Olfactory Testing and Gray Matter Volume: A Combined Approach to Predict the Conversion to Alzheimer
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive and Olfactory Assessment
| COGNITIVE DOMAIN | TESTS |
|---|---|
| LANGUAGE | Boston Naming Test [25] |
| Phonemics verbal fluency [26] | |
| Semantic verbal fluency [26] | |
| CONSTRUCTIONAL PRAXIS | Rey–Osterrieth Complex Figure Test [27] |
| MEMORY | Babcock Story Recall Test [28] |
| Paired-associate Words learning [28] | |
| Free and Cued Selective Reminding Test (FCRST), comprising Immediate Free Recall (IFR), Immediate Total Recall (ITR), Index of Sensitivity of Cueing (ISC) [29] | |
| Recall of Rey–Osterrieth Complex Figure Test [27] | |
| Digit Span test [30] | |
| Corsi’s Spatial Span test [30] | |
| LEARNING | Corsi’s Spatial Supra-Span test [31] |
| REASONING | Raven’s Cognitive Progressive Matrices [32] |
| Similarities Test (Wechsler Adult Intelligence Scale-IV) [33] | |
| ATTENTION | Visual Search/Attentive Matrices test [34] |
| EXECUTIVE FUNCTION | Frontal Assessment Battery (FAB) [35] |
| Cognitive estimates (time and weights) [36] | |
| Stroop test [37] | |
| Trial Making Test (TMT) [38] |
2.3. Imaging Data Collection
2.4. Data Analyses
2.4.1. Behavioural Data Analyses
2.4.2. MRI Data Analysis
- tissue segmentation in gray matter—GM, white matter—WM, and cerebral spinal fluid—CSF;
- spatial normalization, using the Dartel algorithm to build a group-specific template and to warp participants’ scans onto this template, creating the flow field, which stores the deformation information;
- spatial smoothing with an isotropic Gaussian FWHM kernel of 8 × 8 × 8 mm.
3. Results
3.1. Socio-Demographic Results
3.2. Behavioural Results
3.2.1. Burghart Sniffin Sticks Tests
3.2.2. Receiver Operating Characteristic (ROC) Curve and Area Under Curve (AUC) Analyses
3.2.3. Correlational Analyses Between Olfactory and Cognitive Assessment
3.3. Voxel-Based Morphometry Results
3.4. ROIs and Correlational Analyses
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| MCI | Mild Cognitive Impairment |
| cMCI | Converter Mild Cognitive Impairment |
| sMCI | Stable Mild Cognitive Impairment |
| HC | Healthy Controls |
| TT | Threshold Test |
| DT | Discrimination Test |
| IT | Identification Test |
| VBM | Voxel-Based Morphometry |
| DMN | Default Mode Network |
| UPSIT | University of Pennsylvania Smell Identification Test |
| SD | Standard Deviation |
| MMSE | Mini Mental State Examination |
| MRI | Magnetic Resonance Imaging |
| F | Female |
| M | Male |
| FCRST | Free and Cued Selective Reminding Test |
| IFR | Immediate Free Recall |
| ITR | Immediate Total Recall |
| ISC | Index of Sensitivity of Cueing |
| FAB | Frontal Assessment Battery |
| TMT | Trial Making Test |
| TR | Repetition time |
| TE | Echo time |
| ANCOVA | Analysis of Covariance |
| ln | natural logarithm |
| ROC | Receiver Operating Characteristic |
| AUC | Areas Under Curve |
| GM | Gray Matter |
| WM | White Matter |
| CSF | Cerebral Spinal Fluid |
| FWHM | Full Width Half Maximum |
| TIV | Total Intracranial Volume |
| ROI | Regions Of Interest |
| BA | Brodmann Area |
| L | Left |
| R | Right |
| MNI | Montreal Neurological Institute coordinate system |
| PASA | Posterior–Anterior Shifting in Aging |
References
- Jellinek, J.S. Proust remembered: Has Proust’s account of odor-cued autobiographical memory recall really been investigated? Chem. Senses 2004, 29, 455–458; author reply 459–461. [Google Scholar] [CrossRef]
- Sela, L.; Sobel, N. Human olfaction: A constant state of change-blindness. Exp. Brain Res. 2010, 205, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E.; Fransen, E.; Dickson, C.; Alonso, A.A. Computational modeling of entorhinal cortex. Ann. NY Acad. Sci. 2000, 911, 418–446. [Google Scholar] [CrossRef] [PubMed]
- Porcherot, C.; Delplanque, S.; Raviot-Derrien, S.; Calvé, B.L.; Chrea, C.; Gaudreau, N.; Cayeux, I. How do you feel when you smell this? optimization of a verbal measurement of odor-elicited emotions. Food Qual. Prefer. 2010, 21, 938–947. [Google Scholar] [CrossRef]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfaction and Aging: A Mini-Review. Gerontology 2015, 61, 485–490. [Google Scholar] [CrossRef]
- Doty, R.L.; Kamath, V. The influences of age on olfaction: A review. Front. Psychol. 2014, 5, 20. [Google Scholar] [CrossRef]
- Bothwell, A.R.; Resnick, S.M.; Ferrucci, L.; Tian, Q. Associations of olfactory function with brain structural and functional outcomes. A systematic review. Ageing Res. Rev. 2023, 92, 102095. [Google Scholar] [CrossRef]
- Kamath, V.; Senjem, M.L.; Spychalla, A.J.; Chen, H.; Palta, P.; Mosley, T.H.; Windham, B.G.; Griswold, M.; Knopman, D.S.; Gottesman, R.F.; et al. The Neuroanatomic Correlates of Olfactory Identification Impairment in Healthy Older Adults and in Persons with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2022, 89, 233–245. [Google Scholar] [CrossRef]
- Bouhaben, J.; Delgado-Lima, A.H.; Delgado-Losada, M.L. The role of olfactory dysfunction in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Arch. Gerontol. Geriatr. 2024, 123, 105425. [Google Scholar] [CrossRef]
- Chandra, A.; Dervenoulas, G.; Politis, M. Alzheimer’s Disease Neuroimaging Initiative. Magnetic resonance imaging in Alzheimer’s disease and mild cognitive impairment. J. Neurol. 2019, 266, 1293–1302. [Google Scholar] [CrossRef]
- Jobin, B.; Boller, B.; Frasnelli, J. Volumetry of Olfactory Structures in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and a Meta-Analysis. Brain Sci. 2021, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Ni, J.; Cao, T.; Wang, S.; Shi, J.; Tian, J. The correlation between olfactory test and hippocampal volume in alzheimer’s disease and mild cognitive impairment patients: A meta-analysis. Front. Aging Neurosci. 2021, 13, 755160. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità. Linee Guida e Trattamento di Demenza e Mild Cognitive Impairment. Available online: https://www.demenze.it/it-schede-18-documentazione_sulle_demenze (accessed on 11 February 2025).
- National Institute for Health and Care Excellence. Guidelines; National Institute for Health and Care Excellence (NICE): London, UK, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11822/ (accessed on 11 February 2025).
- Eyler, L.T.; Elman, J.A.; Hatton, S.N.; Gough, S.; Mischel, A.K.; Hagler, D.J.; Franz, C.E.; Docherty, A.; Fennema-Notestine, C.; Gillespie, N.; et al. Resting State Abnormalities of the Default Mode Network in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2019, 70, 107–120. [Google Scholar] [CrossRef]
- Audronyte, E.; Sutnikiene, V.; Pakulaite-Kazliene, G.; Kaubrys, G. Brief Test of Olfactory Dysfunction Based on Diagnostic Features of Specific Odors in Early-Stage Alzheimer Disease. Med Sci Monit. 2023, 29, e940363-1. [Google Scholar] [CrossRef] [PubMed]
- Audronytė, E.; Sutnikiene, V.; Pakulaite-Kazliene, G.; Kaubrys, G. Olfactory memory in mild cognitive impairment and alzheimer’s disease. Front. Neurol. 2023, 14, 1165594. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Kobal, G.; Hummel, T.; Sekinger, B.; Barz, S.; Roscher, S.; Wolf, S. "Sniffin’ sticks": Screening of olfactory performance. Rhinology 1996, 34, 222–226. [Google Scholar]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’ sticks’. Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Kobal, G.; Palisch, K.; Wolf, S.R.; Meyer, E.D.; Hüttenbrink, K.B.; Roscher, S.; Wagner, R.; Hummel, T. A threshold-like measure for the assessment of olfactory sensitivity: The “random” procedure. Eur. Arch. Otorhinolaryngol. 2001, 258, 168–172. [Google Scholar] [CrossRef]
- D’Agostino, L. Taratura su Soggetti Normali di Prove di Denominazione per L’afasia. Ph.D. Thesis, Università Degli Studi di Modena, Facoltà di Medicina e Chirurgia, Istituto di Clinica Neurologica, Modena, Italy, 1985. [Google Scholar]
- Costa, A.; Bagoj, E.; Monaco, M.; Zabberoni, S.; De Rosa, S.; Papantonio, A.M.; Mundi, C.; Caltagirone, C.; Carlesimo, G.A. Standardization and normative data obtained in the Italian population for a new verbal fluency instrument, the phonemic/semantic alternate fluency test. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2014, 35, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2002, 22, 443–447. [Google Scholar] [CrossRef]
- De Renzi, E.; Faglioni, P.; Ruggerini, C. Prove di memoria verbale di impiego clinico per la diagnosi di amnesia. Arch. Psicol. Neurol. Psichiatr. 1977, 38, 303–318. [Google Scholar]
- Girtler, N.; Carli, F.; Amore, M.; Arnaldi, D.; Bosia, L.; Bruzzaniti, C.; Cappa, S.; Cocito, L.; Colazzo, G.; Ghio, L.; et al. A normative study of the Italian printed word version of the free and cued selective reminding test. Neurol. Sci. 2015, 36, 1127e1134. [Google Scholar] [CrossRef]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2013, 34, 749–754. [Google Scholar] [CrossRef]
- Capitani, E.; Laiacona, M.; Ciceri, E. Sex differences in spatial memory: A reanalysis of block tapping long-term memory according to the short-term memory level. Ital. J. Neurol. Sci. 1991, 12, 461–466. [Google Scholar] [CrossRef]
- Spinnler, H.; Tognin, G. Standardizzazione e taratura italiana di test neuropsicologici. Ital. J. Neurol. Sci. 1987, 6, 62–65. [Google Scholar]
- Wechsler, D. WAIS-IV: Wechsler Adult Intelligence Scale. 2008. Available online: https://psycnet.apa.org/doiLanding?doi=10.1037%2Ft15169-000 (accessed on 10 October 2019).
- Della Sala, S.; Laiacona, M.; Spinnler, H.; Ubezio, C. A cancellation test: Its reliability in assessing attentional deficits in Alzheimer’s disease. Psychol. Med. 1992, 22, 885–901. [Google Scholar] [CrossRef]
- Aiello, E.N.; Esposito, A.; Gramegna, C.; Gazzaniga, V.; Zago, S.; Difonzo, T.; Appollonio, I.M.; Bolognini, N. The Frontal Assessment Battery (FAB) and its sub-scales: Validation and updated normative data in an Italian population sample. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022, 43, 979–984. [Google Scholar] [CrossRef]
- Della Sala, S.; MacPherson, S.E.; Phillips, L.H.; Sacco, L.; Spinnler, H. How many camels are there in Italy? Cognitive estimates standardised on the Italian population. Neurol. Sci. 2003, 24, 10–15. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Una versione abbreviata del test di Stroop: Dati normativi nella popolazione italiana. Nuova Riv. Neurol. 2002, 12, 111–115. [Google Scholar]
- Siciliano, M.; Chiorri, C.; Battini, V.; Sant’Elia, V.; Altieri, M.; Trojano, L.; Santangelo, G. Regression-based normative data and equivalent scores for Trail Making Test (TMT): An updated Italian normative study. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2019, 40, 469–477. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Why voxel-based morphometry should be used. Neuroimage 2001, 14, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.A.; Friston, K.J.; Frackowiak, R.S.J. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. Neuroimage 2001, 14, 21–36. [Google Scholar] [CrossRef]
- Brett, M.; Anton, J.-L.; Valabregue, R.; Poline, J.-B. Region of interest analysis using an SPM toolbox. In Proceedings of the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, 2–6 June 2002; Available on CD-ROM in NeuroImage. Volume 16. No 2. [Google Scholar]
- Grober, E.; Buschke, H.; Crystal, H.; Bang, S.; Dresner, R. Screening for dementia by memory testing. Neurology 1988, 38, 900–903. [Google Scholar] [CrossRef]
- Grober, E.; Lipton, R.B.; Hall, C.; Crystal, H. Memory impairment on free and cued selective reminding predicts dementia. Neurology 2000, 54, 827–832. [Google Scholar] [CrossRef]
- Grande, G.; Vanacore, N.; Vetrano, D.L.; Cova, I.; Rizzuto, D.; Mayer, F.; Maggiore, L.; Ghiretti, R.; Cucumo, V.; Mariani, C.; et al. Free and cued selective reminding test predicts progression to Alzheimer’s disease in people with mild cognitive impairment. Neurol. Sci. 2018, 39, 1867–1875. [Google Scholar] [CrossRef]
- Kollndorfer, K.; Reichert, J.; Braunsteiner, J.; Schöpf, V. Assessment of Olfactory Memory in Olfactory Dysfunction. Perception 2017, 46, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Devanand, D.P.; Lee, S.; Luchsinger, J.A.; Andrews, H.; Goldberg, T.; Huey, E.D.; Schupf, N.; Manly, J.; Stern, Y.; Kreisl, W.C.; et al. Intact global cognitive and olfactory ability predicts lack of transition to dementia. Alzheimer’s Dement. 2020, 16, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Schlintl, C.; Schienle, A. Reduced Olfactory Memory Performance Is Associated with Executive Function Deficits in Older Adults. Exp. Aging Res. 2023, 49, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Broks, P.; Lines, C.; Atchison, L.; Challenor, J.; Traub, M.; Foster, C.; Sagar, H. Neuropsychological investigation of anterior and posterior cortical function in early-stage probable Alzheimer’s disease. Behav. Neurol. 1996, 9, 135–148. [Google Scholar] [CrossRef]
- Razani, J.; Casas, R.; Wong, J.T.; Lu, P.; Alessi, C.; Josephson, K. Relationship between executive functioning and activities of daily living in patients with relatively mild dementia. Appl. Neuropsychol. 2007, 14, 208–214. [Google Scholar] [CrossRef]
- Binetti, G.; Magni, E.; Padovani, A.; Cappa, S.F.; Bianchetti, A.; Trabucchi, M. Executive dysfunction in early Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1996, 60, 91–93. [Google Scholar] [CrossRef]
- Bondi, M.W.; Serody, A.B.; Chan, A.S.; Eberson-Shumate, S.C.; Delis, D.C.; Hansen, L.A.; Salmon, D.P. Cognitive and neuropathologic correlates of Stroop Color-Word Test performance in Alzheimer’s disease. Neuropsychology 2002, 16, 335–343. [Google Scholar] [CrossRef]
- Amieva, H.; Phillips, L.H.; Della Sala, S.; Henry, J.D. Inhibitory functioning in Alzheimer’s disease. Brain 2004, 127, 949–964. [Google Scholar] [CrossRef]
- Djordjevic, J.; Jones-Gotman, M.; De Sousa, K.; Chertkow, H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2008, 29, 693–706. [Google Scholar] [CrossRef]
- Lehrner, J.; Pusswald, G.; Gleiss, A.; Auff, E.; Dal Bianco, P. Odor identification and selfreported olfactory functioning in patients with subtypes of mild cognitive impairment. Clin. Neuropsychol. 2009, 23, 818–830. [Google Scholar] [CrossRef]
- Makizako, M.; Makizako, H.; Doi, T.; Uemura, K.; Tsutsumimoto, K.; Miyaguchi, H.; Shimada, H. Olfactory identification and cognitive performance in community-dwelling older adults with mild cognitive impairment. Chem. Senses 2014, 39, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Royall, D.R.; Chiodo, L.K.; Polk, M.J.; Jaramillo, C.J. Severe dysosmia is specifically associated with Alzheimer-like memory deficits in nondemented elderly retirees. Neuroepidemiology 2002, 21, 68–73. [Google Scholar] [CrossRef]
- Ward, A.M.; Calamia, M.; Thiemann, E.; Dunlap, J.; Tranel, D. Association between olfaction and higher cortical functions in Alzheimer’s disease, mild cognitive impairment, and healthy older adults. J. Clin. Exp. Neuropsychol. 2017, 39, 646–658. [Google Scholar] [CrossRef]
- Challakere Ramaswamy, V.M.; Schofield, P.W. Olfaction and Executive Cognitive Performance: A Systematic Review. Front. Psychol. 2022, 13, 871391. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Savva, G.M.; Wharton, S.B.; Ince, P.G.; Forster, G.; Matthews, F.E.; Brayne, C. Medical Research Council Cognitive Function and Ageing Study. Age, neuropathology, and dementia. N. Engl. J. Med. 2009, 360, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Scahill, R.I.; Schott, J.M.; Stevens, J.M.; Rossor, M.N.; Fox, N.C. Mapping the evolution of regional atrophy in Alzheimer’s disease: Unbiased analysis of fluid-registered serial MRI. Proc. Natl. Acad. Sci. USA 2002, 99, 4703–4707. [Google Scholar] [CrossRef]
- Lehéricy, S.; Baulac, M.; Chiras, J.; Piérot, L.; Martin, N.; Pillon, B.; Deweer, B.; Dubois, B.; Marsault, C. Amygdalohippocampal MR volume measurements in the early stages of Alzheimer disease. AJNR Am. J. Neuroradiol. 1994, 15, 929–937. [Google Scholar]
- Chan, D.; Fox, N.C.; Scahill, R.I.; Crum, W.R.; Whitwell, J.L.; Leschziner, G.; Rossor, A.M.; Stevens, J.M.; Cipolotti, L.; Rossor, M.N. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann. Neurol. 2001, 49, 433–442. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Goncharova, I.; Sullivan, M.P.; Forchetti, C.; Wilson, R.S.; Bennett, D.A.; Beckett, L.A.; deToledo-Morrell, L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol. Aging 2001, 22, 747–754. [Google Scholar] [CrossRef]
- Killiany, R.J.; Hyman, B.T.; Gomez-Isla, T.; Moss, M.B.; Kikinis, R.; Jolesz, F.; Tanzi, R.; Jones, K.; Albert, M.S. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology 2002, 58, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Buschhüter, D.; Smitka, M.; Puschmann, S.; Gerber, J.C.; Witt, M.; Abolmaali, N.D.; Hummel, T. Correlation between olfactory bulb volume and olfactory function. Neuroimage 2008, 42, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Gudziol, V.; Buschhüter, D.; Abolmaali, N.; Gerber, J.; Rombaux, P.; Hummel, T. Increasing olfactory bulb volume due to treatment of chronic rhinosinusitis—A longitudinal study. Brain 2009, 132, 3096–3101. [Google Scholar] [CrossRef]
- Gottfried, J.A. Central mechanisms of odour object perception. Nat. Rev. Neurosci. 2010, 11, 628–641. [Google Scholar] [CrossRef]
- Li, W.; Howard, J.D.; Gottfried, J.A. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer’s disease. Brain 2010, 133, 2714–2726. [Google Scholar] [CrossRef] [PubMed]
- Prestia, A.; Drago, V.; Rasser, P.E.; Bonetti, M.; Thompson, P.M.; Frisoni, G.B. Cortical changes in incipient Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 22, 1339–1349. [Google Scholar] [CrossRef]
- Davis, S.W.; Dennis, N.A.; Daselaar, S.M.; Fleck, M.S.; Cabeza, R. Que PASA? The posterior-anterior shift in aging. Cereb. Cortex 2008, 18, 1201–1209. [Google Scholar] [CrossRef]


| Whole Group (n = 27) | cMCI (n = 13) | sMCI (n = 14) | Group Comparison | |
|---|---|---|---|---|
| Age (years) | 71.4 (±7.5) | 71.9 (±7.4) | 70.9 (±7.5) | p = 0.7 |
| Gender F/M | 14:13 | 7:6 | 7:7 | p = 1 |
| MMSE | 27 (±2) | 26.5 (±1.9) | 27 (±1.8) | p = 0.5 |
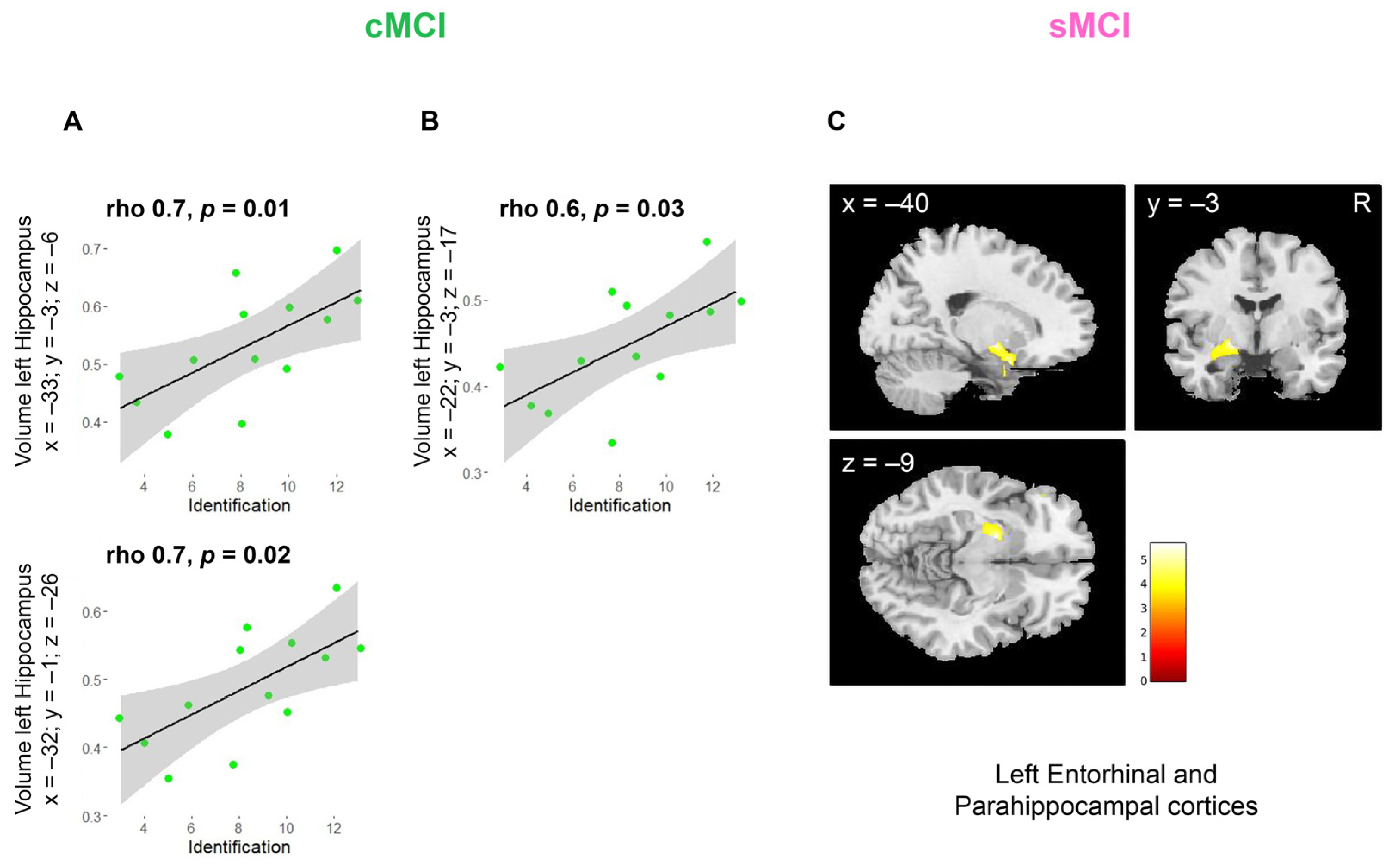
| Group | Olfactory Battery Tests | Neuropsychological Tests | Spearman’s Rho |
|---|---|---|---|
| sMCI | DT | IFR | 0.8 ** |
| ITR | 0.8 ** | ||
| ISC | 0.8 ** | ||
| Raven Matrices | 0.6 * | ||
| sMCI | IT | IFR | 0.9 *** |
| ITR | 0.9 *** | ||
| ISC | 0.8 *** | ||
| Raven Matrices | 0.8 ** | ||
| Boston Naming Test | 0.7 ** | ||
| Stroop (Rate of Errors) | −0.6 * | ||
| cMCI | DT | Paired-Associate Words Test | 0.6 * |
| FAB | 0.7 * | ||
| cMCI | IT | Weight Estimate Score | 0.8 ** |
| Total Cognitive Estimates Scores | 0.6 * |
| Anatomical Regions | BA | Side | Cluster | Voxel Level | MNI Coordinates | ||
|---|---|---|---|---|---|---|---|
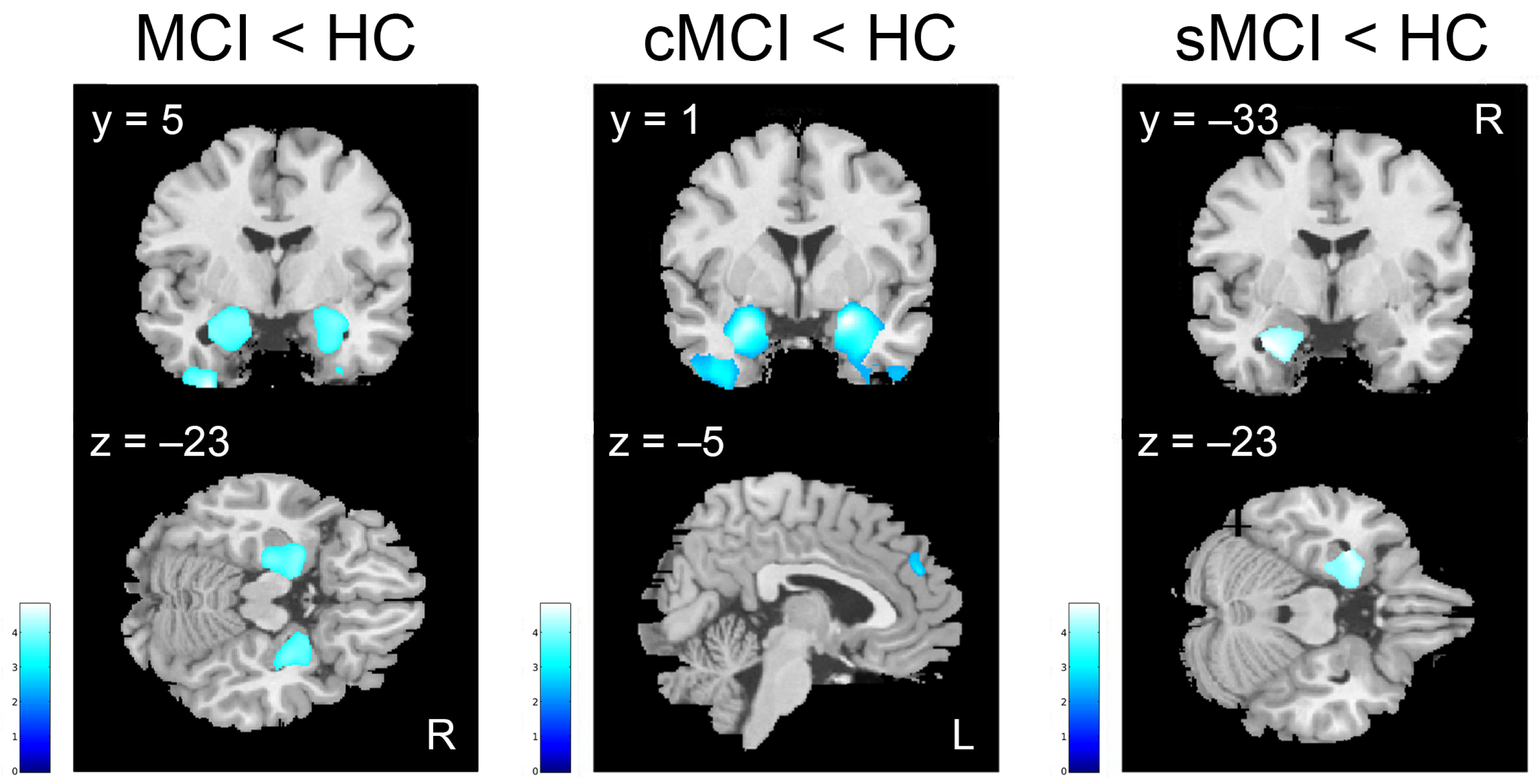
| MCI < HC (k ≥ 543) | k | T | x | y | z | ||
| Superior, Middle, Inferior Temporal gyri, Fusiform gyrus, Uncus | 38, 21, 20 | L | 2907 | 4.81 | −35 | −3 | −50 |
| Hippocampus, Parahippocampal gyrus, Amygdala, Uncus, Superior Temporal gyrus, Fusiform gyrus, Temporal Pole | 37, 38 | L | 6101 | 4.22 | −32 | −1 | 26 |
| Hippocampus, Parahippocampal gyrus, Amygdala, Caudate, Uncus, Superior Temporal gyrus, Fusiform gyrus, Temporal Pole | 37, 38 | R | 5832 | 4.17 | 28 | 5 | −23 |
| Superior, Middle, Inferior Temporal gyri, Fusiform gyrus, Uncus | 38 | R | 1616 | 4.12 | 46 | 7 | −47 |
| cMCI < HC (k ≥ 602) | k | T | x | y | z | ||
| Hippocampus, Parahippocampal gyrus, Amygdala, Uncus, Precuneus, Superior, Middle, Inferior Temporal gyri, Temporal Pole, Fusiform gyrus, Lingual gyrus, Inferior Frontal gyrus, Olfactory cortex | 20, 21, 27, 30, 47 | R | 17,225 | 6.99 | 22 | 1 | −18 |
| Hippocampus, Parahippocampal gyrus, Amygdala, Insula, Putamen, Uncus, Precuneus, Superior, Middle, Inferior Temporal gyri, Fusiform gyrus, Lingual gyrus, Temporal Pole, Inferior Frontal gyrus, Olfactory cortex, Cerebellum | 20, 21, 27, 37, 38, 47 | L | 19,029 | 6.72 | −22 | −3 | −17 |
| Medial Frontal gyrus | 6, 8, 9 | L | 656 | 4.22 | −5 | 47 | 28 |
| Cingulate gyrus | 23, 24, 31 | R | 1225 | 4.18 | 1 | −28 | 41 |
| Insula, Putamen | 44 | R | 712 | 4.16 | 35 | 12 | 3 |
| Middle Temporal gyrus | 21 | L | 768 | 4.08 | −62 | −28 | −14 |
| sMCI < HC (k ≥ 538) | k | T | x | y | z | ||
| Hippocampus, Parahippocampal gyrus, Amygdala, Uncus, Precuneus, Superior Temporal gyrus, Temporal Pole, Fusiform gyrus | 38 | L | 2698 | 4.08 | −33 | −3 | −26 |
| Anatomical Regions | BA | Side | Cluster | Voxel Level | MNI Coordinates | ||
|---|---|---|---|---|---|---|---|
| k | T | x | y | z | |||
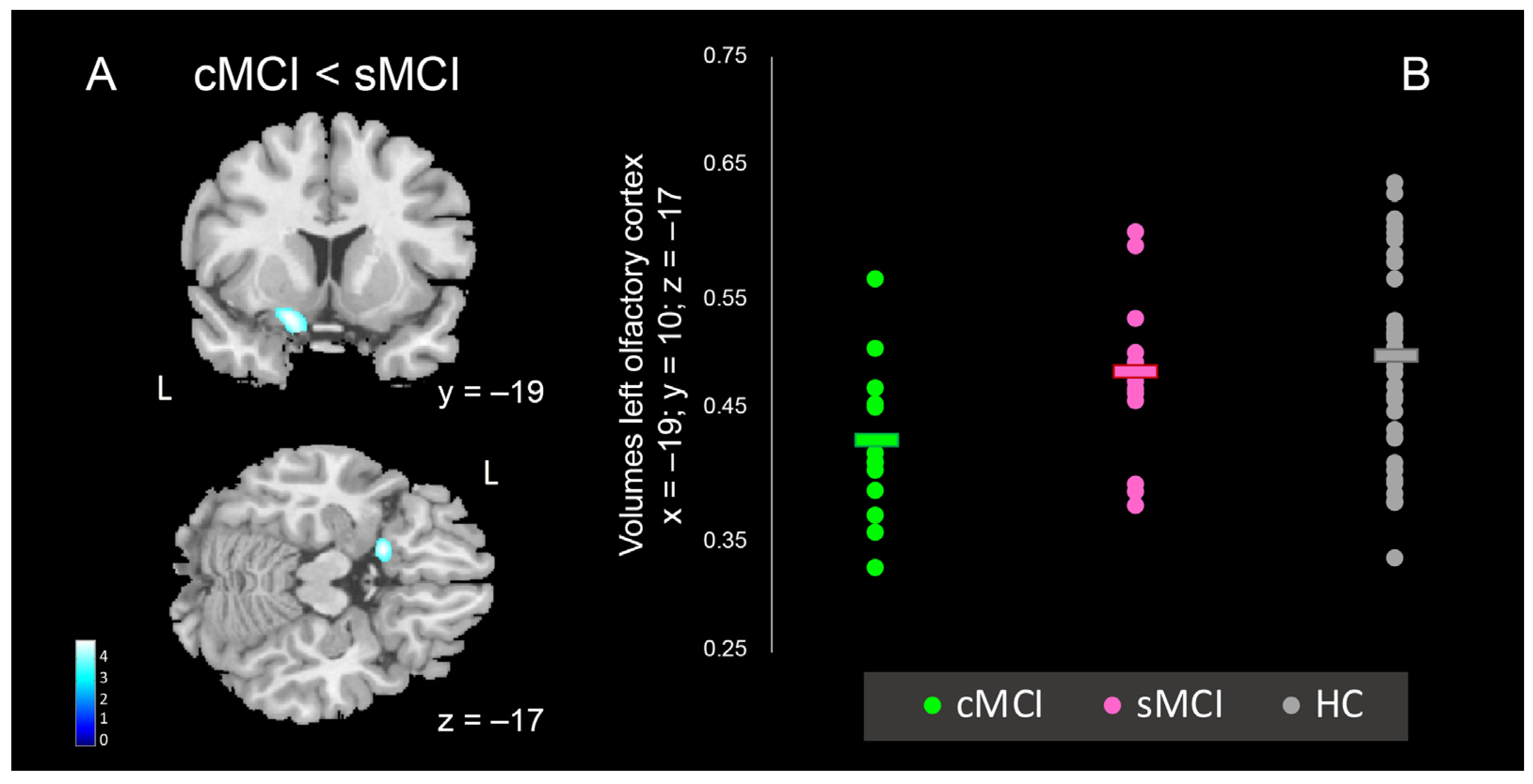
| Inferior Frontal gyrus, gyrus Rectus, Olfactory cortex, Insula | 47 | L | 833 | 4.83 | −19 | 10 | −17 |
| Middle, Inferior Temporal gyri, Middle Occipital gyrus | 19, 37, 21 | R | 1002 | 4.8 | 54 | −60 | 6 |
| Anatomical Regions | Cluster Peak In MNI Coordinates | VBM Group Analyses | ||
|---|---|---|---|---|
| x | y | z | contrasts | |
| Left Hippocampus | −32 | −1 | −26 | HC vs. MCI |
| Right Hippocampus | 28 | 5 | −23 | HC vs. MCI |
| Left Hippocampus | −33 | −3 | −6 | HC vs. sMCI |
| Left Hippocampus | −22 | −3 | −17 | HC vs. cMCI |
| Right Hippocampus | 22 | 1 | −18 | HC vs. cMCI |
| Right Insula | 35 | 12 | 3 | HC vs. cMCI |
| Left Medial Frontal Gyrus | −5 | 47 | 28 | HC vs. cMCI |
| Cingulate Gyrus | 1 | −28 | 41 | HC vs. cMCI |
| Left Olfactory Cortex | −19 | 10 | −17 | sMCI vs. cMCI |
| Superior Frontal Gyrus | −24 | 35 | 41 | sMCI vs. cMCI |
| Anatomical Regions Per Test | BA | Side | Cluster | Voxel Level | MNI Coordinates | ||
|---|---|---|---|---|---|---|---|
| k | T | x | y | z | |||
| Parahippocampal gyrus, Amygdala, Hippocampus, Fusiform gyrus, Olfactory cortex, Inferior Frontal gyrus, Insula | 28, 37, 20, 34, 47 | L | 4291 | 5.6 | −20 | −3 | −9 |
| Inferior and Middle Frontal gyrus | 47, 45 | L | 688 | 4.4 | −47 | 31 | 1 |
| Anatomical Regions Per Test | BA | Side | Cluster | Voxel Level | MNI Coordinates | ||
|---|---|---|---|---|---|---|---|
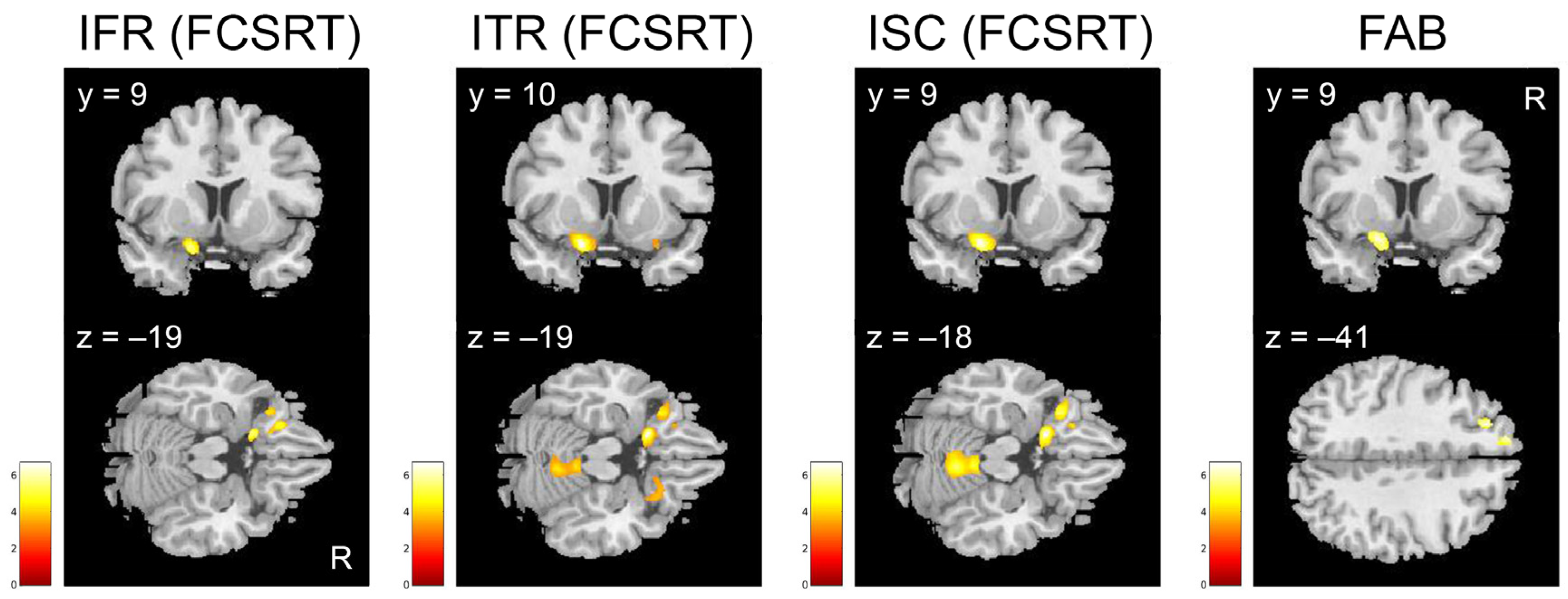
| IFR from FCSRT (k ≥ 681) | k | T | x | y | z | ||
| Inferior and Middle Frontal gyrus, Olfactory cortex, gyrus Rectus, Insula | 11, 47 | L | 2410 | 5.9 | −19 | 9 | −19 |
| Superior, Middle and Inferior Temporal gyrus | 19, 22, 37 | R | 961 | 5.8 | 52 | −61 | 8 |
| ITR from FCSRT (k ≥ 691) | k | T | x | y | z | ||
| Inferior and Middle Frontal gyrus, Medial Frontal gyrus, Olfactory cortex, gyrus Rectus, Insula | 47, 11 | L | 4954 | 7.7 | −19 | 10 | −19 |
| Superior Parietal Lobule, Precuneus | 7 | R | 1320 | 5.8 | 20 | −58 | 65 |
| Cerebellum | R | 2542 | 5.1 | 5 | −54 | −18 | |
| Superior, Middle and Inferior Temporal gyrus | 39, 37, 22 | R | 1086 | 4.8 | 53 | −60 | 7 |
| Inferior Frontal gyrus, Olfactory cortex, gyrus Rectus, Insula, Superior Temporal gyrus, Amygdala | 47, 25, 34, | R | 831 | 4.3 | 19 | 20 | −21 |
| ISC from FCSRT (k ≥ 701) | k | T | x | y | z | ||
| Middle and Inferior Frontal gyrus, Olfactory cortex, gyrus Rectus, Insula | 47, 11, 25 | L | 5104 | 6.8 | −19 | 10 | −18 |
| Superior Parietal lobule, Precuneus | 7 | R | 1312 | 5.8 | 20 | −58 | 65 |
| Cerebellum | R | 3826 | 5.6 | 5 | −53 | −18 | |
| FAB (k ≥ 685) | k | T | x | y | z | ||
| Superior and Middle Frontal gyrus, Medial Frontal gyrus | 9, 8 | L | 776 | 5.2 | −24 | 35 | 41 |
| Olfactory cortex, Inferior Frontal gyrus, gyrus Rectus, Insula, Medial Frontal gyrus | 34, 47, 25 | L | 721 | 4.9 | −19 | 9 | −16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casadio, C.; Ballotta, D.; Ricci, F.; Zanelli, V.; Carpentiero, O.; Corni, M.G.; Bardi, E.; Filippini, N.; Lui, F.; Nichelli, P.F.; et al. Olfactory Testing and Gray Matter Volume: A Combined Approach to Predict the Conversion to Alzheimer. Brain Sci. 2025, 15, 310. https://doi.org/10.3390/brainsci15030310
Casadio C, Ballotta D, Ricci F, Zanelli V, Carpentiero O, Corni MG, Bardi E, Filippini N, Lui F, Nichelli PF, et al. Olfactory Testing and Gray Matter Volume: A Combined Approach to Predict the Conversion to Alzheimer. Brain Sciences. 2025; 15(3):310. https://doi.org/10.3390/brainsci15030310
Chicago/Turabian StyleCasadio, Claudia, Daniela Ballotta, Francesco Ricci, Vanessa Zanelli, Omar Carpentiero, Maria Giulia Corni, Elisa Bardi, Nicola Filippini, Fausta Lui, Paolo Frigio Nichelli, and et al. 2025. "Olfactory Testing and Gray Matter Volume: A Combined Approach to Predict the Conversion to Alzheimer" Brain Sciences 15, no. 3: 310. https://doi.org/10.3390/brainsci15030310
APA StyleCasadio, C., Ballotta, D., Ricci, F., Zanelli, V., Carpentiero, O., Corni, M. G., Bardi, E., Filippini, N., Lui, F., Nichelli, P. F., Molinari, M. A., & Benuzzi, F. (2025). Olfactory Testing and Gray Matter Volume: A Combined Approach to Predict the Conversion to Alzheimer. Brain Sciences, 15(3), 310. https://doi.org/10.3390/brainsci15030310








