Survival Prediction in Brain Metastasis Patients Treated with Stereotactic Radiosurgery: A Hybrid Machine Learning Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Follow-Up and Endpoints
2.2. Stereotactic Radiotherapy Technique
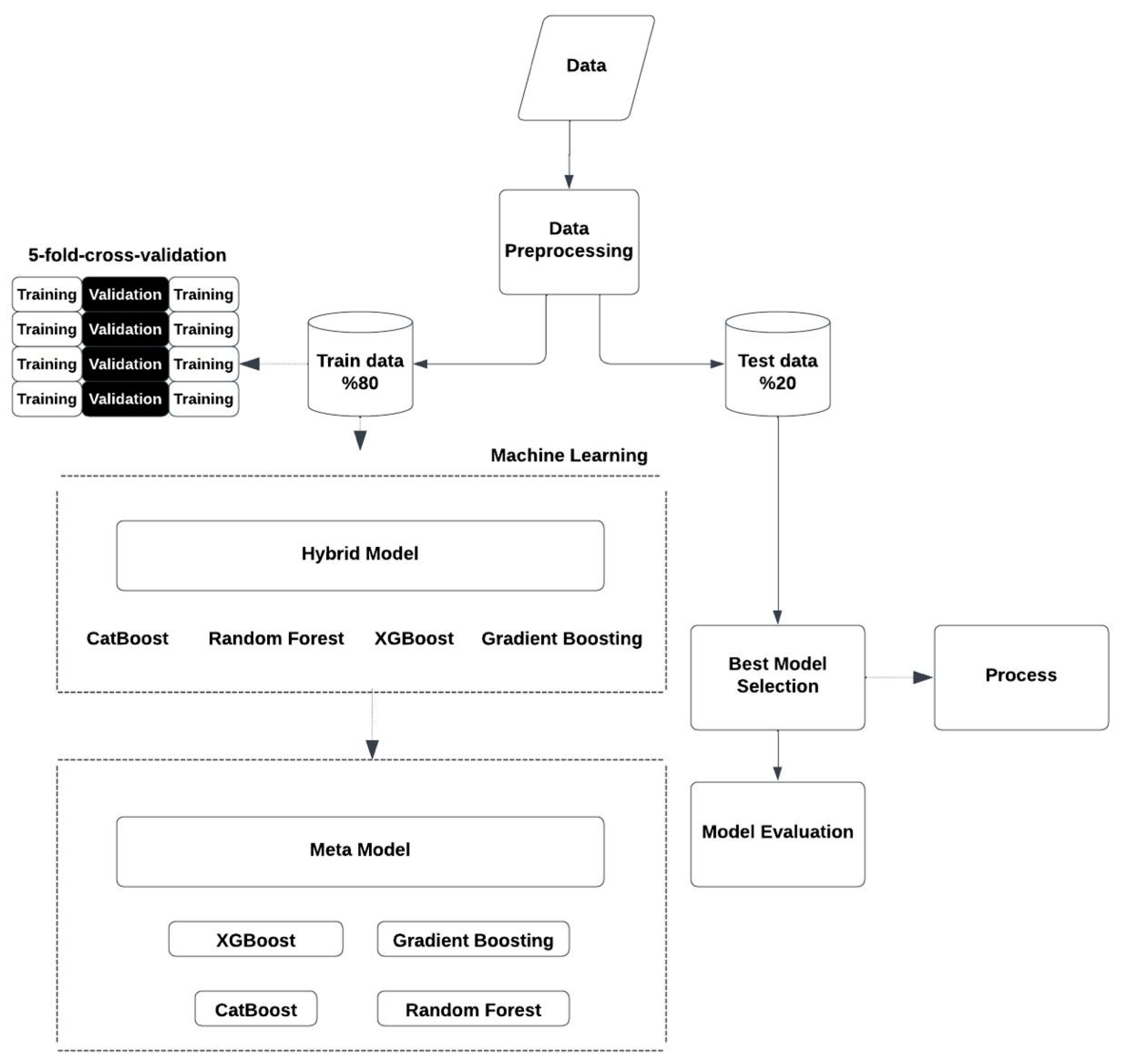
2.3. Hybrid Model Approach
- XGBoost Regressor: A gradient boosting algorithm that is mostly preferred to obtain high-quality estimates [14]. During research, the hyperparameters (n_estimators = 100), learning rate (learning_rate = 0.05), and the maximum tree depth (max_depth = 7) were optimized.
- Gradient Boosting Regressor: Implementation of the same idea was used, but instead to create smaller trees [15]. With regard to adequate predictive outcome, the optimal parameters were specified as n_estimators = 100, learning_rate = 0.1, and max_depth = 4.
- Random Forest Regressor: This is a combination of several decision trees in the model [16]. The optimized hyperparameters for this model included the number of trees to be used (n_estimators = 100), the highest depth of trees (max_depth = 4), and the minimum number of samples needed to split a node (min_samples_split = 5).
- CatBoost Regressor: This is an algorithm particularly good with categorical datasets [17]. After hyperparameter optimization, we obtained the following parameters: depth = 5, learning_rate = 0.05, and iterations = 100 for effective and accurate predictions.
2.4. Stacking Meta-Model
2.5. Evaluation Metrics
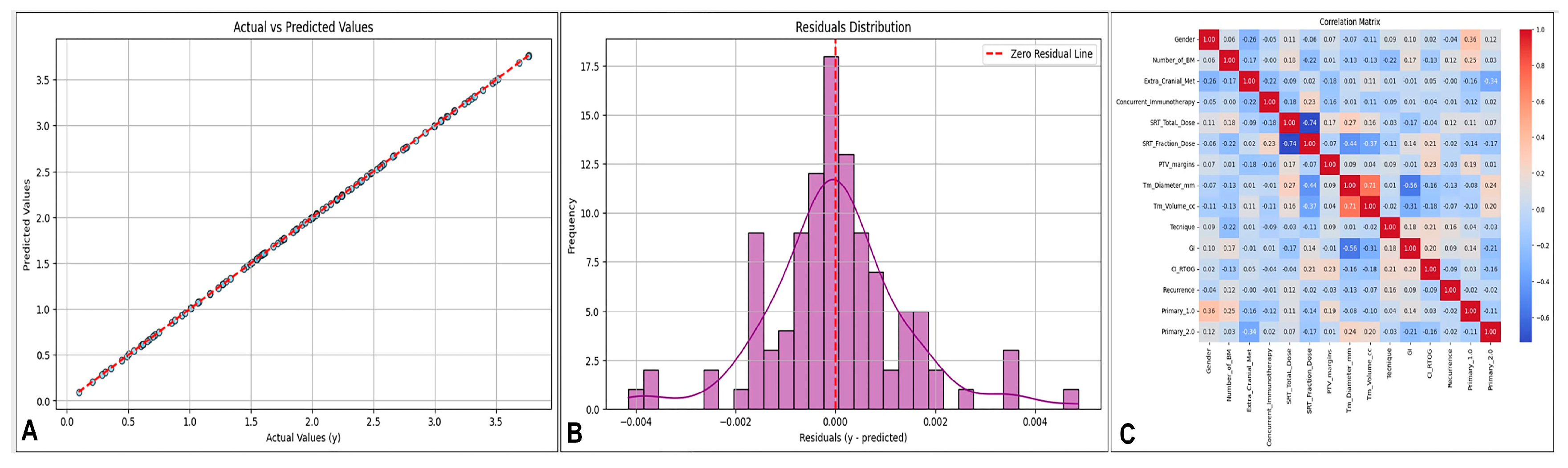
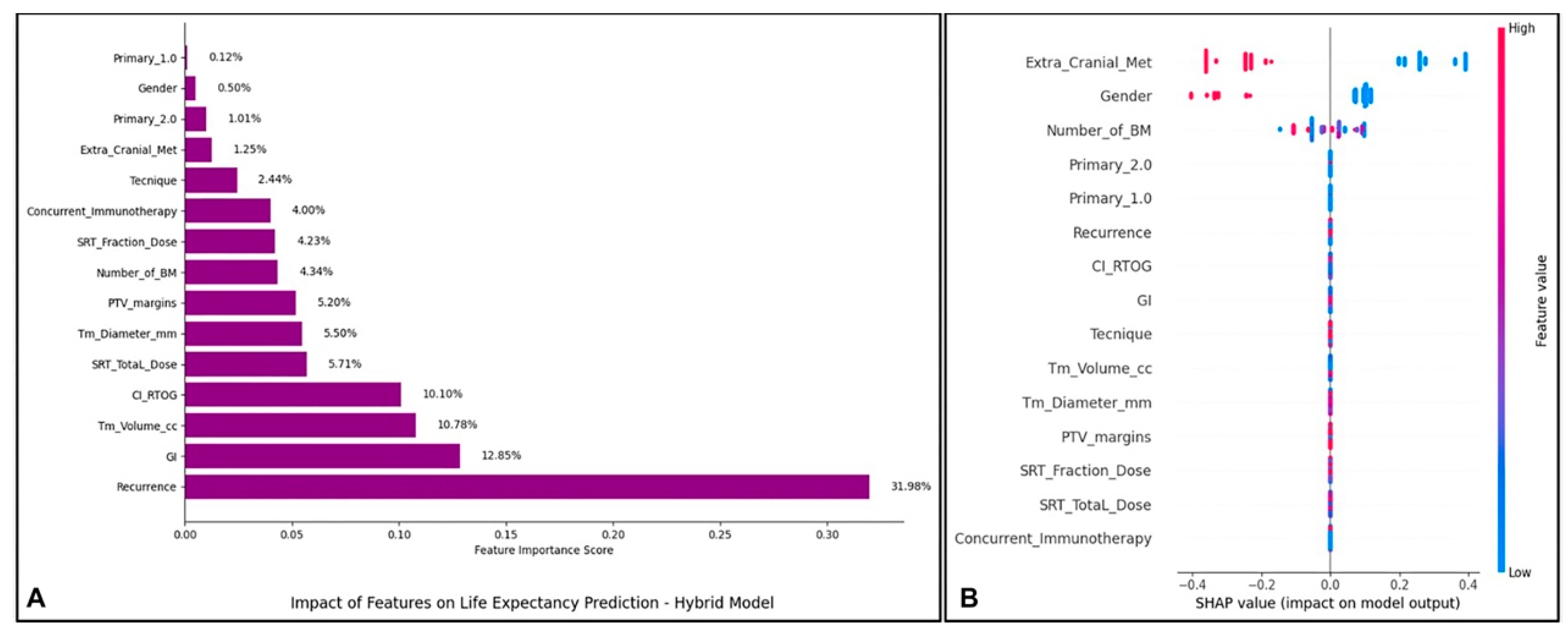
2.6. Visualization and Analysis of Model Performance and Feature Relationships
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SRT | Stereotactic Radiotherapy |
| OS | Overall Survival |
| SBRT | Stereotactic Body Radiotherapy |
| MSE | Mean Squared Error |
| RMSE | Root Mean Squared Error |
| MAPE | Mean Absolute Percentage Error |
| R² | R-squared |
| C-index | Concordance Index |
| SHAP | SHapley Additive exPlanations |
| GI | Gradient Index |
| CI | Conformity Index |
| BM | Brain Metastasis |
References
- Kotecha, R.; Gondi, V.; Ahluwalia, M.S.; Brastianos, P.K.; Mehta, M.P. Recent advances in managing brain metastasis. F1000Res 2018, 7, F1000. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Armocida, D.; Ius, T.; Zancana, G.; Bianconi, A.; Cofano, F.; Tartara, F.; Frati, A.; Garbossa, D.; Salvati, M. Anamnestic radiological metastases outcome surgical score (ARMO-S). A purpose of a predictive surgical scoring system for brain metastases. J. Clin. Neurosci. 2024, 125, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Abdulhaleem, M.; Ruiz, J.; Cramer, C.; Xing, F.; Lo, H.W.; Su, J.; Chan, M.D. Brain metastasis prognostic nomograms and brain metastasis velocity: A narrative review. Chin. Clin. Oncol. 2022, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Fangerau, H. Artifical intelligence in surgery: Ethical considerations in the light of social trends in the perception of health and medicine. EFORT Open Rev. 2024, 9, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Lachman, P. Looking to the Future. In Textbook of Patient Safety and Clinical Risk Management [Internet]; Donaldson, L., Ricciardi, W., Sheridan, S., Tartaglia, R., Eds.; Springer: Cham, Switzerland, 2021; Chapter 4. [Google Scholar] [PubMed]
- Barbour, A.B.; Frush, J.M.; Gatta, L.A.; McManigle, W.C.; Keah, N.M.; Bejarano-Pineda, L.; Guerrero, E.M. Artificial Intelligence in Health Care: Insights From an Educational Forum. J. Med. Educ. Curric. Dev. 2020, 6, 2382120519889348. [Google Scholar] [CrossRef] [PubMed]
- Haug, C.J.; Drazen, J.M. Artificial Intelligence and Machine Learning in Clinical Medicine, 2023. N. Engl. J. Med. 2023, 388, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- MacEachern, S.J.; Forkert, N.D. Machine learning for precision medicine. Genome 2021, 64, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Ting Sim, J.Z.; Fong, Q.W.; Huang, W.; Tan, C.H. Machine learning in medicine: What clinicians should know. Singapore Med. J. 2023, 64, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Gong, L.; Hu, D.; Jiang, Y.; Ding, X. A Hybrid Missing Data Imputation Method for Batch Process Monitoring Dataset. Sensors 2023, 23, 8678. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.R. Analysis of Survival Data, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 1984. [Google Scholar] [CrossRef]
- Jung, Y.; Hu, J. A K-fold Averaging Cross-validation Procedure. J. Nonparametr. Stat. 2015, 27, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. XGBoost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Otchere, D.A.; Ganat, T.O.A.; Ojero, J.O.; Tackie-Otoo, B.N.; Taki, M.Y. Application of gradient boosting regression model for the evaluation of feature selection techniques in improving reservoir characterisation predictions. J. Pet. Sci. Eng. 2022, 208, 109244. [Google Scholar] [CrossRef]
- Segal, M.R. Machine Learning Benchmarks and Random FOREST Regression. 2004. Available online: https://escholarship.org/uc/item/35x3v9t4 (accessed on 1 December 2024).
- Jeganathan, S.; Lakshminarayanan, A.R.; Parthasarathy, S.; Khan, A.A.A.; Sathick, K.J. OptCatB: Optuna Hyperparameter Optimization Model to Forecast the Educational Proficiency of Immigrant Students based on CatBoost Regression. J. Internet Serv. Inf. Secur. 2024, 14, 111–132. [Google Scholar] [CrossRef]
- Hyndman, R.J.; Athanasopoulos, G. Forecasting: Principles and Practice, 2nd ed.; OTexts: Melbourne, Australia, 2018. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Makridakis, S.; Hibon, M. The M3-Competition: Results, conclusions and implications. Int. J. Forecast. 2000, 16, 451–476. [Google Scholar] [CrossRef]
- Seber, G.A.F.; Lee, A.J. Linear Regression Analysis, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Friedman, J.; Hastie, T.; Tibshirani, R. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Harrell, F.E.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the yield of medical tests. J. Am. Med. Assoc. 1982, 247, 2543–2546. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 4765–4774. [Google Scholar]
- Belsley, D.A.; Kuh, E.; Welsch, R.E. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity; Wiley: Hoboken, NJ, USA, 1980; ISBN 978-0471058294. [Google Scholar]
- Cleveland, W.S. The Elements of Graphing Data; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1985; ISBN 978-0534085024. [Google Scholar]
- Lukas, R.V.; Lesniak, M.S.; Salgia, R. Brain metastases in non-small-cell lung cancer: Better outcomes through current therapies and utilization of molecularly targeted approaches. CNS Oncol. 2014, 3, 61–75. [Google Scholar] [CrossRef]
- Zhu, F.Y.; Sun, Y.F.; Yin, X.P.; Zhang, Y.; Xing, L.H.; Ma, Z.P.; Xue, L.Y.; Wang, J.N. Using machine learning-based radiomics to differentiate between glioma and solitary brain metastasis from lung cancer and its subtypes. Discov. Oncol. 2023, 14, 224. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, J.; Wang, L. Machine Learning Based Prediction of Brain Metastasis of Patients with IIIA-N2 Lung Adenocarcinoma by a Three-miRNA Signature. Transl. Oncol. 2018, 11, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Armandi, A.; Sanavia, T.; Younes, R.; Caviglia, G.P.; Rosso, C.; Govaere, O.; Liguori, A.; Francione, P.; Gallego-Duràn, R.; Ampuero, J.; et al. Serum ferritin levels can predict long-term outcomes in patients with metabolic dysfunction-associated steatotic liver disease. Gut 2024, 73, 825–834. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, X.; Xu, X.; Chen, J.; Christiani, D.C.; Chen, F.; Zhang, R.; Wei, Y. Serial platelet count as a dynamic prediction marker of hospital mortality among septic patients. Burns Trauma. 2024, 12, tkae016. [Google Scholar] [CrossRef]
- Xiao, Z.; Song, Q.; Wei, Y.; Fu, Y.; Huang, D.; Huang, C. Use of survival support vector machine combined with random survival forest to predict the survival of nasopharyngeal carcinoma patients. Transl. Cancer Res. 2023, 12, 3581–3590. [Google Scholar] [CrossRef]
- Schmauch, B.; Elsoukkary, S.S.; Moro, A.; Raj, R.; Wehrle, C.J.; Sasaki, K.; Calderaro, J.; Sin-Chan, P.; Aucejo, F.; Roberts, D.E. Combining a deep learning model with clinical data better predicts hepatocellular carcinoma behavior following surgery. J. Pathol. Inform. 2023, 15, 100360. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, Y.; Wu, J.; Li, C.; Zheng, L.; Zhu, B.; Min, Y.; Ling, T.; Liu, X. Association between systemic inflammatory indicators with the survival of chronic kidney disease: A prospective study based on NHANES. Front. Immunol. 2024, 15, 1365591. [Google Scholar] [CrossRef]
- Zhou, L.; Ji, Q.; Peng, H.; Chen, F.; Zheng, Y.; Jiao, Z.; Gong, J.; Li, W. Automatic image segmentation and online survival prediction model of medulloblastoma based on machine learning. Eur. Radiol. 2024, 34, 3644–3655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Y.; Li, M.; Bai, J.; Wang, H.; Pang, X.; Du, R.; Wang, J.; Huang, X. Prognostic Value of Systemic Inflammation, Nutritional Status and Sarcopenia in Patients With Amyotrophic Lateral Sclerosis. J. Cachexia Sarcopenia Muscle 2024, 15, 2743–2755. [Google Scholar] [CrossRef] [PubMed]
- Minardi, M.; Bianconi, A.; Mesin, L.; Salvati, L.F.; Griva, F.; Narducci, A. Proposal of a Machine Learning Based Prognostic Score for Ruptured Microsurgically Treated Anterior Communicating Artery Aneurysms. J. Clin. Med. 2025, 14, 578. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wan, J.; Zeng, Y. Use machine learning to predict pulmonary metastasis of esophageal cancer: A population-based study. J. Cancer Res. Clin. Oncol. 2024, 150, 420. [Google Scholar] [CrossRef]
- Munai, E.; Zeng, S.; Yuan, Z.; Yang, D.; Jiang, Y.; Wang, Q.; Wu, Y.; Zhang, Y.; Tao, D. Machine learning-based prediction model for brain metastasis in patients with extensive-stage small cell lung cancer. Sci. Rep. 2024, 14, 28790. [Google Scholar] [CrossRef]
- Habibi, M.A.; Rashidi, F.; Habibzadeh, A.; Mehrtabar, E.; Arshadi, M.R.; Mirjani, M.S. Prediction of the treatment response and local failure of patients with brain metastasis treated with stereotactic radiosurgery using machine learning: A systematic review and meta-analysis. Neurosurg. Rev. 2024, 47, 199. [Google Scholar] [CrossRef]
- Yichu, S.; Fei, L.; Ying, L.; Youyou, X. Potential of radiomics analysis and machine learning for predicting brain metastasis in newly diagnosed lung cancer patients. Clin. Radiol. 2024, 79, e807–e816. [Google Scholar] [CrossRef]
- Rappaport, J.; Chen, Q.; McGuire, T.; Daugherty-Lopès, A.; Goldszmid, R. Machine learning approach to assess brain metastatic burden in preclinical models. Methods Cell Biol. 2024, 190, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, C.; Chen, X.; Feng, Y.; Feng, J.; Zhang, R.; Ouyang, F.; Li, X.; Tan, Z.; Deng, L.; et al. Noninvasive prediction of perineural invasion in intrahepatic cholangiocarcinoma by clinicoradiological features and computed tomography radiomics based on interpretable machine learning: A multicenter cohort study. Int. J. Surg. 2024, 110, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, M.; Ramamurthy, K.; Shanmugam, S.; Prasanna, G.; S, V.; Won, D. A hybrid model for the classification of Autism Spectrum Disorder using Mu rhythm in EEG. Technol. Health Care 2024, 32, 4485–4503. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, R.; Azeman, N.H.; Mokhtar, M.H.H.; Mobarak, N.N.; Abu Bakar, M.H.; Bakar, A.A.A. Hybrid ensemble-based machine learning model for predicting phosphorus concentrations in hydroponic solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 304, 123327. [Google Scholar] [CrossRef]
- Izonin, I.; Tkachenko, R.; Gurbych, O.; Kovac, M.; Rutkowski, L.; Holoven, R. A non-linear SVR-based cascade model for improving prediction accuracy of biomedical data analysis. Math. Biosci. Eng. 2023, 20, 13398–13414. [Google Scholar] [CrossRef]
| Metric | XGBoost Meta-Model | CatBoost Meta-Model | Gradient Boosting Meta-Model | Random Forest Meta-Model |
|---|---|---|---|---|
| MSE | 0.0000 | 0.0211 | 0.0699 | 0.1439 |
| RMSE | 0.0014 | 0.1451 | 0.2643 | 0.3794 |
| MAE | 0.0010 | 0.1133 | 0.2104 | 0.3023 |
| MAPE | 0.0931 | 10.3640 | 19.2890 | 33.5808 |
| MedAE | 0.0007 | 0.0866 | 0.1951 | 0.2526 |
| R² Score | 0.9998 | 0.9755 | 0.9188 | 0.8328 |
| Explained Variance Score | 0.9998 | 0.9755 | 0.9188 | 0.8328 |
| C-index | 1.0000 | 0.9684 | 0.9405 | 0.9212 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öznacar, T.; Aral, İ.P.; Zengin, H.Y.; Tezcan, Y. Survival Prediction in Brain Metastasis Patients Treated with Stereotactic Radiosurgery: A Hybrid Machine Learning Approach. Brain Sci. 2025, 15, 266. https://doi.org/10.3390/brainsci15030266
Öznacar T, Aral İP, Zengin HY, Tezcan Y. Survival Prediction in Brain Metastasis Patients Treated with Stereotactic Radiosurgery: A Hybrid Machine Learning Approach. Brain Sciences. 2025; 15(3):266. https://doi.org/10.3390/brainsci15030266
Chicago/Turabian StyleÖznacar, Tuğçe, İpek Pınar Aral, Hatice Yağmur Zengin, and Yılmaz Tezcan. 2025. "Survival Prediction in Brain Metastasis Patients Treated with Stereotactic Radiosurgery: A Hybrid Machine Learning Approach" Brain Sciences 15, no. 3: 266. https://doi.org/10.3390/brainsci15030266
APA StyleÖznacar, T., Aral, İ. P., Zengin, H. Y., & Tezcan, Y. (2025). Survival Prediction in Brain Metastasis Patients Treated with Stereotactic Radiosurgery: A Hybrid Machine Learning Approach. Brain Sciences, 15(3), 266. https://doi.org/10.3390/brainsci15030266






