Evaluating Cognitive Function and Brain Activity Patterns via Blood Oxygen Level-Dependent Transformer in N-Back Working Memory Tasks
Abstract
1. Introduction
2. Methods and Materials
2.1. Datasets Description
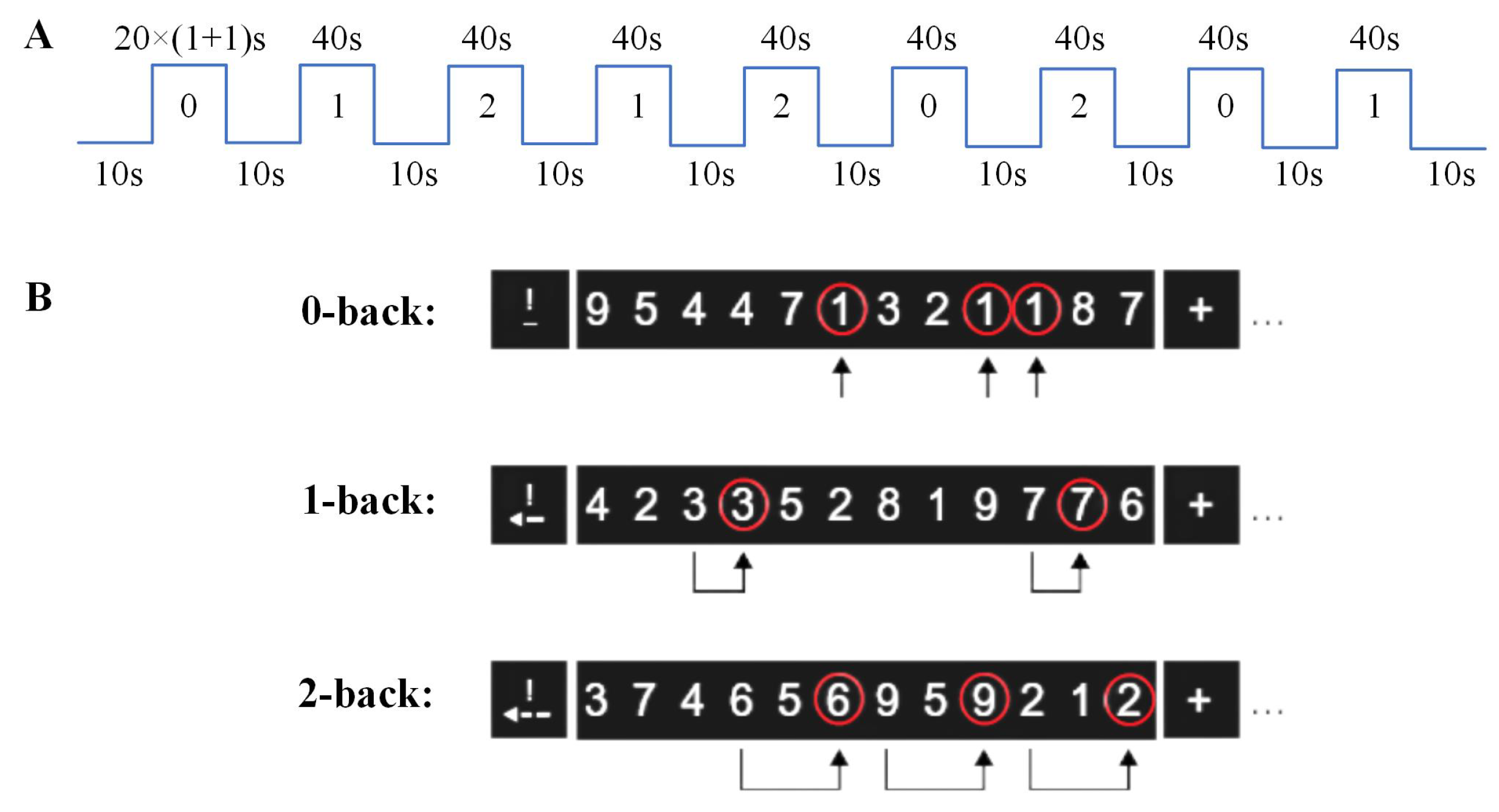
2.2. N-Back Task Design
2.3. Data Preprocessing
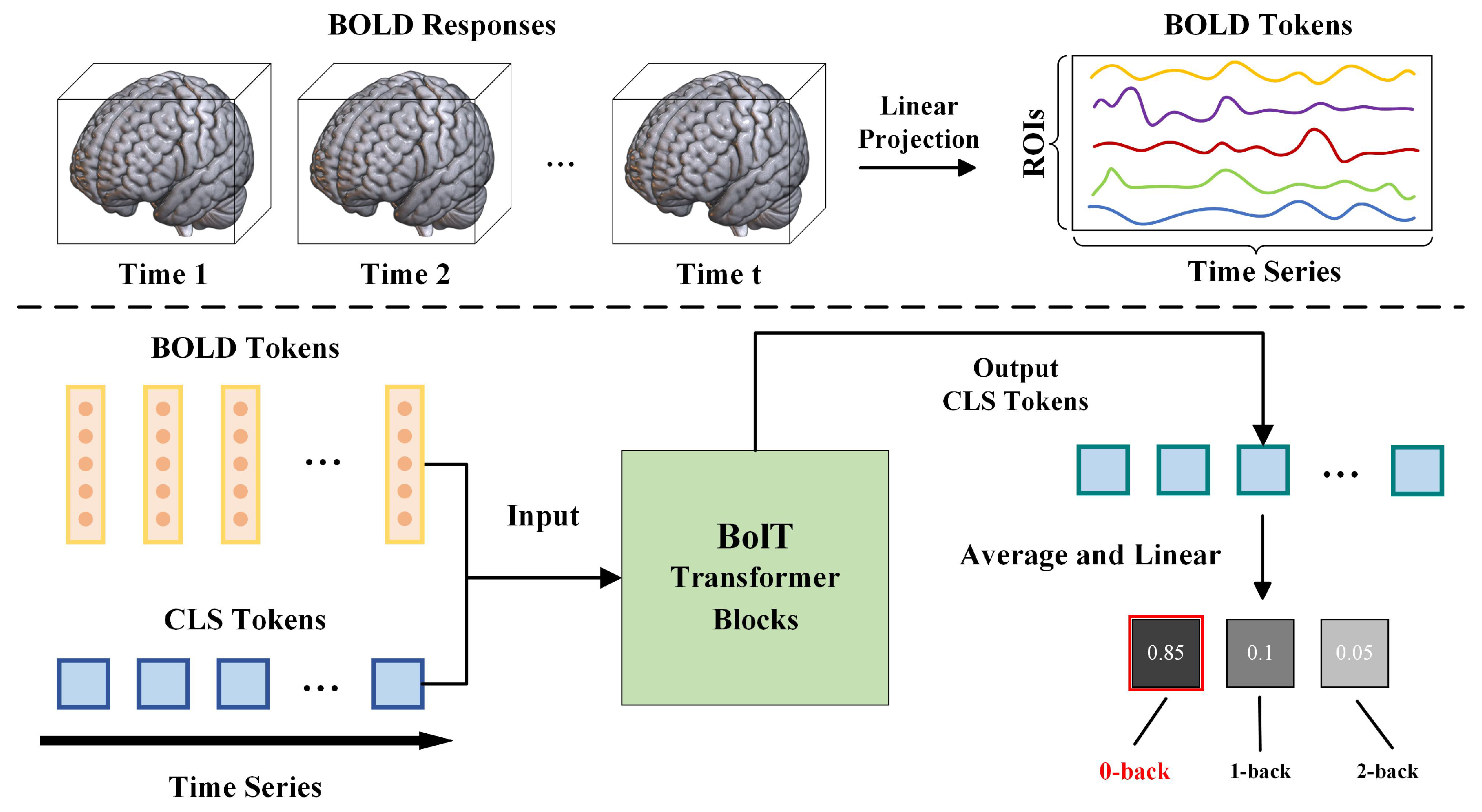
2.4. Analysis Techniques
2.5. Implementation Details
3. Results
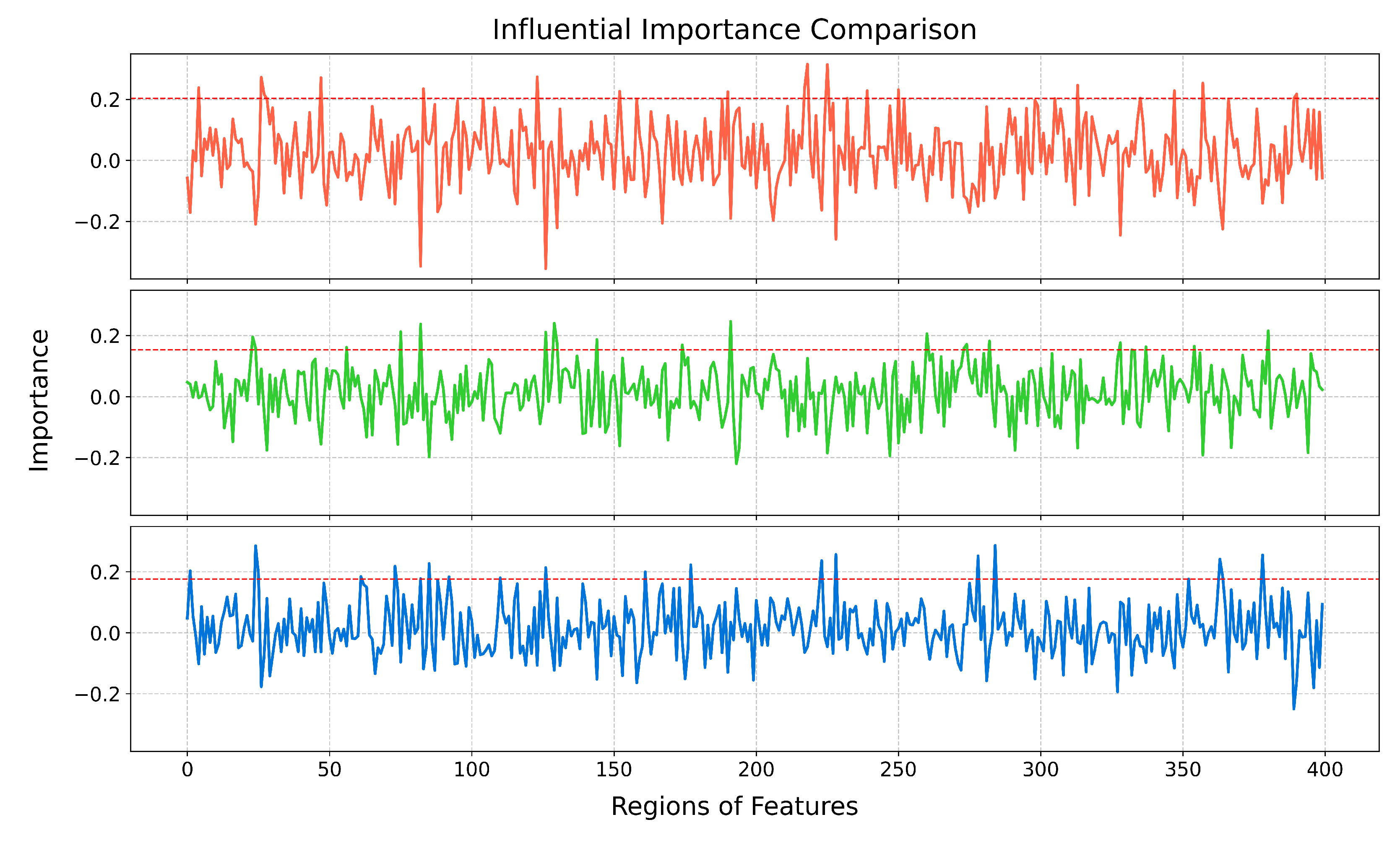
3.1. Importance Weight Characteristics of Brain Regions
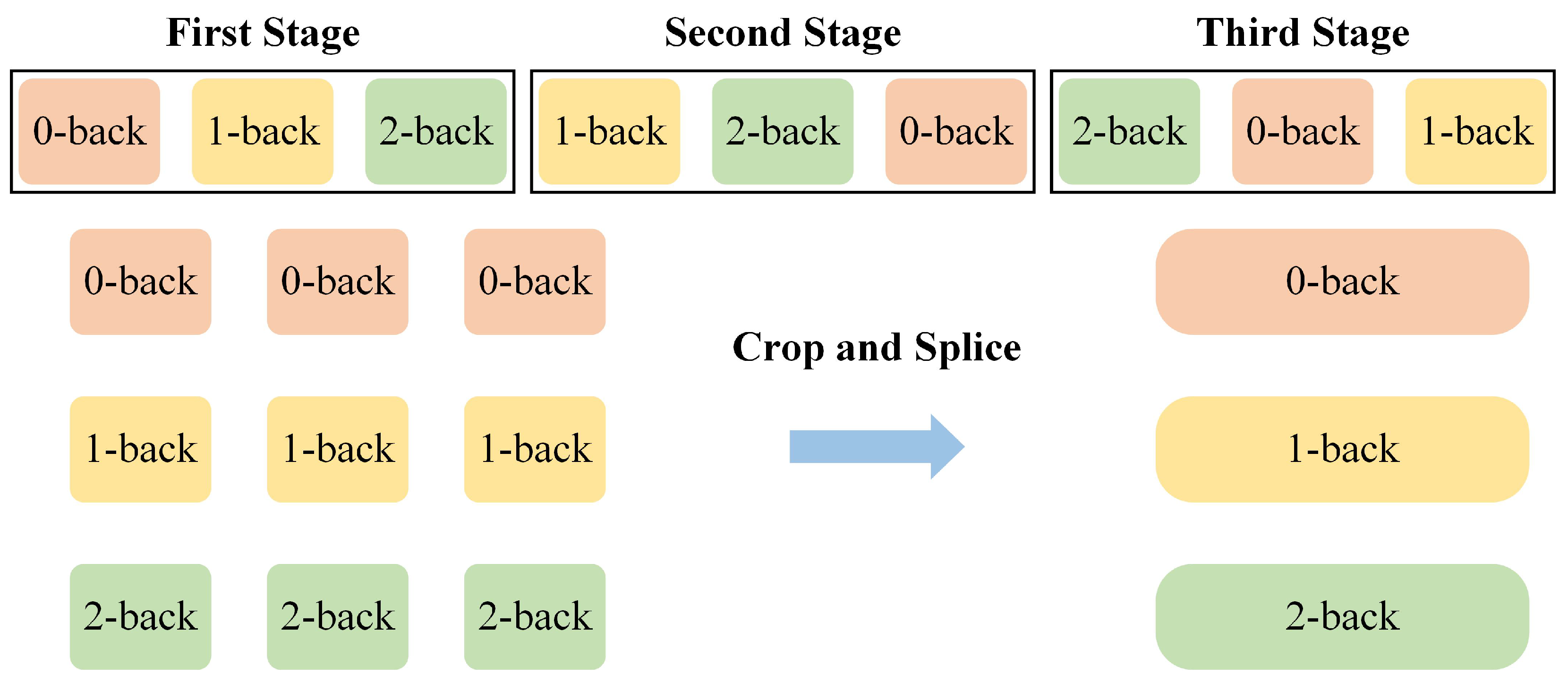
3.2. Comparative Experiments in Different Prior Knowledge Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Rank | Brain Regions | Side | Centroid Coordinates (R A S) | Importance Weight |
|---|---|---|---|---|
| 1 | Visual 19 | R | 9 −74 9 | 0.315 |
| 2 | Visual 26 | R | 27 −87 21 | 0.314 |
| 3 | Limbic Temporal Pole 6 | L | −40 −21 −27 | 0.274 |
| 4 | Visual 27 | L | −12 −71 20 | 0.272 |
| 5 | Somatomotor 17 | L | −51 −7 43 | 0.271 |
| 6 | Frontoparietal Control Cingulate 1 | R | 6 −26 28 | 0.253 |
| 7 | Salience Ventromedial Attention Medial 4 | R | 12 −34 43 | 0.247 |
| 8 | Visual 5 | L | −23 −73 −10 | 0.238 |
| 9 | Visual 18 | R | 35 −89 2 | 0.238 |
| 10 | Dorsal Attention Posterior 16 | L | −20 −57 66 | 0.234 |
| 11 | Somatomotor 21 | R | 52 −13 49 | 0.231 |
| 12 | Somatomotor 10 | R | 41 −29 18 | 0.228 |
| 13 | Frontoparietal Control Lateral Prefrontal Cortex 8 | R | 48 18 23 | 0.228 |
| 14 | Default Temporal 5 | L | −53 6 −11 | 0.226 |
| 15 | Default Precuneus Posterior Cingulate Cortex 2 | L | −13 −61 19 | 0.225 |
| 16 | Default Dorsal Prefrontal cortex an Medial Prefrontal Cortex 13 | R | 12 20 63 | 0.217 |
| 17 | Visual 28 | L | −32 −84 27 | 0.216 |
| 18 | Frontoparietal Control Parietal 5 | R | 54 −33 51 | 0.204 |
| 19 | Somatomotor 3 | R | 53 −14 6 | 0.203 |
| 20 | Default Dorsal Prefrontal cortex and Medial Prefrontal Cortex 12 | R | 12 −55 15 | 0.203 |
| Rank | Brain Regions | Side | Centroid Coordinates (R A S) | Importance Weight |
|---|---|---|---|---|
| 1 | Default Precuneus Posterior Cingulate Cortex 3 | L | −4 −53 20 | 0.246 |
| 2 | Frontoparietal Control Parietal 4 | L | −35 −62 48 | 0.241 |
| 3 | Dorsal Attention Posterior 15 | L | −7 −59 63 | 0.237 |
| 4 | Limbic Temporal Pole 2 | L | 7 42 4 | 0.216 |
| 5 | Dorsal Attention Posterior 8 | L | −46 −29 44 | 0.212 |
| 6 | Frontoparietal Control Parietal 1 | L | −29 −74 42 | 0.211 |
| 7 | Somatomotor 31 | R | 29 −11 65 | 0.206 |
| 8 | Visual 24 | L | −11 −97 17 | 0.195 |
| 9 | Frontoparietal Control Precuneus 2 | L | −5 −64 52 | 0.187 |
| 10 | Dorsal Attention Posterior 13 | R | 35 −36 51 | 0.181 |
| 11 | Limbic Temporal Pole 5 | R | 29 12 −30 | 0.176 |
| 12 | Frontoparietal Control Parietal 5 | L | −42 −52 49 | 0.174 |
| 13 | Dorsal Attention Posterior 5 | R | 32 −66 35 | 0.171 |
| 14 | Default Prefrontal Cortex 10 | L | −53 19 11 | 0.169 |
| 15 | Frontoparietal Control Lateral Prefrontal Cortex 15 | R | 24 10 58 | 0.165 |
| 16 | Frontoparietal Control Temporal 1 | R | 62 −28 −20 | 0.163 |
| 17 | Somatomotor 26 | L | −36 −19 65 | 0.161 |
| 18 | Visual 25 | L | −3 −84 24 | 0.159 |
| 19 | Dorsal Attention Posterior 4 | R | 45 −75 31 | 0.157 |
| 20 | Frontoparietal Control Parietal 2 | R | 56 −41 48 | 0.153 |
| Rank | Brain Regions | Side | Centroid Coordinates (R A S) | Importance Weight |
|---|---|---|---|---|
| 1 | Dorsal Attention Posterior 15 | R | 8 −71 53 | 0.287 |
| 2 | Visual 25 | L | −3 −84 24 | 0.285 |
| 3 | Visual 29 | R | 16 −87 36 | 0.256 |
| 4 | Visual 19 | L | 5 41 −11 | 0.255 |
| 5 | Dorsal Attention Posterior 9 | R | 45 −28 42 | 0.252 |
| 6 | Default Parietal 3 | R | 53 −53 26 | 0.241 |
| 7 | Visual 24 | R | 16 −66 19 | 0.237 |
| 8 | Dorsal Attention Frontal Eye Fields 1 | L | −40 −3 51 | 0.227 |
| 9 | Default Prefrontal Cortex 13 | L | −4 51 28 | 0.223 |
| 10 | Dorsal Attention Posterior 6 | L | −55 −32 45 | 0.218 |
| 11 | Frontoparietal Control Parietal 1 | L | −29 −74 42 | 0.214 |
| 12 | Visual 2 | L | −30 −33 −18 | 0.203 |
| 13 | Default Parietal 4 | L | −47 −64 31 | 0.200 |
| 14 | Visual 26 | L | −12 −71 20 | 0.195 |
| 15 | Somatomotor 31 | L | −19 −24 67 | 0.185 |
| 16 | Salience Ventromedial Attention Parietal Operculum 2 | L | −58 −44 27 | 0.183 |
| 17 | Default Parietal 4 | R | 55 −45 33 | 0.182 |
| 18 | Salience Ventromedial Attention Medial 5 | L | −13 −41 47 | 0.180 |
| 19 | Dorsal Attention Posterior 15 | L | −7 −59 63 | 0.177 |
| 20 | Frontoparietal Control Lateral Prefrontal Cortex 13 | R | 43 7 51 | 0.176 |
References
- Baddeley, A. Working memory: Theories, models, and controversies. Annu. Rev. Psychol. 2012, 63, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Yaple, Z.; Arsalidou, M. N-back working memory task: Meta-analysis of normative fMRI studies with children. Child Dev. 2018, 89, 2010–2022. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, W.K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958, 55, 352. [Google Scholar] [CrossRef]
- Kane, M.J.; Conway, A.R.; Miura, T.K.; Colflesh, G.J. Working memory, attention control, and the N-back task: A question of construct validity. J. Exp. Psychol. Learn. Mem. Cogn. 2007, 33, 615. [Google Scholar] [CrossRef]
- Jaeggi, S.M.; Buschkuehl, M.; Perrig, W.J.; Meier, B. The concurrent validity of the N-back task as a working memory measure. Memory 2010, 18, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, M.; McCarley, R.W.; Nestor, P.G.; Huh, T.; Kikinis, R.; Shenton, M.E.; Wible, C.G. An fMRI study of semantic processing in men with schizophrenia. Neuroimage 2003, 20, 1923–1933. [Google Scholar] [CrossRef]
- Papma, J.M.; Smits, M.; De Groot, M.; Mattace Raso, F.U.; van der Lugt, A.; Vrooman, H.A.; Niessen, W.J.; Koudstaal, P.J.; van Swieten, J.C.; van der Veen, F.M.; et al. The effect of hippocampal function, volume and connectivity on posterior cingulate cortex functioning during episodic memory fMRI in mild cognitive impairment. Eur. Radiol. 2017, 27, 3716–3724. [Google Scholar] [CrossRef]
- Mensch, A.; Mairal, J.; Bzdok, D.; Thirion, B.; Varoquaux, G. Learning neural representations of human cognition across many fMRI studies. Adv. Neural Inf. Process. Syst. 2017, 30, 1–11. [Google Scholar]
- Hillman, E.M. Coupling mechanism and significance of the BOLD signal: A status report. Annu. Rev. Neurosci. 2014, 37, 161–181. [Google Scholar] [CrossRef]
- Rajapakse, J.C.; Kruggel, F.; Maisog, J.M.; Yves von Cramon, D. Modeling hemodynamic response for analysis of functional MRI time-series. Hum. Brain Mapp. 1998, 6, 283–300. [Google Scholar] [CrossRef]
- Li, K.; Guo, L.; Nie, J.; Li, G.; Liu, T. Review of methods for functional brain connectivity detection using fMRI. Comput. Med. Imaging Graph. 2009, 33, 131–139. [Google Scholar] [CrossRef]
- Venkataraman, A.; Van Dijk, K.R.; Buckner, R.L.; Golland, P. Exploring functional connectivity in fMRI via clustering. In Proceedings of the 2009 IEEE International Conference on Acoustics, Speech and Signal Processing, Taipei, Taiwan, 19–24 April 2009; pp. 441–444. [Google Scholar]
- Nishimoto, S.; Vu, A.T.; Naselaris, T.; Benjamini, Y.; Yu, B.; Gallant, J.L. Reconstructing visual experiences from brain activity evoked by natural movies. Curr. Biol. 2011, 21, 1641–1646. [Google Scholar] [CrossRef]
- Simon, O.; Kherif, F.; Flandin, G.; Poline, J.B.; Riviere, D.; Mangin, J.F.; Le Bihan, D.; Dehaene, S. Automatized clustering and functional geometry of human parietofrontal networks for language, space, and number. Neuroimage 2004, 23, 1192–1202. [Google Scholar] [CrossRef]
- Rogers, B.P.; Morgan, V.L.; Newton, A.T.; Gore, J.C. Assessing functional connectivity in the human brain by fMRI. Magn. Reson. Imaging 2007, 25, 1347–1357. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E.T. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Rottschy, C.; Langner, R.; Dogan, I.; Reetz, K.; Laird, A.R.; Schulz, J.B.; Fox, P.T.; Eickhoff, S.B. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage 2012, 60, 830–846. [Google Scholar] [CrossRef]
- Wang, H.; He, W.; Wu, J.; Zhang, J.; Jin, Z.; Li, L. A coordinate-based meta-analysis of the n-back working memory paradigm using activation likelihood estimation. Brain Cogn. 2019, 132, 1–12. [Google Scholar] [CrossRef]
- McKeown, M.J.; Sejnowski, T.J. Independent component analysis of fMRI data: Examining the assumptions. Hum. Brain Mapp. 1998, 6, 368–372. [Google Scholar] [CrossRef]
- Svensén, M.; Kruggel, F.; Benali, H. ICA of fMRI group study data. NeuroImage 2002, 16, 551–563. [Google Scholar] [CrossRef]
- Pereira, F.; Mitchell, T.; Botvinick, M. Machine learning classifiers and fMRI: A tutorial overview. Neuroimage 2009, 45, S199–S209. [Google Scholar] [CrossRef]
- De Martino, F.; Valente, G.; Staeren, N.; Ashburner, J.; Goebel, R.; Formisano, E. Combining multivariate voxel selection and support vector machines for mapping and classification of fMRI spatial patterns. Neuroimage 2008, 43, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, B.; Ma, X.; Xu, L. Resting-state whole-brain functional connectivity networks for MCI classification using L2-regularized logistic regression. IEEE Trans. Nanobioscience 2015, 14, 237–247. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, Z.; Wu, J. Functional connectivity-based classification of autism and control using SVM-RFECV on rs-fMRI data. Phys. Medica 2019, 65, 99–105. [Google Scholar] [CrossRef]
- Heinsfeld, A.S.; Franco, A.R.; Craddock, R.C.; Buchweitz, A.; Meneguzzi, F. Identification of autism spectrum disorder using deep learning and the ABIDE dataset. NeuroImage Clin. 2018, 17, 16–23. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Tang, Z.; Lei, B. Deep spatial-temporal feature fusion from adaptive dynamic functional connectivity for MCI identification. IEEE Trans. Med. Imaging 2020, 39, 2818–2830. [Google Scholar] [CrossRef]
- Mlynarski, P.; Delingette, H.; Criminisi, A.; Ayache, N. Deep learning with mixed supervision for brain tumor segmentation. J. Med. Imaging 2019, 6, 034002. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, J.; Brown, C.J.; Miller, S.P.; Booth, B.G.; Chau, V.; Grunau, R.E.; Zwicker, J.G.; Hamarneh, G. BrainNetCNN: Convolutional neural networks for brain networks; towards predicting neurodevelopment. NeuroImage 2017, 146, 1038–1049. [Google Scholar] [CrossRef]
- Parisot, S.; Ktena, S.I.; Ferrante, E.; Lee, M.; Guerrero, R.; Glocker, B.; Rueckert, D. Disease prediction using graph convolutional networks: Application to autism spectrum disorder and Alzheimer’s disease. Med. Image Anal. 2018, 48, 117–130. [Google Scholar] [CrossRef]
- Fan, L.; Su, J.; Qin, J.; Hu, D.; Shen, H. A deep network model on dynamic functional connectivity with applications to gender classification and intelligence prediction. Front. Neurosci. 2020, 14, 881. [Google Scholar] [CrossRef]
- Wang, L.; Li, K.; Hu, X.P. Graph convolutional network for fMRI analysis based on connectivity neighborhood. Netw. Neurosci. 2021, 5, 83–95. [Google Scholar] [CrossRef]
- Kan, X.; Dai, W.; Cui, H.; Zhang, Z.; Guo, Y.; Yang, C. Brain network transformer. Adv. Neural Inf. Process. Syst. 2022, 35, 25586–25599. [Google Scholar]
- Malkiel, I.; Rosenman, G.; Wolf, L.; Hendler, T. Self-supervised transformers for fmri representation. In Proceedings of the International Conference on Medical Imaging with Deep Learning, PMLR, Zurich, Switzerland, 6–8 July 2022; pp. 895–913. [Google Scholar]
- Deng, X.; Zhang, J.; Liu, R.; Liu, K. Classifying ASD based on time-series fMRI using spatial–temporal transformer. Comput. Biol. Med. 2022, 151, 106320. [Google Scholar] [CrossRef] [PubMed]
- Bedel, H.A.; Sivgin, I.; Dalmaz, O.; Dar, S.U.; Çukur, T. BolT: Fused window transformers for fMRI time series analysis. Med. Image Anal. 2023, 88, 102841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, K.; Yang, C.; Li, X.; Li, H.; Zhang, J.; Wei, D.; Xia, J.; Tao, W.; Lu, P. Beijing aging brain rejuvenation initiative: Aging with grace. Sci. Sin. Vitae 2018, 48, 721–734. [Google Scholar]
- Hutchison, R.M.; Womelsdorf, T.; Allen, E.A.; Bandettini, P.A.; Calhoun, V.D.; Corbetta, M.; Della Penna, S.; Duyn, J.H.; Glover, G.H.; Gonzalez-Castillo, J.; et al. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 2013, 80, 360–378. [Google Scholar] [CrossRef]
- Schaefer, A.; Kong, R.; Gordon, E.M.; Laumann, T.O.; Zuo, X.N.; Holmes, A.J.; Eickhoff, S.B.; Yeo, B.T. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex 2018, 28, 3095–3114. [Google Scholar] [CrossRef]
- Chefer, H.; Gur, S.; Wolf, L. Generic attention-model explainability for interpreting bi-modal and encoder-decoder transformers. In Proceedings of the IEEE/CVF International Conference on Computer Vision, Montreal, QC, Canada, 10–17 October 2021; pp. 397–406. [Google Scholar]
- Fang, J.; Jin, Z.; Wang, Y.; Li, K.; Kong, J.; Nixon, E.E.; Zeng, Y.; Ren, Y.; Tong, H.; Wang, Y.; et al. The salient characteristics of the central effects of acupuncture needling: Limbic-paralimbic-neocortical network modulation. Hum. Brain Mapp. 2009, 30, 1196–1206. [Google Scholar] [CrossRef]
- Al-sharoa, E.; Al-khassaweneh, M.A.; Aviyente, S. Tensor Based Temporal and Multilayer Community Detection for Studying Brain Dynamics During Resting State fMRI. IEEE Trans. Biomed. Eng. 2019, 66, 695–709. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, B.; Roberts, N.; Wang, S.; Luo, Y.; Tian, F.; Yue, S. Shared and distinct brain fMRI response during performance of working memory tasks in adult patients with schizophrenia and major depressive disorder. Hum. Brain Mapp. 2021, 42, 5458–5476. [Google Scholar] [CrossRef]
- Daamen, M.; Bäuml, J.G.; Scheef, L.; Sorg, C.; Busch, B.; Baumann, N.; Bartmann, P.; Wolke, D.; Wohlschläger, A.M.; Boecker, H. Working memory in preterm-born adults: Load-dependent compensatory activity of the posterior default mode network. Hum. Brain Mapp. 2015, 36, 1121–1137. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, R.J.; Shi, H. Altered Default Mode Network and Salience Network Functional Connectivity in Patients with Generalized Anxiety Disorders: An ICA-Based Resting-State fMRI Study. Evid.-Based Complement. Altern. Med. eCAM 2020, 2020, 4048916. [Google Scholar] [CrossRef]
- Chao, T.H.H.; Lee, B.; Hsu, L.M.; Cerri, D.H.; Zhang, W.; Wang, T.W.W.; Ryali, S.; Menon, V.; Shih, Y.Y.I. Neuronal dynamics of the default mode network and anterior insular cortex: Intrinsic properties and modulation by salient stimuli. Sci. Adv. 2023, 9, eade5732. [Google Scholar] [CrossRef]
- Kim, H. Neural activity during working memory encoding, maintenance, and retrieval: A network-based model and meta-analysis. Hum. Brain Mapp. 2019, 40, 4912–4933. [Google Scholar] [CrossRef]
- Ischebeck, A.; Hiebel, H.; Miller, J.; Höfler, M.; Gilchrist, I.D.; Körner, C. Target processing in overt serial visual search involves the dorsal attention network: A fixation-based event-related fMRI study. Neuropsychologia 2021, 153, 107763. [Google Scholar] [CrossRef]
- Pennock, I.M.L.; Schmidt, T.T.; Zorbek, D.; Blankenburg, F. Representation of visual numerosity information during working memory in humans: An fMRI decoding study. Hum. Brain Mapp. 2021, 42, 2778–2789. [Google Scholar] [CrossRef]
- Capotosto, P.; Sulpizio, V.; Galati, G.; Baldassarre, A. Visuo-spatial attention and semantic memory competition in the parietal cortex. Sci. Rep. 2023, 13, 6218. [Google Scholar] [CrossRef]
- Gilmore, A.W. Perceiving Oldness in Parietal Cortex: fMRI Characterization of a Parietal Memory Network. Ph.D. Thesis, Washington University in St. Louis, St. Louis, MO, USA, 2016. [Google Scholar]
- Beffara, B.; Hadj-Bouziane, F.; Hamed, S.B.; Boehler, C.N.; Chelazzi, L.; Santandrea, E.; Macaluso, E. Separate and overlapping mechanisms of statistical regularities and salience processing in the occipital cortex and dorsal attention network. Hum. Brain Mapp. 2023, 44, 6439–6458. [Google Scholar] [CrossRef]
- Machner, B.; Braun, L.; Imholz, J.; Koch, P.J.; Münte, T.; Helmchen, C.; Sprenger, A. Resting-State Functional Connectivity in the Dorsal Attention Network Relates to Behavioral Performance in Spatial Attention Tasks and May Show Task-Related Adaptation. Front. Hum. Neurosci. 2022, 15, 757128. [Google Scholar] [CrossRef]
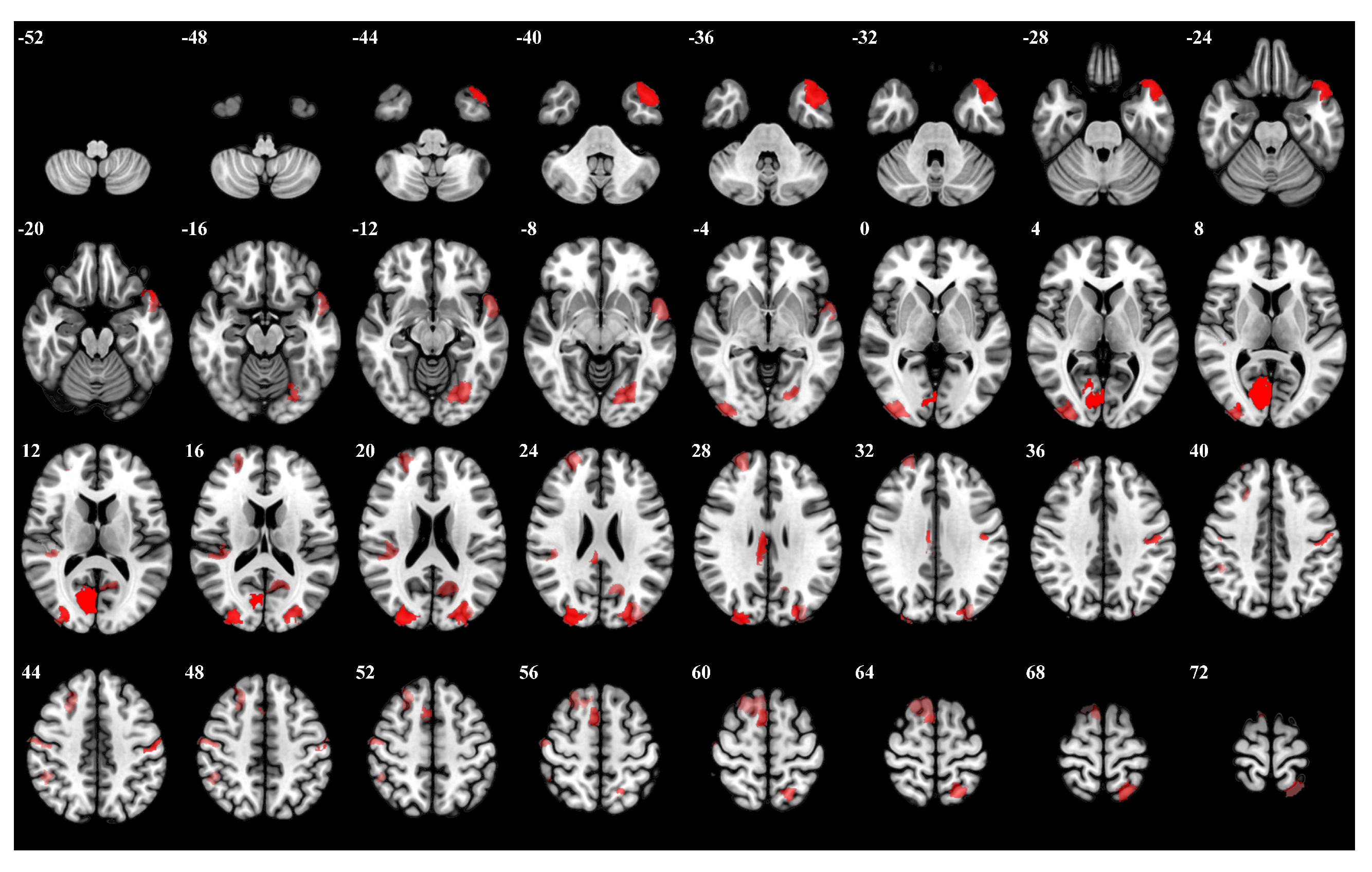
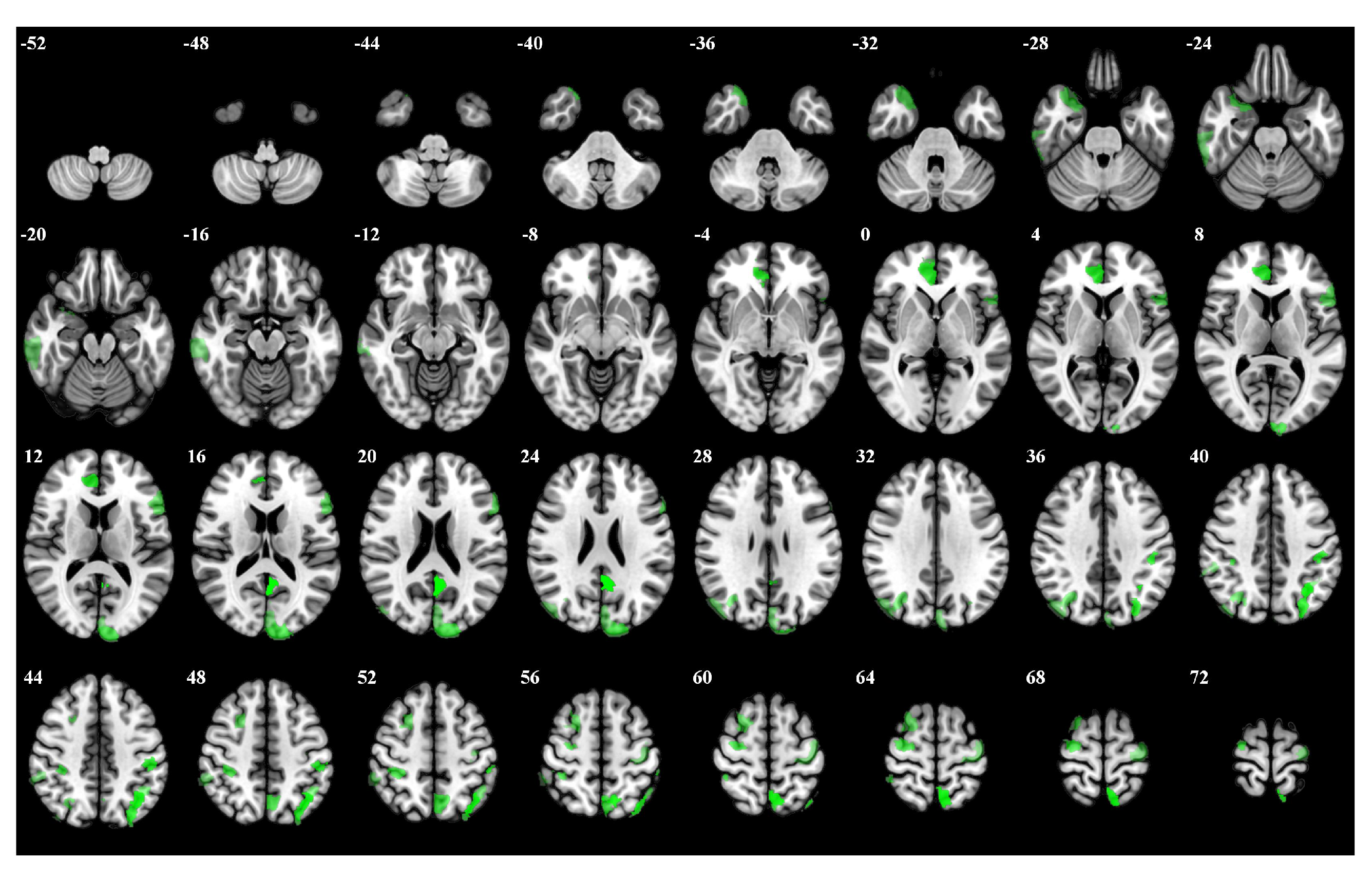
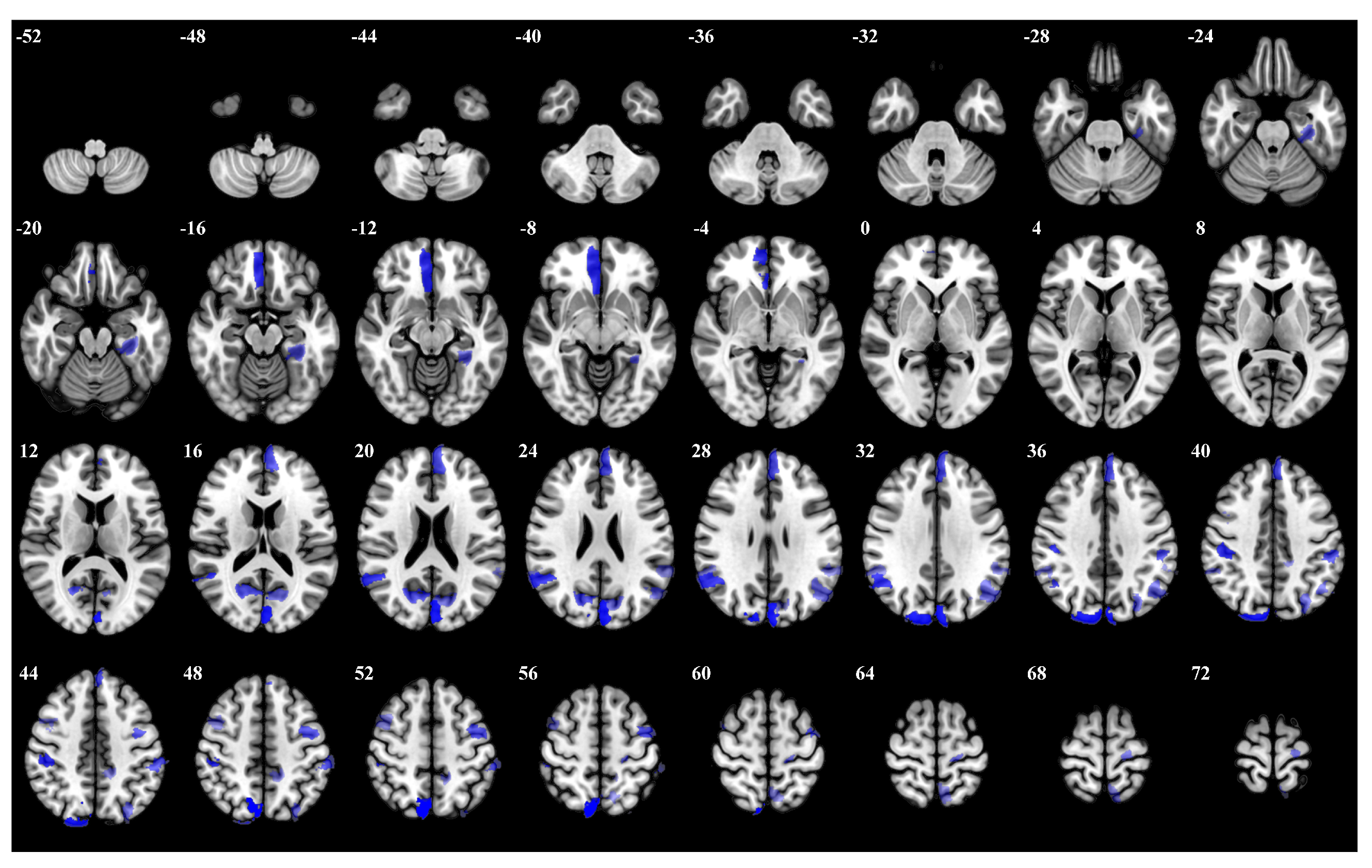
| D | N | W | Accuracy | ROC | |
|---|---|---|---|---|---|
| 20 | 4 | 20 | 0.4 | 57.12% | 74.94% |
| 30 | 4 | 20 | 0.4 | 58.17% | 75.66% |
| 40 | 4 | 20 | 0.4 | 61.96% | 80.30% |
| 50 | 4 | 20 | 0.4 | 63.66% | 83.50% |
| 50 | 4 | 10 | 0.4 | 66.41% | 84.93% |
| 50 | 4 | 5 | 0.4 | 70.72% | 86.93% |
| 50 | 4 | 5 | 0.6 | 71.37% | 86.91% |
| 50 | 6 | 5 | 0.6 | 72.29% | 88.00% |
| 50 | 6 | 5 | 0.8 | 73.07% | 88.46% |
| 50 | 12 | 5 | 0.8 | 73.86% | 89.02% |
| Task Type | Accuracy | ROC |
|---|---|---|
| 0-back | 64.31% | 82.62% |
| 1-back | 56.07% | 75.21% |
| 2-back | 41.05% | 58.80% |
| Task Stage | Accuracy | ROC |
|---|---|---|
| 012 | 67.58% | 84.46% |
| 120 | 63.53% | 82.22% |
| 201 | 63.79% | 82.32% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Chen, Y.; Men, A.; Jiang, Z. Evaluating Cognitive Function and Brain Activity Patterns via Blood Oxygen Level-Dependent Transformer in N-Back Working Memory Tasks. Brain Sci. 2025, 15, 277. https://doi.org/10.3390/brainsci15030277
Zhang Z, Chen Y, Men A, Jiang Z. Evaluating Cognitive Function and Brain Activity Patterns via Blood Oxygen Level-Dependent Transformer in N-Back Working Memory Tasks. Brain Sciences. 2025; 15(3):277. https://doi.org/10.3390/brainsci15030277
Chicago/Turabian StyleZhang, Zhenming, Yaojing Chen, Aidong Men, and Zhuqing Jiang. 2025. "Evaluating Cognitive Function and Brain Activity Patterns via Blood Oxygen Level-Dependent Transformer in N-Back Working Memory Tasks" Brain Sciences 15, no. 3: 277. https://doi.org/10.3390/brainsci15030277
APA StyleZhang, Z., Chen, Y., Men, A., & Jiang, Z. (2025). Evaluating Cognitive Function and Brain Activity Patterns via Blood Oxygen Level-Dependent Transformer in N-Back Working Memory Tasks. Brain Sciences, 15(3), 277. https://doi.org/10.3390/brainsci15030277






