Comparison of ERlangen Score with pTau/Aβ1-42 Ratio for Predicting Cognitive Decline and Conversion to Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Neuropsychological Assessment
2.3. CSF ELISA
2.4. Statistics
3. Results
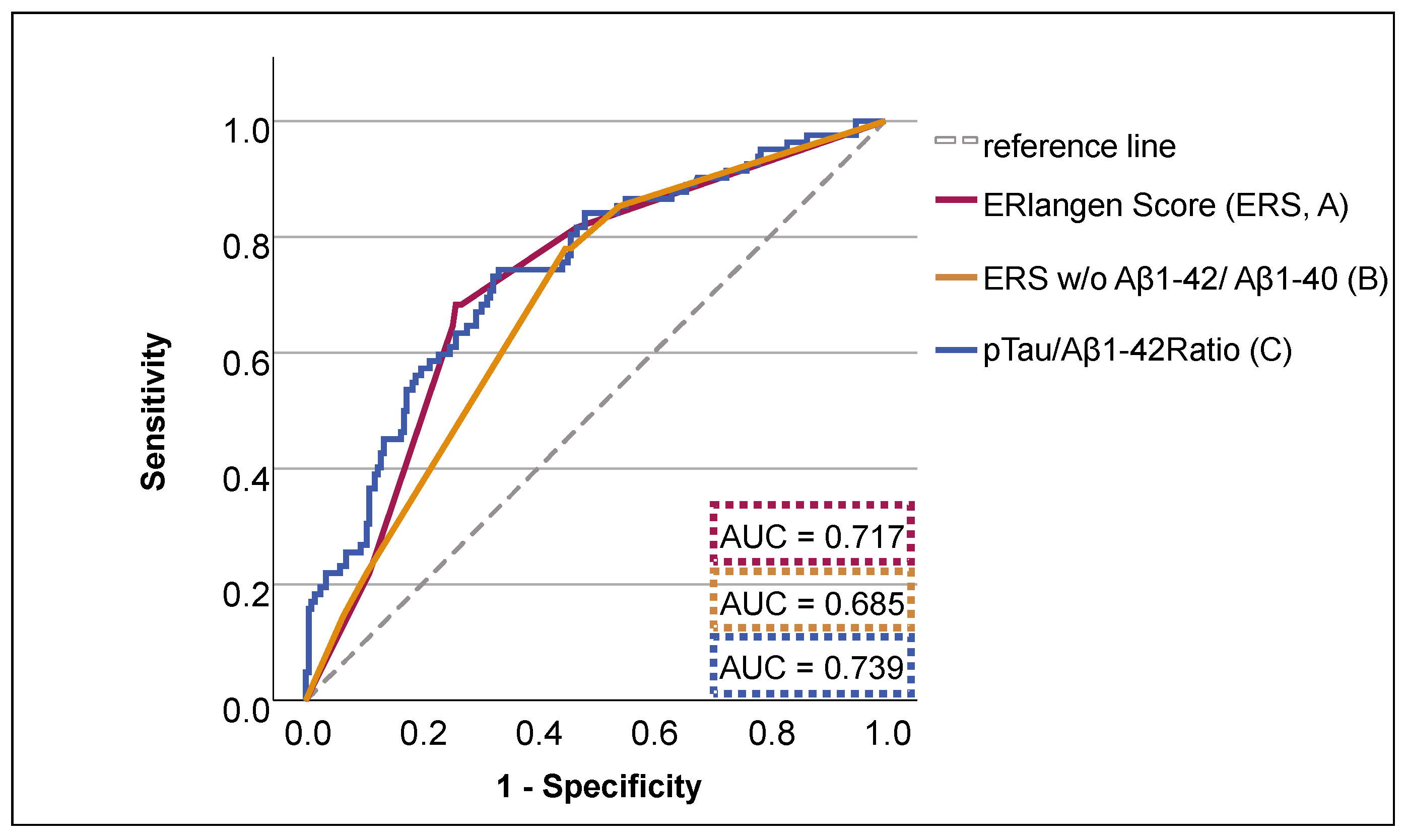
3.1. Prediction Model of Cognitive Decline Using ERlangen Score or pTau/Aβ1-42 Ratio
3.2. Discriminative Ability of ERS and pTau/Aβ1-42 Group for Dementia Risk
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AIC | Akaike Information Criterion |
| AUC | Area under the curve |
| BIC | Bayesian Information Criterion |
| CSF | Cerebrospinal fluid |
| ES | Erlangen Score |
| ERS | ERlangen Score |
| MMSE | Mini-Mental Status Examination |
| RMSE | Root Mean Squared Error |
| SEM | Standard error of means |
| SNAP | Suspected non-Alzheimer pathology |
References
- Mattsson, N.; Andreasson, U.; Persson, S.; Carrillo, M.C.; Collins, S.; Chalbot, S.; Cutler, N.; Dufour-Rainfray, D.; Fagan, A.M.; Heegaard, N.H.; et al. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimer’s Dement. 2013, 9, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Verwey, N.A.; van der Flier, W.M.; Blennow, K.; Clark, C.; Sokolow, S.; De Deyn, P.P.; Galasko, D.; Hampel, H.; Hartmann, T.; Kapaki, E.; et al. A worldwide multicentre comparison of assays for cerebrospinal fluid biomarkers in Alzheimer’s disease. Ann. Clin. Biochem. 2009, 46, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Matsubara, E.; Kanai, M.; Watanabe, M.; Nakamura, T.; Tomidokoro, Y.; Shizuka, M.; Wakabayashi, K.; Igeta, Y.; Ikeda, Y.; et al. Combination assay of CSF tau, Aβ1-40 and Aβ1-42 (43) as a biochemical marker of Alzheimer’s disease. J. Neurol. Sci. 1998, 158, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Esselmann, H.; Otto, M.; Maler, J.M.; Henkel, A.W.; Henkel, M.K.; Eikenberg, O.; Antz, C.; Krause, W.R.; Reulbach, U.; et al. Neurochemical diagnosis of Alzheimer’s dementia by CSF Aβ42, Aβ42/Aβ40 ratio and total tau. Neurobiol. Aging 2004, 25, 273–281. [Google Scholar] [CrossRef]
- Buchhave, P.; Minthon, L.; Zetterberg, H.; Wallin, Å.K.; Blennow, K.; Hansson, O. Cerebrospinal fluid levels ofβ-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch. Gen. Psychiatry 2012, 69, 98–106. [Google Scholar] [CrossRef]
- Fagan, A.M.; Xiong, C.; Jasielec, M.S.; Bateman, R.J.; Goate, A.M.; Benzinger, T.L.S.; Ghetti, B.; Martins, R.N.; Masters, C.L.; Mayeux, R.; et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci. Transl. Med. 2014, 6, ra30–ra226. [Google Scholar] [CrossRef]
- Willemse, E.A.; Tijms, B.M.; van Berckel, B.N.; Le Bastard, N.; van der Flier, W.M.; Scheltens, P.; Teunissen, C.E. Comparing CSF amyloid-beta biomarker ratios for two automated immunoassays, Elecsys and Lumipulse, with amyloid PET status. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12182. [Google Scholar] [CrossRef]
- Doecke, J.D.; Ward, L.; Burnham, S.C.; Villemagne, V.L.; Li, Q.-X.; Collins, S.; Fowler, C.J.; Manuilova, E.; Widmann, M.; The AIBL Research Group; et al. Elecsys CSF biomarker immunoassays demonstrate concordance with amyloid-PET imaging. Alzheimer’s Res. Ther. 2020, 12, 36. [Google Scholar] [CrossRef]
- Lewczuk, P.; Matzen, A.; Blennow, K.; Parnetti, L.; Molinuevo, J.L.; Eusebi, P.; Kornhuber, J.; Morris, J.C.; Fagan, A.M. Cerebrospinal fluid Aβ 42/40 corresponds better than Aβ 42 to amyloid PET in Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 55, 813–822. [Google Scholar] [CrossRef]
- De Strooper, B. Aph-1, Pen-2, and nicastrin with presenilin generate an active γ-secretase complex. Neuron 2003, 38, 9–12. [Google Scholar] [CrossRef]
- Duits, F.H.; Teunissen, C.E.; Bouwman, F.H.; Visser, P.J.; Mattsson, N.; Zetterberg, H.; Blennow, K.; Hansson, O.; Minthon, L.; Andreasen, N.; et al. The cerebrospinal fluid “Alzheimer profile”: Easily said, but what does it mean? Alzheimer’s Dement. 2014, 10, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Seibyl, J.; Stomrud, E.; Zetterberg, H.; Trojanowski, J.Q.; Bittner, T.; Lifke, V.; Corradini, V.; Eichenlaub, U.; Batrla, R.; et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer’s Dement. 2018, 14, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Utz, J.; Olm, P.; Jablonowski, J.; Siegmann, E.-M.; Spitzer, P.; Lewczuk, P.; Kornhuber, J.; Maler, J.M.; Oberstein, T.J. Reconceptualization of the Erlangen Score for the Assessment of Dementia Risk: The ERlangen Score. J. Alzheimer’s Dis. 2023, 96, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Schindler, S.E.; Gray, J.D.; Gordon, B.A.; Xiong, C.; Batrla-Utermann, R.; Quan, M.; Wahl, S.; Benzinger, T.L.; Holtzman, D.M.; Morris, J.C.; et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimer’s Dement. 2018, 14, 1460–1469. [Google Scholar] [CrossRef]
- Caroli, A.; Prestia, A.; Galluzzi, S.; Ferrari, C.; Van Der Flier, W.M.; Ossenkoppele, R.; Van Berckel, B.; Barkhof, F.; Teunissen, C.; Wall, A.E.; et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP) Prediction of progression. Neurology 2015, 84, 508–515. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Oberstein, T.J.; Schmidt, M.A.; Florvaag, A.; Haas, A.-L.; Siegmann, E.-M.; Olm, P.; Utz, J.; Spitzer, P.; Doerfler, A.; Lewczuk, P.; et al. Amyloid-β levels and cognitive trajectories in non-demented pTau181-positive subjects without amyloidopathy. Brain 2022, 145, 4032–4041. [Google Scholar] [CrossRef]
- Berres, M.; Monsch, A.U.; Bernasconi, F.; Thalmann, B.; Stahelin, H. Normal ranges of neuropsychological tests for the diagnosis of Alzheimer’s disease. Stud. Health Technol. Inform. 2000, 77, 195–199. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Burnham, S.C.; Bourgeat, P.; Doré, V.; Savage, G.; Brown, B.; Laws, S.; Maruff, P.; Salvado, O.; Ames, D.; Martins, R.N.; et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: A longitudinal study. Lancet Neurol. 2016, 15, 1044–1053. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [PubMed]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar]
- Available online: https://adni.loni.usc.edu/ (accessed on 23 October 2024).
- Hansson, O.; Lehmann, S.; Otto, M.; Zetterberg, H.; Lewczuk, P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimer’s Res. Ther. 2019, 11, 1–15. [Google Scholar]
- Lewczuk, P.; Zimmermann, R.; Wiltfang, J.; Kornhuber, J. Neurochemical dementia diagnostics: A simple algorithm for interpretation of the CSF biomarkers. J. Neural Transm. 2009, 116, 1163–1167. [Google Scholar]
- de Leon, M.J.; Pirraglia, E.; Osorio, R.S.; Glodzik, L.; Saint-Louis, L.; Kim, H.-J.; Fortea, J.; Fossati, S.; Laska, E.; Siegel, C.; et al. Alzheimer’s Disease Neuroimaging Initiative, National Alzheimer’s Coordinating Center. The nonlinear relationship between cerebrospinal fluid Aβ42 and tau in preclinical Alzheimer’s disease. PLoS ONE 2018, 13, e0191240. [Google Scholar] [CrossRef]
- Lewczuk, P.; Łukaszewicz-Zając, M.; Kornhuber, J.; Mroczko, B. Clinical significance of plasma candidate biomarkers of Alzheimer’s Disease. Neurol. Neurochir. Pol. 2024, 58, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.; Rainey-Smith, S.R.; Bird, S.; Bomke, J.; Bourgeat, P.; Brown, B.M.; Burnham, S.C.; Bush, A.I.; Chadunow, C.; Collins, S.; et al. Fifteen years of the Australian Imaging, Biomarkers and Lifestyle (AIBL) study: Progress and observations from 2359 older adults spanning the spectrum from cognitive normality to Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2021, 5, 443–468. [Google Scholar]
- Potashman, M.; Buessing, M.; Benea, M.L.; Cummings, J.; Borson, S.; Pemberton-Ross, P.; Epstein, A.J. Estimating progression rates across the spectrum of Alzheimer’s disease for amyloid-positive individuals using national Alzheimer’s coordinating center data. Neurol. Ther. 2021, 10, 941–953. [Google Scholar]
| Normal Biomarkers | Alzheimer’s Continuum | SNAP1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R–2A–T−N– | R+3A–T–N– | R–A+T–N– | R+A+T–N– | R+A–T–N+ | R+A+T–N+ | R+A–T+N– | R+A+T+N– | R+A–T+N+ | R+A+T+N+ | R–A–T–N+ | R–A–T+N– | R–A–T+N+ | Total | |||
| pTau/Aβ1-42 ratio | Low | 87 | 1 | 5 | 2 | 3 | 0 | 1 | 0 | 4 | 0 | 9 | 16 | 29 | 157 | |
| High | 0 | 0 | 0 | 3 | 0 | 2 | 1 | 3 | 54 | 30 | 0 | 2 | 7 | 102 | ||
| ERS | Low | 0 | 87 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 9 | 3 | 107 |
| 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 9 | 33 | 49 | ||
| High4 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | |
| 3 | 0 | 0 | 0 | 4 | 1 | 0 | 1 | 0 | 57 | 0 | 0 | 0 | 0 | 63 | ||
| 4 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 1 | 30 | 0 | 0 | 0 | 36 | ||
| pTau/Aβ1-42 Ratio | ERS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | |||||||||||||
| n | % | n | % | n | % | n | % | |||||||||
| Total (female) | 157 | (55) | 61 | (21) | 102 | (49) | 39 | (19) | 156 | (56) | 60 | (22) | 103 | (48) | 40 | (19) |
| Total (MCI) | 117 | (40) | 56 | (47) | 91 | (45) | 44 | (53) | 117 | (39) | 56 | (46) | 91 | (46) | 44 | (54) |
| Total (SCI) | 40 | (16) | 78 | (84) | 11 | (3) | 22 | (16) | 39 | (16) | 76 | (84) | 12 | (3) | 24 | (16) |
| mean | SEM | mean | SEM | mean | SEM | mean | SEM | |||||||||
| Age [years] | 63.5 | 0.7 | 71.8 | 0.8 | 63.3 | 0.7 | 72.0 | 0.8 | ||||||||
| Education [years] | 14.3 | 0.2 | 13.2 | 0.3 | 14.3 | 0.2 | 13.3 | 0.3 | ||||||||
| MMSE | 27.4 | 0.2 | 26.0 | 0.2 | 27.3 | 0.2 | 26.2 | 0.2 | ||||||||
| Follow-Up [years] | 4.9 | 0.3 | 3.3 | 0.2 | 4.8 | 0.3 | 3.5 | 0.2 | ||||||||
| A | Fixed Effects | B | Fixed Effects | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SEM | t | p | 95% CI | Estimate | SEM | t | p | 95% CI | ||||
| Intercept | −1.30 | 0.09 | −14.79 | <0.001 | −1.47 | −1.12 | Intercept | −1.32 | 0.09 | −15.25 | <0.001 | −1.49 | −1.15 |
| Follow-up [Y] | −0.07 | 0.03 | −2.31 | 0.028 | −0.17 | −0.01 | Follow-up [Y] | −0.11 | 0.04 | −2.85 | 0.008 | −0.19 | −0.03 |
| Pathologic ERS | −0.40 | 0.15 | −2.45 | 0.015 | −0.65 | −0.07 | Pathologic pTau/Aβ1−42 ratio | −0.30 | 0.15 | −1.99 | 0.048 | −0.60 | 0.00 |
| Pathologic ERS × Follow-up [Y] | −0.40 | 0.06 | −6.13 | <0.001 | −0.57 | −0.29 | Pathologic pTau/Aβ1−42 ratio × follow-up [Y] | −0.44 | 0.08 | −5.80 | <0.001 | −0.59 | −0.29 |
| Model fit | Model fit | ||||||||||||
| AIC | 2346.69 | AIC | 2371.68 | ||||||||||
| BIC | 2405.16 | BIC | 2412.15 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, J.A.; Schulz, P.; Utz, J.; Rudtke, L.; Jablonowski, J.; Klement, N.; Lewczuk, P.; Kornhuber, J.; Maler, J.M.; Oberstein, T.J. Comparison of ERlangen Score with pTau/Aβ1-42 Ratio for Predicting Cognitive Decline and Conversion to Alzheimer’s Disease. Brain Sci. 2025, 15, 334. https://doi.org/10.3390/brainsci15040334
Schwarz JA, Schulz P, Utz J, Rudtke L, Jablonowski J, Klement N, Lewczuk P, Kornhuber J, Maler JM, Oberstein TJ. Comparison of ERlangen Score with pTau/Aβ1-42 Ratio for Predicting Cognitive Decline and Conversion to Alzheimer’s Disease. Brain Sciences. 2025; 15(4):334. https://doi.org/10.3390/brainsci15040334
Chicago/Turabian StyleSchwarz, Julian Alexander, Pauline Schulz, Janine Utz, Laura Rudtke, Johannes Jablonowski, Neele Klement, Piotr Lewczuk, Johannes Kornhuber, Juan Manuel Maler, and Timo Jan Oberstein. 2025. "Comparison of ERlangen Score with pTau/Aβ1-42 Ratio for Predicting Cognitive Decline and Conversion to Alzheimer’s Disease" Brain Sciences 15, no. 4: 334. https://doi.org/10.3390/brainsci15040334
APA StyleSchwarz, J. A., Schulz, P., Utz, J., Rudtke, L., Jablonowski, J., Klement, N., Lewczuk, P., Kornhuber, J., Maler, J. M., & Oberstein, T. J. (2025). Comparison of ERlangen Score with pTau/Aβ1-42 Ratio for Predicting Cognitive Decline and Conversion to Alzheimer’s Disease. Brain Sciences, 15(4), 334. https://doi.org/10.3390/brainsci15040334







