD pathology extends beyond the basal ganglia to include brainstem structures such as the superior colliculus and frontal eye fields, disruptions in these circuits likely contribute to the oculomotor disturbances observed in affected individuals [4,5,6,7].
These disruptions in eye movement of Parkinson’s patients are closely linked to the degeneration of the dopaminergic system, which affects the basal ganglia and frontal eye fields, both critical for voluntary eye movements. The altered saccades, in particular, involve difficulties in accurately shifting gaze towards a target, often resulting in incomplete eye movements that require compensatory corrective saccades.
These oculomotor dysfunctions are not only indicative of motor impairments but also show a significant relationship with cognitive dysfunction, particularly in executive control and attention. In addition to these well-known eye movement deficits, the relationship between ocular and manual coordination, or eye–hand coupling, is of great interest in PD. Although our review focuses on the eye-tracking measures of eye movement abnormalities, it is important to recognize how these deficits extend to broader motor activities. Alterations in visual processing, such as delayed or impaired saccades, have been shown to affect eye–hand coordination, impairing the ability to accurately reach and grasp objects, a crucial task for daily functioning in PD patients. This connection between eye movement and motor actions highlights the relevance of eye tracking as a tool for assessing the broader functional impairments in PD.
In recent years, eye-tracking technology has gained considerable interest as a non-invasive tool for assessing oculomotor function in neurological disorders. Unlike traditional clinical assessments, which rely on subjective observation, eye tracking provides high-precision data that can be analyzed in real time, allowing researchers and clinicians to monitor subtle changes in eye movements that may serve as early biomarkers of disease onset and progression [8].
Numerous studies have explored the potential of eye tracking in PD research, particularly for early detection, differential diagnosis, and disease monitoring. Given that eye movement impairments are closely linked to cognitive and motor dysfunctions, tracking these deficits over time may provide valuable insights into neurodegenerative trajectories and help predict cognitive decline in patients with PD [8,9]. The integration of eye-tracking data with artificial intelligence (AI) and machine learning algorithms has the potential to enhance diagnostic accuracy, facilitating personalized treatment approaches [10].
Oculomotor disturbances in PD, including hypometric saccades, prolonged saccadic latency, and fixation instability, are not only important for diagnostic purposes but also offer significant implications for therapeutic interventions. Recent studies suggest that eye-tracking technology can be used to monitor the effectiveness of various treatments, such as dopaminergic therapy [9], cognitive rehabilitation [11], and visual–motor training [11]. Emerging therapies integrating virtual reality (VR) and biofeedback aim to enhance oculomotor function and eye–hand coordination, potentially improving both motor and cognitive outcomes in PD patients [11,12]. As such, eye tracking could play a pivotal role in the development of personalized rehabilitation strategies tailored to the specific needs of PD patients, offering a more targeted approach to treatment [13].
This narrative review synthesizes the current literature on eye movement impairments in Parkinson’s disease, focusing on saccadic abnormalities, fixation instability, smooth pursuit deficits, and pupillary changes. We discuss how these impairments correlate with disease severity, cognitive dysfunction, and neurophysiological alterations. We also emphasize the clinical implications of eye-tracking technology for both diagnosis and therapeutic intervention. We also explore emerging trends in AI-driven analysis, virtual reality-based rehabilitation programs, and the integration of eye tracking into multimodal neurophysiological assessments.
Through a comprehensive examination of existing studies, this review underscores the importance of eye movement analysis as a key component in PD research, advocating for the broader implementation of eye-tracking technologies in both clinical practice and experimental investigations. By establishing standardized assessment protocols and leveraging technological advancements, eye tracking could play a pivotal role in shaping the future of Parkinson’s disease management. It offers a promising avenue for early detection, progression monitoring, and therapeutic innovation.
In contrast to previous reviews that have focused primarily on diagnostic or cognitive aspects of oculomotor dysfunction in Parkinson’s disease, the present review offers a broader synthesis that includes motor–cognitive interactions, therapeutic implications, and innovative applications of emerging technologies such as AI-based analysis and VR-integrated rehabilitation. Several previous reviews have addressed aspects of oculomotor function in Parkinson’s disease. For example, Liao et al. [2] and Gibbs et al. [6] provided overviews of eye movement abnormalities with a focus on diagnostic potential. Others, such as Tsitsi et al. [14], have examined specific parameters like fixation duration and pupillary size in clinical populations. However, these reviews often focus on isolated features or specific clinical applications.
In contrast, the present work offers a comprehensive and integrative synthesis of the literature on saccadic impairments, fixation instability, smooth pursuit deficits, and pupillary dynamics, all assessed through eye tracking in PD. It also expands on prior work by discussing the role of machine learning, the clinical and therapeutic implications of these markers, and their relevance to rehabilitation strategies. This broader perspective highlights how eye tracking is evolving from a diagnostic tool to a potential instrument for monitoring disease progression and guiding personalized interventions. This comprehensive perspective aims to bridge the gap between clinical observations and technological innovation, providing a more holistic understanding of how eye-tracking metrics can inform both diagnosis and treatment.
2. Methods
This narrative review aims to synthesize and discuss the existing literature on eye movement abnormalities in Parkinson’s disease (PD) and explore the potential applications of eye-tracking technology for early diagnosis, disease monitoring, and rehabilitation. The literature search was performed between September 2024 and January 2025, focusing on peer-reviewed studies available at that time. Given the complexity of oculomotor dysfunction in PD, this review integrates findings from clinical studies, experimental research, and technological advancements, providing a comprehensive and interpretative synthesis of current knowledge rather than a systematic meta-analysis.
The literature included in this review was selected based on its relevance to the key themes of the study, specifically focusing on saccadic impairments, fixation instability, smooth pursuit deficits, and pupillary changes in PD. Studies exploring the clinical and technological applications of eye tracking in PD were also prioritized, with particular attention to early detection, disease progression monitoring, and neurorehabilitation strategies. The selection of articles was guided by thematic relevance rather than rigid inclusion or exclusion criteria, as the objective was to critically discuss diverse perspectives and emerging trends in the field.
Relevant studies were identified through structured searches in PubMed, Scopus, Web of Science, and Google Scholar, using tailored keyword combinations and Boolean operators (AND, OR) to optimize the search results. The search strategies applied were as follows: (1) PubMed: (“Parkinson’s disease” OR “PD”) AND (“eye-tracking” OR “oculomotor” OR “saccadic” OR “fixation instability” OR “smooth pursuit” OR “pupillary response”); (2) Scopus/Web of Science: TITLE-ABS-KEY (“Parkinson’s disease” AND “eye movements”) AND (“saccades” OR “fixation” OR “oculomotor” OR “eye-tracking” OR “pupil”); (3) Google Scholar: “eye-tracking in Parkinson’s disease” + “saccadic latency” + “fixation instability” + “cognitive impairment”. Additional articles were retrieved through citation tracking, particularly from review papers and key experimental studies in the field.
The discussion is structured around the primary oculomotor deficits observed in PD, analyzing how these impairments correlate with cognitive and motor dysfunction and how eye-tracking metrics can be leveraged for diagnostic and rehabilitative purposes. Given the increasing role of artificial intelligence (AI) in neurodegenerative disease research, studies employing machine learning models for eye-tracking analysis in PD were also reviewed to highlight innovative diagnostic approaches. Particular attention was given to studies that demonstrate the clinical feasibility of eye tracking in PD and those that propose future research directions for integrating eye tracking into clinical practice. A flowchart illustrating the number of records identified, screened, and included in the review is presented in Figure 1, in accordance with the narrative review design. While no formal inclusion/exclusion protocol was applied, studies were selected based on thematic relevance and quality of content.
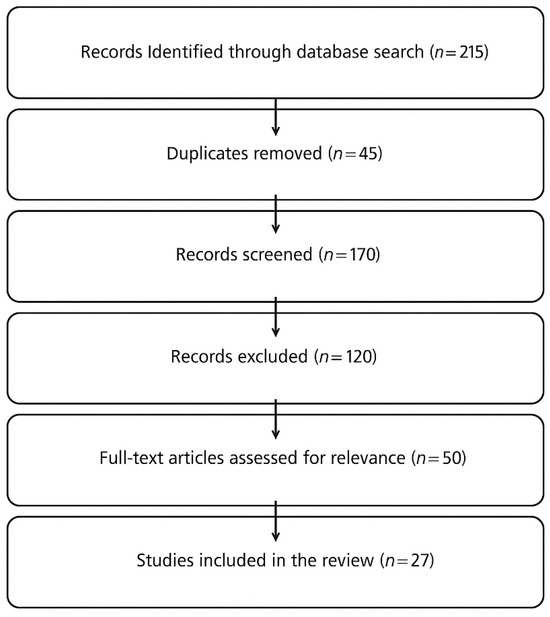
Figure 1.
Flowchart illustrating the literature selection process for the narrative review. A total of 215 records were identified, 45 duplicates were removed, and 27 studies were included based on thematic relevance.
3. Results
In order to better contextualize the findings presented in this section, Table 1 below summarizes the 27 major studies that we extracted from our empirical literature review on eye tracking in PD. These studies, ordered thematically and chronologically, offer insights into saccadic abnormalities, fixation instability, smooth pursuit deficits, and pupillary dysfunctions. The following sections summarize the main findings across the reviewed studies, grouped according to key oculomotor domains.
Figure 2 provides a visual summary of the principal eye movement abnormalities observed in Parkinson’s disease and their associations with motor and cognitive impairment.
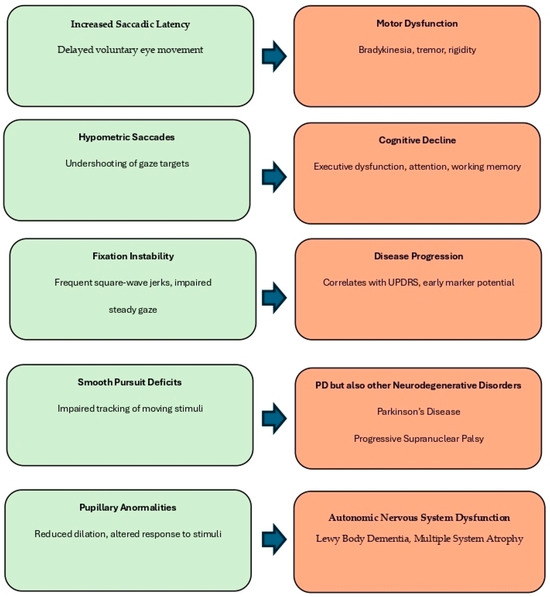
Figure 2.
Summary of oculomotor alterations observed in Parkinson’s disease (PD) and their clinical relevance. Eye movement abnormalities, including increased saccadic latency, hypometric saccades, fixation instability, smooth pursuit deficits, and pupillary abnormalities, reflect dysfunctions across distinct neural circuits. These features are associated with various functional outcomes and disease markers.
Saccadic abnormalities (delayed and hypometric eye movements) and fixation instability (e.g., square-wave jerks) are commonly linked to motor dysfunction and cognitive decline, particularly executive dysfunction and working memory deficits. Smooth pursuit deficits, characterized by impaired tracking of moving stimuli, are associated not only with PD but also with other neurodegenerative disorders such as progressive supranuclear palsy (PSP) and Huntington’s disease, reflecting cerebellar and basal ganglia involvement. Pupillary abnormalities, such as reduced dilation and delayed light response, are indicative of autonomic nervous system dysfunction and are frequently seen in Lewy Body Dementia (LBD) and Multiple System Atrophy (MSA), where dysautonomia is a prominent clinical feature. Together, these oculomotor signs may contribute to disease staging, differential diagnosis, and the identification of early cognitive or motor deterioration in PD and atypical parkinsonian syndromes
3.1. Eye Movement Abnormalities in Parkinson’s Disease
3.1.1. Saccadic Dysfunction in PD
Patients with Parkinson’s disease (PD) exhibited significant increases in saccadic latency, with an average delay of 200 ms (SD = 25 ms) compared to healthy controls (mean = 140 ms, SD = 15 ms) [4,8,15]. This increase in latency was strongly correlated with disease progression, as measured by UPDRS motor scores (r = 0.72, p < 0.001), indicating that saccadic latency may serve as a useful biomarker for monitoring motor decline in PD.
Saccades are rapid, ballistic eye movements that serve to reposition the fovea, the central part of the retina responsible for high-acuity vision, toward new visual stimuli. These movements are essential for visual scanning, reading, and object localization, and their proper execution depends on a complex interplay between cortical, subcortical, and brainstem structures. In Parkinson’s disease (PD), the degeneration of dopaminergic pathways, particularly in the substantia nigra and basal ganglia, leads to dysfunctional saccadic control, resulting in various forms of saccadic impairments [11]. Among these, hypometric saccades are one of the most frequently observed abnormalities, characterized by reduced amplitude, meaning the eyes do not fully reach the intended target, requiring corrective saccades to compensate for incomplete movements [16]. This phenomenon is closely linked to impaired motor initiation and disrupted feedback processing in the basal ganglia, which plays a critical role in controlling voluntary movement. Another common impairment is increased saccadic latency, where PD patients exhibit slower initiation times, leading to delayed responses to visual stimuli. This suggests functional deficits in the fronto-striatal-thalamic circuit, which is responsible for movement planning and execution.
Beyond these deficits in saccadic precision and initiation, PD patients also exhibit square-wave jerks, which are involuntary, small-amplitude eye movements that occur between fixations, disrupting visual stability. These micro-movements have been associated with abnormal inhibitory control mechanisms in the superior colliculus and cerebellum, structures involved in fixation stability and saccadic suppression [17]. PD affects voluntary goal-directed saccades more than reflexive saccades, meaning that stimulus-driven saccades tend to be less impaired than internally guided saccades [18]. This reflects preferential deficits in executive control mechanisms, particularly those regulated by the prefrontal cortex and basal ganglia circuits [19,20].
Numerous studies have examined saccadic dysfunction in PD using pro-saccade and anti-saccade tasks, which assess the ability to generate rapid, goal-directed eye movements and inhibit inappropriate responses [21,22,23]. Pro-saccade tasks require participants to shift their gaze toward a suddenly appearing stimulus, and PD patients often exhibit prolonged reaction times and hypometric saccades, indicating slower sensorimotor processing and disrupted motor output. Anti-saccade tasks, on the other hand, require individuals to suppress a reflexive saccade toward a stimulus and instead look in the opposite direction. PD patients frequently demonstrate increased error rates, reduced accuracy, and prolonged reaction times, which suggest deficits in inhibitory control and executive function, processes mediated by the frontal–striatal network. Hindle et al. and Archibald et al. [23,24] found significant correlations between saccadic performance and cognitive function in PD, particularly in tasks requiring working memory and executive control, reinforcing the idea that oculomotor dysfunction in PD extends beyond motor impairments and reflects underlying cognitive decline [25].
Further evidence from functional neuroimaging and electrophysiology has shown that saccadic impairments are associated with reduced activity in the frontal cortex, basal ganglia, and superior colliculus, supporting the idea that oculomotor deficits serve as biomarkers for deterioration in motor and cognitive functions in PD [26,27]. Given that saccadic abnormalities often emerge early in the disease, eye movement analysis holds significant diagnostic potential. Measuring saccadic latency, accuracy, and error rates could provide quantifiable markers of disease progression, offering a non-invasive, objective alternative to traditional motor assessments [28]. As research continues to refine machine learning algorithms and AI-driven eye-tracking models, integrating oculomotor biomarkers into clinical diagnostics and disease monitoring could greatly enhance early detection, stratification of disease subtypes, and targeted therapeutic interventions in Parkinson’s disease.
3.1.2. Fixation Instability and Microsaccadic Intrusions
Fixation instability, measured by the frequency of square-wave jerks, increased with the severity of the disease. Early PD patients showed an average of 5.4 square-wave jerks per minute (SD = 2.1), while advanced PD patients exhibited a significantly higher average of 12.3 jerks per minute (SD = 3.4) (p = 0.03) [16,25]. This increase was correlated with cognitive decline, as assessed by the MoCA (r = −0.60, p < 0.01), suggesting that fixation instability could serve as an early indicator of motor and cognitive deterioration in PD.
Fixation is a fundamental oculomotor function that allows the eyes to maintain a steady gaze on a target, ensuring visual clarity, spatial stability, and efficient cognitive processing. In healthy individuals, fixation is supported by a delicate balance between involuntary micro-movements (microsaccades) and active control mechanisms, preventing visual fading while maintaining gaze precision [29]. In Parkinson’s disease (PD), fixation stability is significantly compromised, leading to involuntary eye drifts, excessive microsaccades, and frequent square-wave jerks. PD patients often struggle to maintain steady gaze and exhibit a significantly higher frequency of square-wave jerks, whose intrusions disrupt gaze fixation by shifting the eyes away from the target and then rapidly correcting back [30,31]. Shaikh et al. [16] proposed that fixation instability in PD contributes to broader cognitive and attentional deficits, as visual fixation plays a crucial role in maintaining spatial awareness, processing visual information, and sustaining attention. The presence of excessive microsaccades and square-wave jerks can lead to visual discomfort, impaired reading fluency, difficulties in processing complex visual scenes, exacerbating cognitive strain and functional impairment, suggesting deficits in oculomotor inhibitory control mechanisms. PD patients display abnormally frequent and dysregulated microsaccadic movements, which interfere with reading, fine visual discrimination, and attentional focus, making daily tasks more difficult [17].
Fixation control is governed by a network of cortical and subcortical structures, including the superior colliculus (SC), which plays a key role also in reflexive gaze control and visual attention, the frontal eye fields (FEFs), responsible for voluntary eye movement planning, and the basal ganglia, particularly the substantia nigra pars reticulata, which modulates saccadic suppression and fixation control. Krauzlis et al. [30] demonstrated that fixation control involves a dynamic interaction between these regions, with dopaminergic dysfunction in PD disrupting their functional connectivity, leading to deficits in voluntary gaze stabilization, increased oculomotor noise, and decreased ability to suppress involuntary eye movements, which causes instability in visual tracking.
Fixation instability has profound implications for PD patients, significantly affecting daily life activities. Frequent microsaccadic intrusions disrupt text tracking, leading to slower reading speeds and impaired comprehension. Unstable fixation may interfere with sustained attention in tasks requiring detailed visual examination, such as driving, object recognition, and facial identification, further reducing functional independence. Another critical consequence of fixation instability is its potential link to postural instability; impaired gaze fixation control has been associated with poorer balance and gait disturbances, which may contribute to an increased risk of falls and reduced mobility in PD patients [29,31,32].
Given the clinical significance of fixation instability, eye-tracking technology has emerged as a valuable tool for assessing and monitoring fixation impairments in PD. By quantifying fixation duration, microsaccadic frequency, and square-wave jerk rates, researchers and clinicians can gain objective insights into disease progression and its impact on cognitive and motor function. Eye-tracking assessments allow for the differentiation of PD from other neurodegenerative disorders with overlapping motor symptoms, such as progressive supranuclear palsy (PSP), which presents distinct patterns of fixation instability [32]. These tools can help track disease progression and serve as a biomarker for cognitive function, providing valuable information on executive dysfunction and attentional impairments in PD patients [2]. As advancements in machine learning and AI-driven analytics continue to refine eye-tracking methodologies, these tools could be further integrated into routine neurological assessments, allowing for earlier detection, personalized treatment strategies, and improved monitoring of disease progression.
3.1.3. Smooth Pursuit and Convergence Deficits
Smooth pursuit eye movements are continuous, coordinated movements that allow the eyes to track a moving object smoothly rather than through discrete jumps (saccades). This function is essential for visual stability, motion perception, and spatial awareness in everyday activities, such as driving, reading, and navigating dynamic environments. In Parkinson’s disease (PD), smooth pursuit movements are often compromised, resulting in the delayed, fragmented, and inefficient tracking of moving stimuli. Several impairments have been identified in PD patients, including reduced pursuit gain, where the eyes lag behind moving targets, necessitating frequent catch-up saccades to compensate for tracking deficits. PD patients often rely on increased compensatory saccades to maintain fixation on a moving object, which disrupts the fluidity of gaze control and makes it harder to track motion continuously [16]. Another common issue is compromised binocular convergence, where individuals with PD experience difficulty focusing on near objects, a condition known as convergence insufficiency, which affects depth perception, spatial judgment, and near-field vision clarity, leading to difficulties in tasks such as reading and object manipulation [11].
Smooth pursuit movements are controlled by a complex network of brain regions, including the parieto-occipital cortex, which processes motion stimuli, the cerebellum, which fine-tunes the velocity and precision of pursuit movements, the frontal eye fields (FEF) and the basal ganglia. In PD, dopaminergic depletion in the basal ganglia disrupts the interaction between these regions, leading to deficits in pursuit initiation and maintenance. Dysfunction in the superior colliculus and brainstem pathways further impairs motion processing and gaze stabilization mechanisms, contributing to jerky, inefficient pursuit movements [5,30].
These impairments have significant functional consequences, affecting visual perception, motor coordination, and cognition. Difficulties in reading arise because tracking lines of text smoothly becomes challenging, leading to fatigue, slower reading speeds, and comprehension difficulties. Impaired motion perception can cause PD patients to struggle with accurately judging moving objects, which in turn affects spatial orientation and balance [33,34,35]. Navigation difficulties also emerge due to a reduced ability to track moving pedestrians, vehicles, or environmental cues, which increases the risk of falls and accidents [36,37]. Fooken et al. [20] found that PD patients required significantly more corrective saccades to maintain smooth pursuit, leading to disruptions in visual tracking accuracy, placing additional cognitive and motor demands on the individual and potentially contributing to fatigue and visual discomfort.
Binocular convergence results in blurred near vision, making activities such as reading and writing more difficult. In more severe cases, patients experience double vision (diplopia), which affects spatial coordination and increases fall risk. This misalignment also leads to increased eye strain and visual fatigue, as the brain struggles to compensate for conflicting binocular input [11]. Given the diagnostic and functional relevance of smooth pursuit and convergence abnormalities in PD, eye-tracking technology provides a valuable tool for assessing and monitoring these impairments. By quantifying smooth pursuit gain and compensatory saccades, clinicians can help differentiate PD from other neurodegenerative disorders, while tracking convergence ability over time can serve as an indicator of disease progression and cognitive decline.
3.1.4. Pupillary Abnormalities and Cognitive Correlates
Pupillary responses are regulated by autonomic nervous system activity and cognitive processing demands, with pupil size dynamically modulated by sympathetic and parasympathetic pathways. The locus coeruleus–norepinephrine system (LC-NE) plays a crucial role in cognitive arousal, attentional control, and executive functioning [38]. In Parkinson’s disease (PD), disruptions in both autonomic regulation and neurocognitive processing manifest as pupillary dysfunctions, which may serve as biomarkers for disease progression and cognitive decline. Several deficits have been identified in PD patients, including a reduced baseline pupil size, indicative of parasympathetic overactivity and reduced sympathetic tone, possibly reflecting neurodegenerative changes in autonomic control circuits. Another key deficit is the altered pupillary reflex under stable lighting, pointing to brainstem dysfunction affecting autonomic regulation [39,40]. PD patients frequently exhibit shorter fixation durations, linked to dysfunctional gaze control and increased microsaccadic intrusions, which may contribute to visual fatigue, attentional lapses, and reading difficulties [14,25]. Tsitsi et al. [14] demonstrated that pupillary changes in PD correlate with both motor symptoms and cognitive impairments, reinforcing the idea that oculomotor biomarkers could provide insights into the broader neurodegenerative process.
The underlying neurophysiological mechanisms responsible for pupillary abnormalities in PD involve multiple brain regions that govern autonomic regulation and cognitive processing. Among these, the locus coeruleus (LC) modulates arousal, attention, and cognitive flexibility through norepinephrine signaling, while the superior colliculus (SC) integrates visual, motor, and autonomic functions, and the prefrontal cortex (PFC) plays a critical role in cognitive effort and executive decision making. The degeneration of the LC-NE system leads to dysregulated autonomic output and cognitive control impairments, resulting in abnormal pupillary responses that could reflect early neurodegenerative changes affecting these key regulatory pathways [38].
Beyond autonomic impairments, pupillary responses are closely linked to cognitive function in PD, with research suggesting that reduced pupil dilation during cognitive tasks is associated with slower information processing and executive dysfunction [23,38]. Pupil dynamics during attention and memory tasks have been found to predict early cognitive decline in PD patients, highlighting their potential as an early biomarker of neurodegeneration. Aberrant pupillary responses appear more pronounced in PD patients with mild cognitive impairment (MCI) and may serve as a precursor to dementia, further supporting the hypothesis that pupillometry could serve as a non-invasive physiological marker of neurocognitive deterioration [23,36,37].
3.2. Eye Tracking as a Diagnostic and Monitoring Tool in PD
3.2.1. Correlation Between Eye Movements and Progression in Parkinson’s Disease
Eye movement abnormalities in Parkinson’s disease (PD) are increasingly recognized as early indicators of cognitive decline and disease progression. Oculomotor dysfunction, including saccadic impairments, fixation instability, and smooth pursuit deficits, correlates with executive dysfunction, attentional deficits, and working memory impairments in PD patients [23,24,25,26]. As neurodegeneration progresses, these deficits tend to worsen over time. Longitudinal studies have investigated the relationship between oculomotor function and cognitive decline in PD, demonstrating that saccadic parameters can predict disease progression [6,14,25]. Notably, Stuart et al. [8] followed PD patients for 54 months and found that shorter saccadic latency predicted more rapid cognitive deterioration.
The interplay between cognitive and motor functions in PD is well established: eye movements are closely linked to executive function and working memory through frontal-striatal circuits involving the dorsolateral prefrontal cortex (DLPFC), the supplementary eye field (SEF), and the basal ganglia. Dopaminergic depletion in these areas leads to both motor and cognitive impairments. Saccadic deficits serve as a proxy for broader neurodegenerative changes due to mechanisms such as prefrontal cortex dysfunction causing increased variability in saccadic execution.
Given their longitudinal stability and objective nature, oculomotor metrics have potential as standardized biomarkers for PD progression. Regular saccadic testing could help track cognitive and motor decline over time. Identifying patients with rapidly worsening oculomotor function could aid in stratifying individuals at higher risk for developing Parkinson’s disease dementia (PDD). Measuring changes in ocular–motor parameters following pharmacological or rehabilitative interventions can offer valuable insights into the effectiveness of various treatment strategies. By leveraging this technology for early detection and personalized management strategies tailored to motor and cognitive aspects of PD, researchers can improve patient outcomes significantly by enhancing diagnostic precision [2,14,38].
3.2.2. Machine Learning Applications for Automated Diagnosis
Recent advancements in artificial intelligence (AI) and machine learning (ML) have revolutionized biomedical diagnostics, offering automated, objective, and high-precision analysis of complex neurological data. In the context of Parkinson’s disease (PD), AI-driven approaches are increasingly being explored to analyze eye-tracking data for early detection, continuous disease monitoring, and differential diagnosis. Eye movement abnormalities often manifest early in the disease course; thus, AI-based analysis of oculomotor biomarkers, such as saccadic latency, fixation instability, smooth pursuit deficits, and pupillary responses, can enhance diagnostic accuracy and improve patient stratification [39,40].
Machine learning models excel in pattern recognition and have been effective in detecting subtle alterations in eye movements that might precede noticeable clinical symptoms. These approaches allow for the identification of oculomotor dysfunctions before traditional motor symptoms emerge and facilitate differentiation between PD and other neurodegenerative disorders like progressive supranuclear palsy (PSP) and Multiple System Atrophy (MSA). Continuous eye-tracking assessments provide quantitative measures that help track disease progression over time, offering valuable insights into neurodegeneration trajectories [41]. Bredemeyer et al. [42] applied machine learning algorithms to predict disease severity based on oculomotor parameters across different PD stages.
Traditional PD diagnosis relies heavily on clinical examination and subjective symptom reporting, which can lead to variability and delays. AI-driven eye-tracking analysis offers precise metrics that reduce reliance on subjective assessments. Features like fixation duration, saccadic velocity, and pupillary dynamics provide biomarker-level accuracy for standardized diagnostic criteria. Automated classification is another significant advancement; machine learning models trained on large datasets enable clinician-independent classification of PD cases.
AI-based diagnostic tools assist neurologists by generating real-time probability scores for PD presence and severity. This capability is critical for early detection years before significant motor symptoms appear, a window allowing for earlier intervention and potentially slowing disease progression, and improves diagnostic specificity by distinguishing PD-related eye movement abnormalities from those seen in other diseases like PSP where vertical gaze dysfunction is an early hallmark.
Beyond diagnostic improvements, AI-powered eye-tracking analysis is scalable and cost-effective compared to conventional techniques such as MRI PET scans, making it suitable for widespread implementation, including home-based monitoring, reducing the healthcare burden. Various machine learning models, including supervised unsupervised deep learning approaches, offer unique advantages, each enhancing diagnostic precision when integrating multimodal data sources combining oculomotor neuroimaging genetic information [43,44].
3.3. The Role of Eye Tracking in Cognitive Assessment
3.3.1. Eye Movements and Executive Function in PD
Eye movements are increasingly recognized as a window into the broader cognitive impairments associated with Parkinson’s disease (PD), particularly executive dysfunction, which encompasses deficits in attention, inhibitory control, and working memory. Executive function relies on the integrity of the frontal-striatal circuitry, a network affected by progressive neurodegeneration in PD. As the disease advances, impairments manifest not only in motor symptoms but also in cognitive deficits that can be objectively assessed through oculomotor tasks. Research has demonstrated that saccadic eye movements are highly sensitive to executive dysfunction in PD, making them valuable indicators of disease progression and cognitive decline [45].
Liao et al. [2] investigated the relationship between saccadic performance and cognitive impairments in PD. They found higher error rates in anti-saccade tasks due to deficits in inhibitory control, a core aspect of executive function regulated by the dorsolateral prefrontal cortex (DLPFC) and basal ganglia pathways. Increased saccadic latency correlated with slower cognitive processing speeds, indicating delays in goal-directed decision making linked to frontal-striatal dysfunction.
The connection between oculomotor function and executive processing highlights PD’s dual nature as both a movement disorder and a cognitive disorder. Since executive dysfunction often predates severe cognitive decline and Parkinson’s disease dementia (PDD), detecting early changes through eye-tracking technology offers opportunities for early diagnosis, risk stratification, and monitoring of disease progression [6,14].
Beyond diagnostic implications, studying executive dysfunction through eye movements offers potential therapeutic applications. Targeted interventions focusing on improving response inhibition may enhance both oculomotor control and cognitive function. Emerging evidence suggests benefits from computer-based training incorporating real-time eye-tracking feedback could improve executive function early on, potentially slowing its decline [46,47]. This growing body of evidence underscores adopting a multimodal approach considering both motor–cognitive trajectories for improved clinical outcomes using tools like those proposed by Gibbs et al. and Tsitsi [6,48].
3.3.2. Visual Search and Reading Impairments
Patients with Parkinson’s disease (PD) often face significant challenges in visual exploration and reading fluency due to oculomotor impairments that disrupt text processing and the navigation of visually complex environments. Reading requires the coordination of fixation stability, saccadic control, and visual contrast sensitivity, functions disrupted in PD by degeneration in frontal-striatal circuits and associated oculomotor pathways. As a result, text scanning becomes inefficient; PD patients frequently lose their place or experience visual fatigue due to reduced fixation stability, characterized by involuntary eye drifts, microsaccadic intrusions, and square-wave jerks [48,49]. Prolonged saccadic latency further disrupts reading rhythm by delaying the initiation of eye movements. Normal reading involves precise sequences of saccades and fixations to smoothly traverse lines of text. However, in PD, saccades are often hypometric and delayed, requiring additional corrective movements that increase cognitive load and reduce reading efficiency.
Impairments in visual contrast sensitivity also affect letter recognition as PD patients struggle with distinguishing characters under low-contrast conditions [29,49]. These deficits are linked to dopaminergic dysfunction, affecting the retinal and cortical visual processing centers [30]. Given the impact on daily life, there is an urgent need for standardized eye-tracking assessments to evaluate cognitive impairments in PD objectively. Gibbs et al. [6] emphasized that incorporating objective eye-tracking tasks into clinical evaluations as traditional neuropsychological assessments may not fully capture subtle but disabling visual deficits experienced by PD patients.
3.4. Implications for Early Diagnosis and Clinical Interventions
3.4.1. Potential for Early Diagnosis and Disease Monitoring
Eye-tracking technology holds significant potential as a diagnostic biomarker for early detection and continuous disease monitoring in Parkinson’s disease (PD). Unlike traditional clinical assessments, eye tracking provides an objective, quantifiable, and non-invasive method to detect subtle oculomotor dysfunctions before more pronounced motor symptoms emerge [2]. As PD progresses, cognitive and motor dysfunctions become increasingly apparent; measuring saccadic latency, fixation stability, smooth pursuit efficiency, and pupillary responses can serve as valuable markers for disease onset and progression [6]. Specific oculomotor signatures unique to PD, such as hypometric saccades, increased square-wave jerks, and prolonged anti-saccade reaction times, allow clinicians to differentiate PD from other neurodegenerative disorders, like progressive supranuclear palsy (PSP), Multiple System Atrophy (MSA), and Alzheimer’s disease [40].
Bek et al. [7] proposed that predictive models based on eye movement metrics could enable earlier intervention strategies by identifying preclinical PD biomarkers. Leveraging machine learning algorithms with large-scale datasets can facilitate risk stratification and individualized treatment plans before irreversible neurodegeneration occurs. Longitudinal studies could monitor disease progression while assessing therapeutic intervention effectiveness over time. Integrating eye tracking into clinical trials could provide real-time measures of therapeutic response by modulating certain oculomotor deficits through dopaminergic replacement therapy or neurorehabilitation programs [50]. Incorporating this technology into routine assessments has the potential to redefine early PD diagnosis by reducing diagnostic delays.
3.4.2. Integration of Eye Tracking in PD Rehabilitation
The integration of eye-tracking technology into neurorehabilitation programs for Parkinson’s disease (PD) represents a promising approach to addressing oculomotor, cognitive, and motor impairments. Training programs focusing on gaze control, visual exploration, and motor–cognitive coordination could enhance functional outcomes and quality of life by targeting fixation instability, saccadic dysfunction, and impaired smooth pursuit, common challenges faced by PD patients [51]. One potential avenue involves training gaze control to improve reading fluency and visual exploration. Many PD patients struggle with reduced fixation stability, increased microsaccadic intrusions, and prolonged saccadic latencies, disrupting their ability to scan text and efficiently navigate complex environments. Using real-time eye-tracking feedback, patients can stabilize fixation, optimize saccadic efficiency, and reduce involuntary gaze shifts, improving reading speed comprehension and spatial navigation. Structured saccadic exercises could enhance motor–cognitive coordination, as PD patients often exhibit difficulties initiating controlling sequencing voluntary saccades. Training precise goal-directed movements may help strengthen neural pathways involved in movement execution and executive control, potentially counteracting the frontal-striatal dysfunction associated with PD [52,53].
A particularly exciting development is integrating eye tracking into virtual reality (VR)-based neurorehabilitation programs. VR environments combined with biofeedback create engaging adaptive rehabilitation experiences, allowing PD patients to practice real-world tasks in controlled settings. For instance, VR simulations guide patients through visual search tasks, spatial navigation challenges, and dynamic eye–hand coordination exercises, all while tracking adjusting individual oculomotor patterns. These interventions are beneficial in enhancing attentional flexibility, improving depth perception, and reducing the cognitive load associated with visual–motor integration. VR-based training helps reinforce compensatory strategies by teaching individuals to adapt their behavior in response to real-world challenges, such as crossing streets, scanning shelves, and following social cues [10,11,12].

Table 1.
Eye-tracking studies in Parkinson’s disease.
Table 1.
Eye-tracking studies in Parkinson’s disease.
| Thematic Area | Autors | Year | Sample | Objective | Methods | Results | Key Findings | |
|---|---|---|---|---|---|---|---|---|
| 1 | Smooth Pursuiit Deficits | Tanabe, J., Tregellas, J., Miller, D., Ross, R. G., & Freedman, R. | 2002 [34] | PD patients (N = 48, 26 M/22 F) | Study brain activation during smooth pursuit | Neuroimaging smooth pursuit tasks | Disrupted pursuit control mechanisms | Neurophysiological basis of pursuit deficits |
| 2 | Fixation Instability | Shaikh, A.G., Xu-Wilson, M., Grill, S., & Zee D. S. | 2011 [16] | PD patients (N = 40, 22 M/18 F) | Investigate ‘staircase’ square-wave jerks in early PD | Oculomotor testinf | Increased square-wave jerks in early PD | Fixation instability as an early PD marker |
| 3 | Fixation instability | Marx, S., Respondek, G., Stamaleou, M., Dowiasch, S., Stoll, J., Bremmer, F., & Einhäuser, W. | 2012 [32] | PD patients (N = 50, 30 M/20 F) | Differentiate PSP from PD using fixation analysis | Mobile eye.tracking | Distinct fixation instability patterns in PSP vs PD | Eye-tracking helps differentitate neurodegenerative disorders |
| 4 | Pupillary abnormalities | Wang, C.A., & Munoz, D. P. | 2015 [39] | PD patients (N = 52, 30 M/22 F) | Analyze cognitive modulation of pupil size | Pupillometry and neurocognitive tasks | Dysregulates autonomic control | Pupillary changes correlate with cognitive decline |
| 5 | Reading and Visual Impairments | Ekker, M. S., Janssen, S., Seppi, K., Poewe, W., de Vries, N. M., Theelen, T., & Bloem, B. R. | 2017 [54] | PD patients (N = 50, 27 M/23 F) | Analyze ocular disorders in PD | Comprensive visual assessments | High prevalence of visual deficits | Ocular disorders often overlooked in PD |
| 6 | Fixation Instability | Wong, O. W., Chan, A. Y., Wong, A., Lau, C. K., Yeung, J. H., Mok, V. C., ... & Chan, S. | 2018 [25] | PD patients (N = 40, 20 M/20 F) | Examine eye movement parameters and cognitive function | Eye-tracking with cognitive assessments | Fixation instability correlates with cognitive decline | Oculomotor measures predict neurocognitive impairment |
| 7 | Eye-tracking in PD Cognitive Assessment | Luke, S. G., Darowski, E. S., & Gale, S. D. | 2018 [49] | PD patients (N = 40, 20 M/20 F) | Predict cognitive impairments through eye traking | Eye movement tasks | Correlation between eye movements and cognitive decline | Early cognitive impairment detection |
| 8 | Reading and Visual Impairments | Jehangir, N., Yu, C. Y., Song, J., Shariati, M. A., Binder, S., Beyer, J., ... & Liao, Y. J. | 2018 [52] | PD patients (N = 42, 22 M/20 F) | Examine reading difficulties in PD | Saccadic analysis during reading | Slower saccadic reading | Reading impairments linked to oculomotor dysfunction |
| 9 | Saccadic Dysfunction | Stuart, S., Lawson, R. A., Yarnall, A. J., Nell, J., Alcock, L., Duncan, G. W., ... & ICICLE-PD study group. | 2019 [8] | PD patients (N = 75, 40 M/35 F) | Examine pro-saccades as predictor of cognitive decline | Saccadic eye-trackinng tasks | Prolonged saccadic latency predicts cognitive decline | Saccadic metrics correlate with executive dysfunction |
| 10 | Reading and Visual Impairments | Stock, L., Krüger-Zechlin, C., Deeb, Z., Timmermann, L., & Waldthaler, J. | 2020 [53] | PD patients (N = 45, 22 M/23 F) | Investigate reading impairments in PD | Naturalistic reading tasks with eye-tracking | PD patients show reduced reading fluency | Reading difficulties linked to cognitive dysfunction |
| 11 | Pupillary Abnormalities | Kahya, M., Lyons, K. E., Pahwa, R., Akinwuntan, A. E., He, J., & Devos, H. | 2021 [38] | PD patients (N = 50, 30 M/20 F) | Investigate pupillary responses to postural demands | Pupillometry and balance tasks | Abnormal pupillary reflex during postural adjustments | Pupil size linked to autonomic dysfunction |
| 12 | Fixation Instability | Tsitsi, P., Benfatto, M. N., Seimyr, G. Ö., Larsson, O., Svenningsson, P., & Markaki, I. | 2021 [14] | PD patients (N = 55, 28 M/27 F) | Analyze fixation duration and pupil size as PD diagnostic tools | Pupillometry and eye-tracking | Shorter fixation duration, smaller pupils | Oculomotor markers for PD diagnosis |
| 13 | Pupillary abnormalities | Tsitsi, P., Benfatto, M. N., Seimyr, G. Ö., Larsson, O., Svenningsson, P., & Markaki, I. | 2021 [14] | PD patients (N = 55, 28 M/27 F) | Investigate pupil size changes in PD | Eye-tracking and pupillometry | Reduce pupil dilatation in PD | Potential biomarker for cognitive decline |
| 14 | AI in PD diagnosis | Mei, J., Desrosiers, C., & Frasnelli, J. | 2021 [43] | PD patients (N = 85, 50 M/35 F) | Review of machine learning for PD diagnosis | Literature review | Various AI models effective in PD classification | Potential for AI in automated diagnostics |
| 15 | Motor-Ocular Function | Fasano, A., Mazzoni, A., & Falotico, E. | 2022 [37] | PD patients (N = 70, 40 M/30 F) | Asses reaching and grasping movements in PD | Oculomotor and motor coordination tests | Impaired visuomotor integration | Oculomotor deficits affect daily function |
| 16 | Saccadic Dysfunction | Kassavetis, P., Kaski, D., Anderson, T., & Hallett, M. | 2022 [4] | PD patients (N = 50, 30 M/20 F) | Investigate eye movement disorders in PD | Clinical observation eye-tracking analysis | Hypometric saccades, increased latency | Saccadic impairments serve as early biomarkers |
| 17 | Smooth Pursuit Deficits | Fooken, J., Patel, P., Jones, C. B., McKeown, M. J., & Spering, M. | 2022 [20] | PD patients (N = 60, 35 M/25 F) | Assess smoth pursuit impairments | Eye-tracking | Reduced pursuit gain, increased compensatory saccades | Deficits in motion tracking |
| 18 | Saccadic Dysfunction | Waldthaler, J., Vinding, M. C., Eriksson, A., Svenningsson, P., & Lundqvist, D. | 2022 [21] | PD patients (N = 45, 25 M/20 F) | Examine neural correlates of impaired response inhibition | EEG and antisaccade tasks | Altered brain activity during saccade inhibition | Deficits in executive function |
| 19 | Saccadic Dysfunction | Fooken, J., Patel, P., Jones, C. B., McKeown, M. J., & Spering, M. | 2022 [20] | PD patients (N = 60, 35 M/25 F) | Assess stimulus and task-specific preservation of ete movemets | Eye-tracking and neurocognitive assessments | Selective preservation of saccades in PD | Task-dependent variability in eye movements |
| 20 | AI in PD Diagnosis | Przybyszewski, A. W., Śledzianowski, A., Chudzik, A., Szlufik, S., & Koziorowski, D. | 2023 [44] | PD patients (N = 90, 48 M/42 F) | Use machine learning to analyze eye movements in neurodegeneration | AI-based classification models | High accuracy in distinguishing PD from other disorders | Machine Learning improves PD diagnostic |
| 21 | Pupillary Abnormalities | Sun, Y. R., Beylergil, S. B., Gupta, P., Ghasia, F. F., & Shaikh, A. G. | 2023 [11] | PD patients (N = 60, 33 M/27 F) | Analyze pupillary responses in PD | Pupillometry assessments | Reduced pupil dilatation linked to cognitive impairment | Potential biomarker for neurodegeneration |
| 22 | Smooth Pursuit Deficits | Swart, E. K., & Sikkema-de Jong, M. T. | 2023 [33] | PD patients (N = 55, 28 M/27 F) | Examine effects of dopamine levels on smooth pursuit | Pharmacological eye-tracking | Dopamine modulates smooth pursuit accuracy | Dopaminergic treatment improves eye tracking |
| 23 | Saccadic Dysfunction | Riek, H. C., Brien, D. C., Coe, B. C., Huang, J., Perkins, J. E., Yep, R., & Munoz, D. P. | 2023 [15] | PD patients (N = 55, 28 M/27 F) | Examine antisaccade behavior across neurodegenerative diases | Antisaccade eye-tracking tasks | Increased error rates in antisaccade tasks | Executive dysfunction correlates with saccadic impairments |
| 24 | VR- based Rehabilitation | Daniol, M., Hemmerling, D., Sikora, J., Jemiolo, P., Wodzinski, M., & Wojcik-Pedziwiatr, M. | 2024 [12] | PD patients (N = 30, 18 M/12 F) | Assess VR applications in PD neurorehabilitation | Mixed reality and eye tracking | Improved visual search and spatial awareness | VR enhances motor-cognitive coordination |
| 25 | Ai in PD Diagnosis | Chudzik, A., Śledzianowski, A., & Przybyszewski, A. W. | 2024 [10] | PD patients (N = 100, 55 M/45 F) | Assess AI and digital biomarkers in early PD detection | Machine learning analysis of eye-tracking data | High accuracy in early diagnosis | AI enhances diagnostic precision |
| 26 | Fixation Instability | Antoniades, C. A., & Spering, M. | 2024 [28] | PD patients (N = 60, 32 M/28 F) | Investigate neurophysiological mechanisms of fixation instability | Eye tracking with neural recordings | Abnormal inhibitory control of fixational eye movements | Fixation instability as a biomarker for PD |
| 27 | Pupillary Abnormalities | Gibbs, M. C., Huxley, J., Readman, M. R., Polden, M., Bredemeyer, O., Crawford, T. J., & Antoniades, C. A. | 2024 [6] | PD patients (N = 58, 31 M/27 F) | Analyze naturalistic eye movement tasks in PD | Pupillometry and real-word eye tracking | Reduced pupil dilation and impaired gaze control | Naturlistic tasks improve PD assessment |
| 28 | Ai in PD Diagnosis | Liao, X., Yao, J., Tang, H., Xing, Y., Zhao, X., Nie, D., ... & Li, G. | 2024 [2] | PD patients (N = 100, 55 M/45 F) | Use AI-driven eye movement analysis for early PD detection | Machine learning on eye-tracking data | High predictive accuracy for early PD | AI-based eye-tracking enhances diagnostic precision |
| 29 | Motor-Ocular Function | Barbieri, F. A., Polastri, P. F., Barela, J. A., Bonnet, C. T., Brito, M. B., & Rodrigues, S. T. | 2024 [31] | PD patients (N = 45, 26 M/19 F) | Investigate coupling of eye movements and postural stability | Eye tracking with balance assessments | PD patients compensate gaze instability with postural adjustments | Eye movement analisys informs fall risk assessment |
4. Discussion
Eye-tracking technology has emerged as a powerful tool for understanding the neurophysiological mechanisms underlying Parkinson’s disease (PD), offering objective insights into motor, cognitive, and autonomic dysfunctions. Saccadic abnormalities, fixation deficits, and pupillary changes are characteristic features of PD disease progression. Measuring saccadic latency, accuracy, and inhibitory control deficits provides valuable insights into the integrity of the fronto-striatal network, making eye movement metrics relevant correlates of cognitive decline and executive dysfunction in patients with PD [54,55,56]. Given that executive impairments often emerge early in the disease course, eye-tracking assessments may serve as early warning signs of broader cognitive deterioration, including mild cognitive impairment (MCI) and Parkinson’s disease dementia (PDD). Machine learning approaches enhance the potential for automated diagnosis and monitoring by allowing AI-driven models to identify distinct oculomotor signatures and differentiate them from other neurodegenerative disorders, such as progressive supranuclear palsy (PSP) and Multiple System Atrophy (MSA). However, several key research priorities must be addressed to fully integrate eye-tracking technology into clinical practice. Standardizing eye-tracking tasks is crucial, as current methodologies vary significantly across studies, making it difficult to establish universally accepted diagnostic criteria. Developing consistent protocols for measuring saccadic dynamics, fixation control, and pupillary responses will ensure both reproducibility and clinical applicability.
Larger longitudinal studies are needed to validate oculomotor biomarkers, track changes over time, and determine their predictive value for motor and cognitive decline [57,58,59,60,61,62]. Expanding research efforts to include diverse patient populations will help refine oculomotor profiling techniques and establish eye tracking as a standard tool for PD monitoring.
Another critical area of exploration involves developing interventions that integrate gaze stabilization exercises, saccadic training, and VR-based feedback to improve visual exploration, attentional control, and motor coordination. Given the strong relationship between oculomotor control and executive function, designing therapeutic programs that complement pharmacological treatments can offer a multimodal approach to disease management.
These findings open promising avenues for integrating eye-tracking metrics into clinical assessments and designing tailored cognitive–motor rehabilitation protocols. Recent advances in the use of eye tracking and machine learning (ML) for the diagnosis and monitoring of Parkinson’s disease (PD) further support the relevance of the oculomotor markers discussed in this review. For example, Lukos et al. [63] and Zhou et al. [64] highlighted impaired saccadic control and eye–hand coupling in PD, extending the clinical interpretation of oculomotor dysfunction to real-world visuomotor tasks. These findings complement the markers identified in our synthesis, such as increased saccadic latency, fixation instability, and pupillary alterations, by demonstrating how they manifest in functionally meaningful contexts.
In parallel, studies such as those by Przybyszewski et al. [44], Brien et al. [65], and Bredemeyer et al. [43] have applied ML techniques to eye movement data, achieving promising classification accuracy for PD diagnosis. These approaches align with our findings on the consistency and diagnostic potential of specific oculomotor features, particularly in their capacity to serve as input variables for predictive models. Our review contributes to this growing body of evidence by identifying robust and recurrent markers that may support future ML-driven diagnostic tools and inform personalized rehabilitation strategies.
Future interventions should explore how training can enhance existing treatments, enabling a personalized and proactive approach to patient care. By harnessing the power of objective, data-driven assessments, clinicians and researchers can advance toward earlier diagnosis, targeted interventions, and continuous monitoring.
As technology progresses, wearable and mobile systems will enhance remote capabilities, enabling real-time tracking in naturalistic environments. The future of PD diagnosis and treatment lies in leveraging these innovations to develop precision medicine strategies, ensuring timely and individualized interventions that maximize functional independence and quality of life.
5. Conclusions
Eye tracking provides a reliable, cost-effective tool for early PD detection, cognitive assessment, and rehabilitation. Future research should focus on standardizing clinical protocols, validating predictive AI models, and integrating eye tracking into multimodal treatment strategies.
Author Contributions
Conceptualization, P.D. and G.M.; writing—original draft preparation, P.D., G.M., S.V., F.D.S. and S.M.; writing—review and editing, F.D.P., G.M., S.M. and T.D.L.; supervision, S.M., L.F. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that financial support was received for publication of this article. Project ECS0000024 “Ecosistema dell’innovazione—Rome Technopole” financed by EU in NextGenerationEU plan through MUR Decree n. 105123.06.2022 PNRR Missione 4 Componente 2 Investimento 1.5—CUP H33C22000420001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, J.; Zhang, B.; Ren, Q.; Zhong, Q.; Li, Y.; Liu, G.; Ma, X.; Zhao, C. Eye movement especially vertical oculomotor impairment as an aid to assess Parkinson’s disease. Neurol. Sci. 2020, 42, 2337–2345. [Google Scholar] [PubMed]
- Liao, X.; Yao, J.; Tang, H.; Xing, Y.; Zhao, X.; Nie, D.; Luan, P.; Li, G. Deciphering Parkinson’s Disease through Eye Movements: A Promising Tool for Early Diagnosis in the Face of Cognitive Impairment. Int. J. Clin. Pract. 2024, 1, 557923. [Google Scholar]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [PubMed]
- Kassavetis, P.; Kaski, D.; Anderson, T.; Hallett, M. Eye Movement Disorders in Movement Disorders. Mov. Disord. Clin. Pract. 2022, 9, 284–295. [Google Scholar] [CrossRef]
- Terao, Y.; Fukuda, H.; Yugeta, A.; Hikosaka, O.; Nomura, Y.; Segawa, M.; Ugawa, Y. Initiation and inhibitory control of saccades with the progression of Parkinson’s disease—Changes in three major drives converging on the superior colliculus. Neuropsychologia 2011, 49, 1794–1806. [Google Scholar] [CrossRef]
- Gibbs, M.C.; Huxley, J.; Readman, M.R.; Polden, M.; Bredemeyer, O.; Crawford, T.J.; Antoniades, C.A. Naturalistic Eye Movement Tasks in Parkinson’s Disease: A Systematic Review. J. Park Dis. 2024, 14, 1369–1386. [Google Scholar]
- Ştefănescu, E.; Strilciuc, Ş.; Chelaru, V.F.; Chira, D.; Mureşanu, D. Eye tracking assessment of Parkinson’s disease: A clinical retrospective analysis. J. Med. Life 2024, 17, 360–367. [Google Scholar]
- Stuart, S.; Lawson, R.A.; Yarnall, A.J.; Nell, J.; Alcock, L.; Duncan, G.W.; Khoo, T.K.; Barker, R.A.; Rochester, L.; Burn, D.J. Pro-Saccades Predict Cognitive Decline in Parkinson’s Disease: ICICLE-PD. Mov. Disord. 2019, 34, 1690–1698. [Google Scholar]
- Bek, J.; Gowen, E.; Vogt, S.; Crawford, T.J.; Poliakoff, E. Observation and imitation of object-directed hand movements in Parkinson’s disease. Sci. Rep. 2023, 13, 18749. [Google Scholar]
- Chudzik, A.; Śledzianowski, A.; Przybyszewski, A.W. Machine Learning and Digital Biomarkers Can Detect Early Stages of Neurodegenerative Diseases. Sensors 2024, 24, 1572. [Google Scholar] [CrossRef]
- Sun, Y.R.; Beylergil, S.B.; Gupta, P.; Ghasia, F.F.; Shaikh, A.G. Monitoring eye movement in patients with Parkinson’s disease: What can it tell us? Eye Brain 2023, 15, 101–112. [Google Scholar] [PubMed]
- Daniol, M.; Hemmerling, D.; Sikora, J.; Jemiolo, P.; Wodzinski, M.; Wojcik-Pedziwiatr, M. Eye-Tracking in Mixed Reality for Diagnosis of Neurodegenerative Diseases. arXiv 2024, arXiv:2404.12984. [Google Scholar]
- Orlosky, J.; Itoh, Y.; Ranchet, M.; Kiyokawa, K.; Morgan, J.; Devos, H. Emulation of physician tasks in eye-tracked virtual reality for remote diagnosis of neurodegenerative disease. IEEE Trans. Vis. Comput. Graph. 2017, 23, 1302–1311. [Google Scholar] [CrossRef]
- Tsitsi, P.; Benfatto, M.N.; Seimyr, G.Ö.; Larsson, O.; Svenningsson, P.; Markaki, I. Fixation Duration and Pupil Size as Diagnostic Tools in Parkinson’s Disease. J. Park. Dis. 2021, 11, 865–875. [Google Scholar] [CrossRef]
- Riek, H.C.; Brien, D.C.; Coe, B.C.; Huang, J.; Perkins, J.E.; Yep, R.; McLaughlin, P.M.; Orange, J.B.; Peltsch, A.J.; Roberts, A.C.; et al. Cognitive correlates of antisaccade behaviour across multiple neurodegenerative diseases. Brain. Commun. 2023, 5, fcad049. [Google Scholar]
- Shaikh, A.G.; Xu-Wilson, M.; Grill, S.; Zee, D.S. ‘Staircase’ square-wave jerks in early Parkinson’s disease. Br. J. Ophthalmol. 2011, 95, 705–709. [Google Scholar] [CrossRef]
- Rivaud-Péchoux, S.; Vidailhet, M.; Gallouedec, G.; Litvan, I.; Gaymard, B.; Pierrot-Deseilligny, C. Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy. Neurology 2000, 54, 1029–1032. [Google Scholar] [CrossRef]
- MacAskill, M.R.; Anderson, T.J. Eye movements in neurodegenerative diseases. Curr. Opin. Neurol. 2016, 29, 61–68. [Google Scholar]
- Railo, H.; Olkoniemi, H.; Eeronheimo, E.; Pääkkönen, O.; Joutsa, J.; Kaasinen, V. Dopamine and eye movement control in Parkinson’s disease: Deficits in corollary discharge signals? PeerJ 2018, 6, 6038. [Google Scholar] [CrossRef]
- Fooken, J.; Patel, P.; Jones, C.B.; McKeown, M.J.; Spering, M. Preservation of Eye Movements in Parkinson’s Disease Is Stimulus- and Task-Specific. J. Neurosci. 2021, 42, 487–499. [Google Scholar] [CrossRef]
- Waldthaler, J.; Vinding, M.C.; Eriksson, A.; Svenningsson, P.; Lundqvist, D. Neural correlates of impaired response inhibition in the antisaccade task in Parkinson’s disease. Behav. Brain. Res. 2022, 422, 113763. [Google Scholar]
- Jiang, M.; Liu, Y.; Cao, Y.; Xia, S.; Teng, F.; Zhao, W.; Lin, Y.; Liu, W. Diagnosis of Parkinson’s disease by eliciting trait-specific eye movements in multi-visual tasks. J. Transl. Med. 2025, 23, 1. [Google Scholar]
- Hindle, J.V.; Martyr, A.; Clare, L. Cognitive reserve in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2014, 20, 1–7. [Google Scholar] [PubMed]
- Archibald, N.K.; Hutton, S.B.; Clarke, M.P.; Mosimann, U.P.; Burn, D.J. Visual exploration in Parkinson’s disease and Parkinson’s disease dementia. Brain 2013, 136, 739–750. [Google Scholar] [PubMed]
- Wong, O.W.; Chan, A.Y.; Wong, A.; Lau, C.K.; Yeung, J.H.; Mok, V.C.; Lam, L.C.; Chan, S. Eye movement parameters and cognitive functions in Parkinson’s disease patients without dementia. Parkinsonism Relat. Disord. 2018, 52, 43–48. [Google Scholar]
- Pretegiani, E.; Optican, L.M. Eye Movements in Parkinson’s Disease and Inherited Parkinsonian Syndromes. Front. Neurol. 2017, 8, 592. [Google Scholar]
- George, S.; Rey, N.L.; Tyson, T.; Esquibel, C.; Meyerdirk, L.; Schulz, E.; Pierce, S.; Burmeister, A.R.; Madaj, Z.; Steiner, J.A.; et al. Microglia affect α-synuclein cell-to-cell transfer in a mouse model of Parkinson’s disease. Mol. Neurodegener. 2019, 14, 34. [Google Scholar]
- Antoniades, C.A.; Spering, M. Eye movements in Parkinson’s disease: From neurophysiological mechanisms to diagnostic tools. Trends Neurosci. 2024, 47, 71–83. [Google Scholar]
- Wark, H.A.C.; Garell, P.C.; Walker, A.L.; Basso, M.A. A case report on fixation instability in Parkinson’s disease with bilateral deep brain stimulation implants. J. Neurol. Neurosurg. Psychiatry 2008, 79, 443–447. [Google Scholar]
- Krauzlis, R.J.; Goffart, L.; Hafed, Z.M. Neuronal control of fixation and fixational eye movements. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160205. [Google Scholar]
- Barbieri, F.A.; Polastri, P.F.; Barela, J.A.; Bonnet, C.T.; Brito, M.B.; Rodrigues, S.T. People with Parkinson’s disease are able to couple eye movements and postural sway to improve stability. Biomechanics 2024, 4, 460–472. [Google Scholar] [CrossRef]
- Marx, S.; Respondek, G.; Stamelou, M.; Dowiasch, S.; Stoll, J.; Bremmer, F.; Oertel, W.H.; Höglinger, G.U.; Einhäuser, W. Validation of mobile eye-tracking as novel and efficient means for differentiating progressive supranuclear palsy from Parkinson’s disease. Front. Behav. Neurosci. 2012, 6, 88. [Google Scholar]
- Swart, E.K.; Sikkema-de Jong, M.T. The effects of increased dopamine levels on attentional control during reading and reading comprehension. Curr. Psychol. 2023, 42, 11009–11025. [Google Scholar]
- Tanabe, J.; Tregellas, J.; Miller, D.; Ross, R.G.; Freedman, R. Brain activation during smooth-pursuit eye movements. Neuroimage 2002, 17, 1315–1324. [Google Scholar]
- Reilly, J.L.; Lencer, R.; Bishop, J.R.; Keedy, S.; Sweeney, J.A. Pharmacological treatment effects on eye movement control. Brain Cogn. 2008, 68, 415–435. [Google Scholar]
- Caroline, Y.Y.; Lee, T.; Shariati, M.A.; Santini, V.; Poston, K.; Liao, Y.J. Abnormal eye movement behavior during reading in Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 32, 130–132. [Google Scholar]
- Fasano, A.; Mazzoni, A.; Falotico, E. Reaching and grasping movements in Parkinson’s disease: A review. J. Park. Dis. 2022, 12, 1083–1113. [Google Scholar]
- Kahya, M.; Lyons, K.E.; Pahwa, R.; Akinwuntan, A.E.; He, J.; Devos, H. Pupillary response to postural demand in Parkinson’s disease. Front. Bioeng. Biotechnol. 2021, 9, 617028. [Google Scholar]
- Wang, C.A.; Munoz, D.P. A circuit for pupil orienting responses: Implications for cognitive modulation of pupil size. Curr. Opin. Neurobiol. 2015, 33, 134–140. [Google Scholar]
- Tabashum, T.; Zaffer, A.; Yousefzai, R.; Colletta, K.; Jost, M.B.; Park, Y.; Chawla, J.; Gaynes, B.; Albert, M.V.; Xiao, T. Detection of Parkinson’s disease through automated pupil tracking of the post-illumination pupillary response. Front. Med. 2021, 8, 645293. [Google Scholar]
- Smith, C.; Malek, N.; Grosset, K.; Cullen, B.; Gentleman, S.; Grosset, D.G. Neuropathology of dementia in patients with Parkinson’s disease: A systematic review of autopsy studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Bredemeyer, O.; Huxley, J.; Antoniades, C.A. Oculomotor deficits in Parkinson’s disease: Increasing sensitivity using multivariate approaches. Front. Digit. Health 2022, 4, 939677. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Desrosiers, C.; Frasnelli, J. Machine learning for the diagnosis of Parkinson’s disease: A review of literature. Front. Aging Neurosci. 2021, 13, 633752. [Google Scholar] [CrossRef] [PubMed]
- Przybyszewski, A.W.; Śledzianowski, A.; Chudzik, A.; Szlufik, S.; Koziorowski, D. Machine learning and eye movements give insights into neurodegenerative disease mechanisms. Sensors 2023, 23, 2145. [Google Scholar] [CrossRef]
- Wu, P.; Cao, B.; Liang, Z.; Wu, M. The advantages of artificial intelligence-based gait assessment in detecting, predicting, and managing Parkinson’s disease. Front. Aging Neurosci. 2023, 15, 11913. [Google Scholar] [CrossRef]
- Abdollahi, S.; Safa, R. Machine learning and AI for advancing Parkinson’s disease diagnosis: Exploring promising applications. Big Data Comput. Vis. 2024, 4, 12–21. [Google Scholar]
- Godoy Junior, C.A.; Miele, F.; Mäkitie, L.; Fiorenzato, E.; Koivu, M.; Bakker, L.J.; Groot, C.U.; Redekop, W.K.; van Deen, W.K. Attitudes Toward the Adoption of Remote Patient Monitoring and Artificial Intelligence in Parkinson’s Disease Management: Perspectives of Patients and Neurologists. Patient Cent. Outcomes Res. 2024, 17, 275–285. [Google Scholar] [CrossRef]
- Tsitsi, P. Studies on Eye Movements in Parkinson’s Disease. Doctoral Thesis, Karolinska Institutet, Stockholm, Sweden, 2022. Available online: https://hdl.handle.net/10616/48040 (accessed on 4 January 2025).
- Luke, S.G.; Darowski, E.S.; Gale, S.D. Predicting eye-movement characteristics across multiple tasks from working memory and executive control. Mem. Cognit. 2018, 46, 826–839. [Google Scholar] [CrossRef]
- Lavermicocca, V. New Applications of Neurofeedback Techniques for Cognitive Rehabilitation in Parkinson’s Disease. Ph.D. Thesis, Università degli Studi di Trieste, Trieste, Italy, 2015. Available online: http://hdl.handle.net/10077/11018 (accessed on 7 January 2025).
- Gavelin, H.M.; Domellöf, M.E.; Leung, I.; Neely, A.S.; Launder, N.H.; Nategh, L.; Finke, C.; Lampit, A. Computerized cognitive training in Parkinson’s disease: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 80, 101671. [Google Scholar] [CrossRef]
- Jehangir, N.; Yu, C.Y.; Song, J.; Shariati, M.A.; Binder, S.; Beyer, J.; Santini, V.; Poston, K.; Liao, Y.J. Slower saccadic reading in Parkinson’s disease. PLoS ONE 2018, 13, e0191005. [Google Scholar] [CrossRef]
- Stock, L.; Krüger-Zechlin, C.; Deeb, Z.; Timmermann, L.; Waldthaler, J. Natural reading in Parkinson’s disease with and without mild cognitive impairment. Front. Aging Neurosci. 2020, 12, 120. [Google Scholar]
- Ekker, M.S.; Janssen, S.; Seppi, K.; Poewe, W.; de Vries, N.M.; Theelen, T.; Nonnekes, J.; Bloem, B.R. Ocular and visual disorders in Parkinson’s disease: Common but frequently overlooked. Parkinsonism Relat. Disord. 2017, 40, 1–10. [Google Scholar] [PubMed]
- Abasi, A.; Hoseinabadi, R.; Raji, P.; Friedman, J.H.; Hadian, M.R. Evaluating Oculomotor Tests before and after Vestibular Rehabilitation in Patients with Parkinson’s Disease: A Pilot Pre-Post Study. Park. Dis. 2022, 2022, 6913691. [Google Scholar]
- Kelton, C.; Wei, Z.; Ahn, S.; Balasubramanian, A.; Das, S.R.; Samaras, D.; Zelinsky, G. Reading Detection in Real-Time. In Proceedings of the 11th ACM Symposium on Eye Tracking Research & Applications, New York, NY, USA, 25–28 June 2019; pp. 1–5. [Google Scholar]
- Tosti, B.; Corrado, S.; Mancone, S.; Di Libero, T.; Rodio, A.; Andrade, A.; Diotaiuti, P. Integrated use of biofeedback and neurofeedback techniques in treating pathological conditions and improving performance: A narrative review. Front. Neurosci. 2024, 18, 1358481. [Google Scholar]
- D'Ermo, A.; Di Libero, T.; Langiano, E.; Tosti, B.; Corrado, S.; Diotaiuti, P.; Rodio, A. Exergames in neurocognitive disease management in elderly: A narrative review of therapeutic benefits and applications. J. Gerontol. Geriatr. 2024, 72, 204–214. [Google Scholar]
- Di Libero, T.; Langiano, E.; Carissimo, C.; Ferrara, M.; Diotaiuti, P.; Rodio, A. Technological support for people with Parkinson’s disease: A narrative review. J. Gerontol. Geriatr. 2023, 87–101. [Google Scholar] [CrossRef]
- Di Libero, T.; Carissimo, C.; Guerra, F.; Zagaglia, A.; Diotaiuti, P.; Langiano, E. On the benefits of wearable devices for Parkinson’s disease. Clin. Ter. 2022, 173, 50–53. [Google Scholar]
- da Cruz, W.M.; D’Oliveira, A.; Dominski, F.H.; Diotaiuti, P.; Andrade, A. Mental health of older people in social isolation: The role of physical activity at home during the COVID-19 pandemic. Sport Sci. Health 2022, 18, 597–602. [Google Scholar] [CrossRef]
- Tsitsi, P.; Nilsson, M.; Seimyr, G.Ö.; Larsson, O.; Svenningsson, P.; Markaki, I. Reading Alterations in Parkinson’s Disease Indicate Worse Cognitive Status. Mov. Disord. Clin. Pract. 2023, 10, 579–585. [Google Scholar]
- Lukos, J.R.; Anholt, R.V.; Carpenter, R.H.; Fisk, J.D.; Fernandez-Ruiz, J.; Scherberger, H.; Santello, M. Parkinson’s disease patients show impaired corrective grasp control and eye–hand coupling when reaching to grasp virtual objects. Neuroscience 2013, 254, 205–221. [Google Scholar] [CrossRef]
- Zhou, M.-X.; Chen, Y.; Fu, H.-C.; Qian, C.; Shen, X.; Li, Z.-Z.; Xu, C.-H. Oculomotor impairments in de novo Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 985679. [Google Scholar]
- Brien, D.C.; Munoz, D.P.; Riek, H.C. Classification and staging of Parkinson’s disease using video-based eye tracking. Parkinsonism Relat. Disord. 2023, 110, 105316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).