Unveiling the Important Role of Gut Microbiota and Diet in Multiple Sclerosis
Abstract
1. Introduction
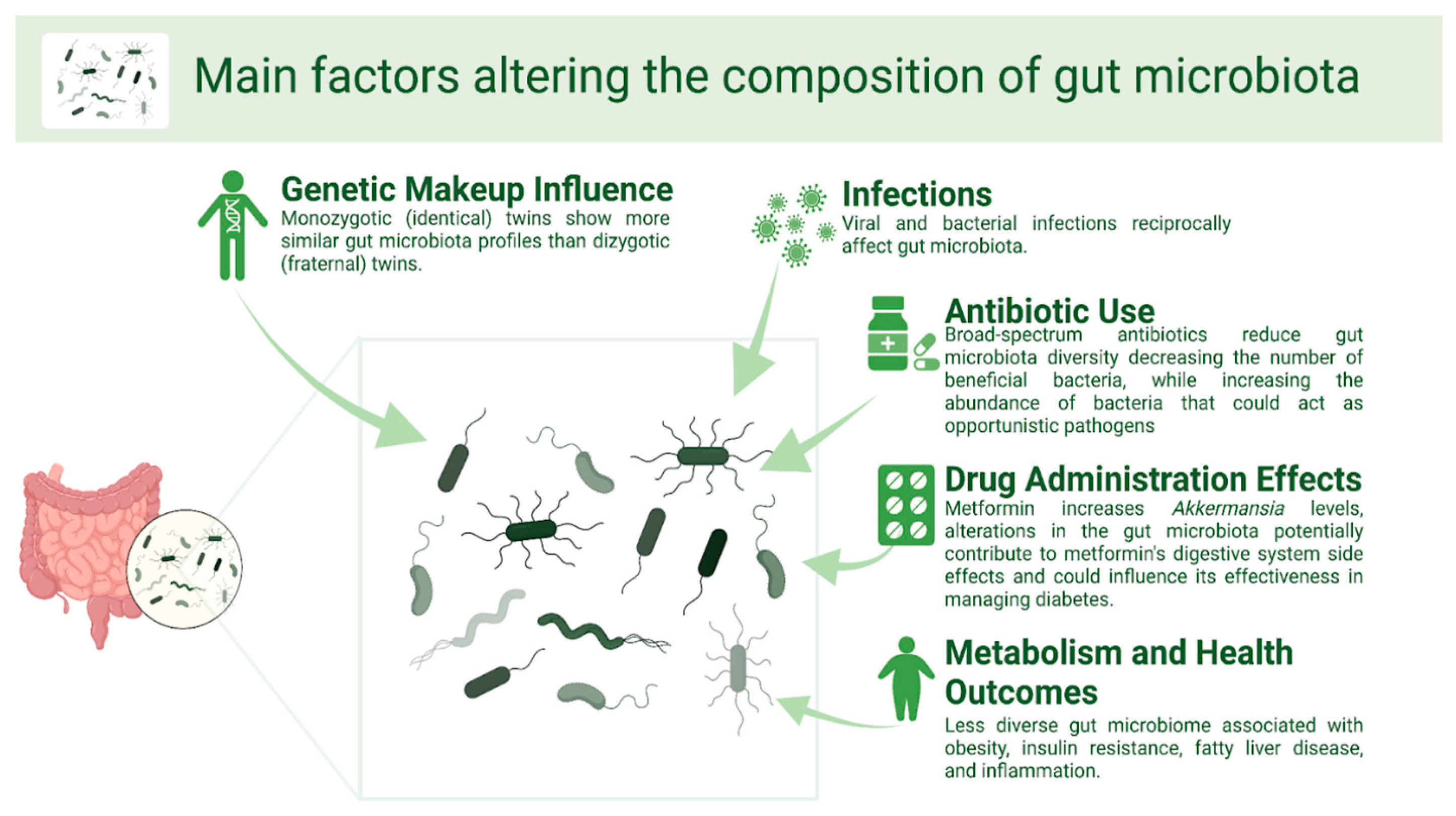
Main Factors Altering the Composition of Gut Microbiota
2. The Complex Relationship Between Gut Microbiota and Multiple Sclerosis
2.1. Gut Microbiota Composition in MS Patients
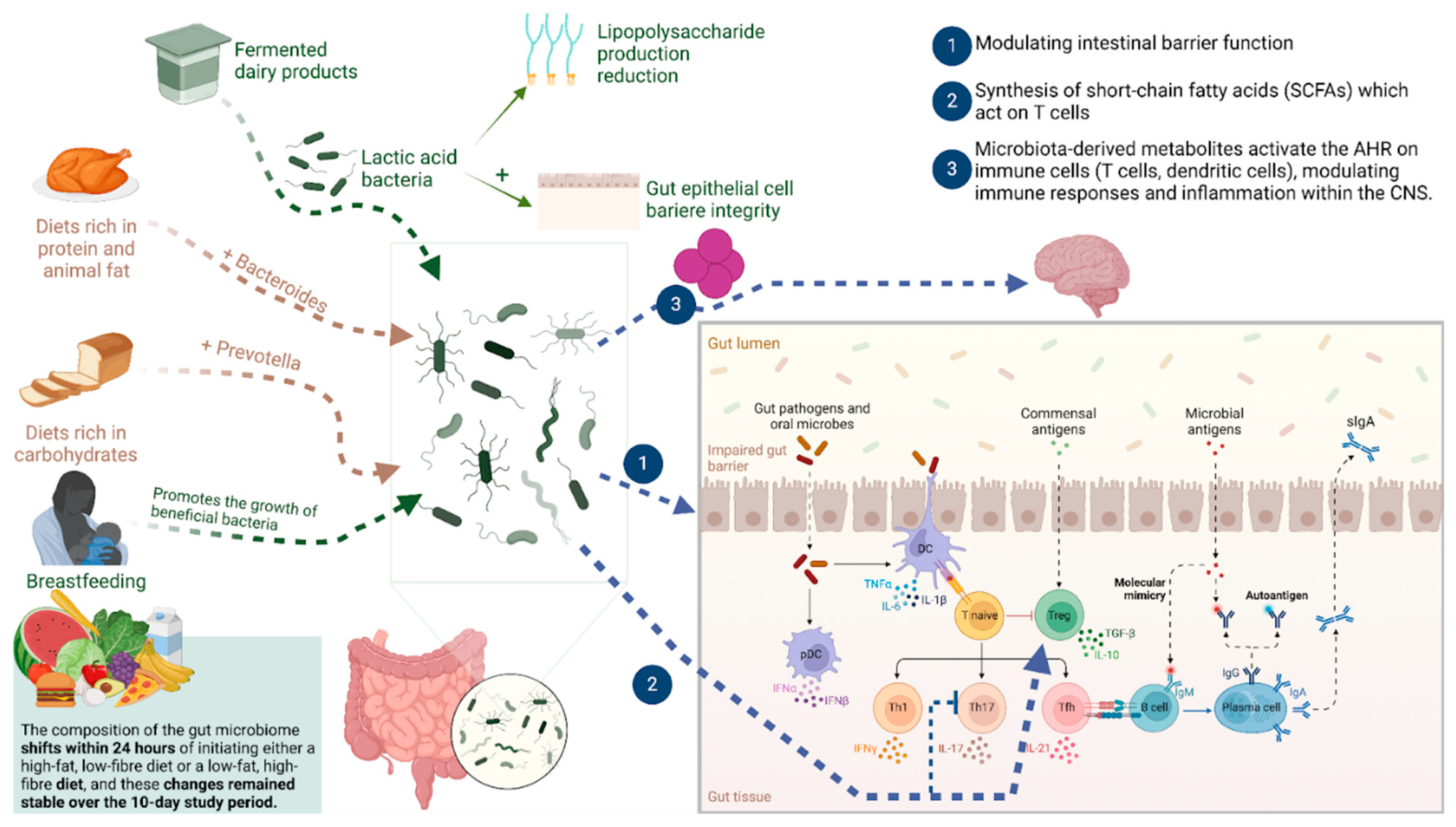
2.2. Underlying Mechanisms of Gut Microbiota Interactions with the Immune System in MS
3. Therapeutic Potential of Gut Microbiota Modulation in MS
3.1. The Mediterranean Diet
3.2. Ketogenic Diet
3.3. Calorie Restriction
3.4. Low-Salt Diet
4. Role of Probiotics, Prebiotics, and Postbiotics in MS
4.1. Probiotics
4.2. Prebiotics
4.3. Postbiotics
5. Gut Dysbiosis in Pediatric-Onset MS
The Role of Nutrition for Patients with Pediatric-Onset MS
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Bien, J.; Palagani, V.; Bozko, P. The intestinal microbiota dysbiosis and Clostridium difficile infection: Is there a relationship with inflammatory bowel disease? Ther. Adv. Gastroenterol. 2013, 6, 53–68. [Google Scholar] [CrossRef]
- Falony, G.; Vlachou, A.; Verbrugghe, K.; De Vuyst, L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl. Environ. Microbiol. 2006, 72, 7835–7841. [Google Scholar] [CrossRef]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Oktaviono, Y.H.; Dyah Lamara, A.; Saputra, P.B.T.; Arnindita, J.N.; Pasahari, D.; Saputra, M.E.; Suasti, N.M.A. The roles of trimethylamine-N-oxide in atherosclerosis and its potential therapeutic aspect: A literature review. Biomol. Biomed. 2023, 23, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, B.; Zhao, L.; Li, H. The gut microbiota: Emerging evidence in autoimmune diseases. Trends Mol. Med. 2020, 26, 862–873. [Google Scholar] [CrossRef]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef]
- Džidić-Krivić, A.; Kusturica, J.; Sher, E.K.; Selak, N.; Osmančević, N.; Karahmet Farhat, E.; Sher, F. Effects of intestinal flora on pharmacokinetics and pharmacodynamics of drugs. Drug Metab. Rev. 2023, 55, 126–139. [Google Scholar] [CrossRef]
- Marchesi, J.; Shanahan, F. The normal intestinal microbiota. Curr. Opin. Infect. Dis. 2007, 20, 508–513. [Google Scholar] [CrossRef]
- Farhat, E.K.; Sher, E.K.; Džidić-Krivić, A.; Banjari, I.; Sher, F. Functional biotransformation of phytoestrogens by gut microbiota with impact on cancer treatment. J. Nutr. Biochem. 2023, 118, 109368. [Google Scholar] [CrossRef]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468s–1475s. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Blekhman, R.; Goodrich, J.K.; Huang, K.; Sun, Q.; Bukowski, R.; Bell, J.T.; Spector, T.D.; Keinan, A.; Ley, R.E.; Gevers, D.; et al. Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 2015, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Chagoyán, O.C.; Maldonado, J.; Gil, A. Aetiology of inflammatory bowel disease (IBD): Role of intestinal microbiota and gut-associated lymphoid tissue immune response. Clin. Nutr. 2005, 24, 339–352. [Google Scholar] [CrossRef]
- Chou, H.H.; Chien, W.H.; Wu, L.L.; Cheng, C.H.; Chung, C.H.; Horng, J.H.; Ni, Y.H.; Tseng, H.T.; Wu, D.; Lu, X.; et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc. Natl. Acad. Sci. USA 2015, 112, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Zilberman-Schapira, G.; Zmora, N.; Itav, S.; Bashiardes, S.; Elinav, H.; Elinav, E. The gut microbiome in human immunodeficiency virus infection. BMC Med. 2016, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Hill, D.A.; Minkah, N.; Kirn, T.; Troy, A.; Artis, D.; Bushman, F. Community-wide response of the gut microbiota to enteropathogenic Citrobacter rodentium infection revealed by deep sequencing. Infect. Immun. 2009, 77, 4668–4678. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, D.; Jiang, C.; Li, Z.; Wang, X.; Peng, Y. Insight into alteration of gut microbiota in Clostridium difficile infection and asymptomatic C. difficile colonization. Anaerobe 2015, 34, 1–7. [Google Scholar] [CrossRef]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014, 312, 1772–1778. [Google Scholar] [CrossRef]
- Bashan, A.; Gibson, T.E.; Friedman, J.; Carey, V.J.; Weiss, S.T.; Hohmann, E.L.; Liu, Y.Y. Universality of human microbial dynamics. Nature 2016, 534, 259–262. [Google Scholar] [CrossRef]
- Seekatz, A.M.; Young, V.B. Clostridium difficile and the microbiota. J. Clin. Investig. 2014, 124, 4182–4189. [Google Scholar] [CrossRef]
- Blanchi, J.; Goret, J.; Mégraud, F. Clostridium difficile Infection: A Model for Disruption of the Gut Microbiota Equilibrium. Dig. Dis. 2016, 34, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Kim, H.G.; Kim, J.S.; Oh, D.G.; Um, Y.J.; Seo, C.S.; Han, J.W.; Cho, H.J.; Kim, G.H.; Jeong, T.C.; et al. The effect of gut microbiota on drug metabolism. Expert. Opin. Drug Metab. Toxicol. 2013, 9, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.H.; Kim, I.S.; Van Le, T.K.; Jung, I.H.; Yoo, H.H.; Kim, D.H. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab. Dispos. 2014, 42, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the gut microbiota. J. Clin. Investig. 2014, 124, 4212–4218. [Google Scholar] [CrossRef]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef]
- Kozyrskyj, A.L.; Ernst, P.; Becker, A.B. Increased risk of childhood asthma from antibiotic use in early life. Chest 2007, 131, 1753–1759. [Google Scholar] [CrossRef]
- Shaw, S.Y.; Blanchard, J.F.; Bernstein, C.N. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am. J. Gastroenterol. 2010, 105, 2687–2692. [Google Scholar] [CrossRef]
- Alatawi, H.; Mosli, M.; Saadah, O.I.; Annese, V.; Al-Hindi, R.; Alatawy, M.; Al-Amrah, H.; Alshehri, D.; Bahieldin, A.; Edris, S. Attributes of intestinal microbiota composition and their correlation with clinical primary non-response to anti-TNF-α agents in inflammatory bowel disease patients. Bosn. J. Basic. Med. Sci. 2022, 22, 412–426. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, Q.; Chen, R. Promotive role of IRF7 in ferroptosis of colonic epithelial cells in ulcerative colitis by the miR-375-3p/SLC11A2 axis. Biomol. Biomed. 2023, 23, 437–449. [Google Scholar] [CrossRef]
- Trasande, L.; Blustein, J.; Liu, M.; Corwin, E.; Cox, L.M.; Blaser, M.J. Infant antibiotic exposures and early-life body mass. Int. J. Obes. 2013, 37, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Mahana, D.; Trent, C.M.; Kurtz, Z.D.; Bokulich, N.A.; Battaglia, T.; Chung, J.; Müller, C.L.; Li, H.; Bonneau, R.A.; Blaser, M.J. Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome Med. 2016, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Ussar, S.; Clish, C.; Devkota, S.; Dreyfuss, J.M.; Sakaguchi, M.; Soto, M.; Konishi, M.; Softic, S.; Altindis, E.; et al. Antibiotic effects on gut microbiota and metabolism are host dependent. J. Clin. Investig. 2016, 126, 4430–4443. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, D.; Goossens, G.H.; Hermes, G.D.; Neis, E.P.; van der Beek, C.M.; Most, J.; Holst, J.J.; Lenaerts, K.; Kootte, R.S.; Nieuwdorp, M.; et al. Effects of Gut Microbiota Manipulation by Antibiotics on Host Metabolism in Obese Humans: A Randomized Double-Blind Placebo-Controlled Trial. Cell Metab. 2016, 24, 63–74. [Google Scholar] [CrossRef]
- Membrez, M.; Blancher, F.; Jaquet, M.; Bibiloni, R.; Cani, P.D.; Burcelin, R.G.; Corthesy, I.; Macé, K.; Chou, C.J. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. Faseb J. 2008, 22, 2416–2426. [Google Scholar] [CrossRef]
- Chou, C.J.; Membrez, M.; Blancher, F. Gut decontamination with norfloxacin and ampicillin enhances insulin sensitivity in mice. In Nestle Nutrition Workshop Series Pediatric Program; Nestec: Basel, Switzerland, 2008; Volume 62, pp. 127–137; discussion 137–140. [Google Scholar] [CrossRef]
- Han, J.; Lin, H.; Huang, W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med. Sci. Monit. 2011, 17, Ra164–Ra167. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem. Cell Biol. 2015, 93, 479–486. [Google Scholar] [CrossRef]
- Hou, K.; Zhang, S.; Wu, Z.; Zhu, D.; Chen, F.; Lei, Z.N.; Liu, W.; Xiao, C.; Chen, Z.S. Reconstruction of intestinal microecology of type 2 diabetes by fecal microbiota transplantation: Why and how. Bosn. J. Basic. Med. Sci. 2022, 22, 315–325. [Google Scholar] [CrossRef]
- Lee, H.; Ko, G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 2014, 80, 5935–5943. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Jette, S.; Tremlett, H. Disease-modifying therapies used to treat multiple sclerosis and the gut microbiome: A systematic review. J. Neurol. 2024, 271, 1108–1123. [Google Scholar] [CrossRef]
- Field, A.E.; Willett, W.C.; Lissner, L.; Colditz, G.A. Dietary fat and weight gain among women in the Nurses’ Health Study. Obesity 2007, 15, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Winzell, M.S.; Ahrén, B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53 (Suppl. S3), S215–S219. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Obesity, diabetes, and gut microbiota: The hygiene hypothesis expanded? Diabetes Care 2010, 33, 2277–2284. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Mackowiak, P.A. Recycling metchnikoff: Probiotics, the intestinal microbiome and the quest for long life. Front. Public Health 2013, 1, 52. [Google Scholar] [CrossRef]
- Merenstein, D.J.; Tan, T.P.; Molokin, A.; Smith, K.H.; Roberts, R.F.; Shara, N.M.; Mete, M.; Sanders, M.E.; Solano-Aguilar, G. Safety of Bifidobacterium animalis subsp. lactis (B. lactis) strain BB-12-supplemented yogurt in healthy adults on antibiotics: A phase I safety study. Gut Microbes 2015, 6, 66–77. [Google Scholar] [CrossRef]
- Uyeno, Y.; Sekiguchi, Y.; Kamagata, Y. Impact of consumption of probiotic lactobacilli-containing yogurt on microbial composition in human feces. Int. J. Food Microbiol. 2008, 122, 16–22. [Google Scholar] [CrossRef]
- Bronzini, M.; Maglione, A.; Rosso, R.; Matta, M.; Masuzzo, F.; Rolla, S.; Clerico, M. Feeding the gut microbiome: Impact on multiple sclerosis. Front. Immunol. 2023, 14, 1176016. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Y.; Zhang, Y.; Liu, P.; Liu, M.; Zhang, M.; Wu, R. S1P/S1PR signaling pathway advancements in autoimmune diseases. Biomol. Biomed. 2023, 23, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Rodriguez, A.; Roman, P.; Rueda-Ruzafa, L.; Campos-Rios, A.; Cardona, D. Changes in Gut Microbiota and Multiple Sclerosis: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 4624. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Mendozzi, L.; Rossi, V.; Mazzali, F.; Piancone, F.; LaRosa, F.; Marventano, I.; Caputo, D.; Felis, G.E.; Clerici, M. Immunological and Clinical Effect of Diet Modulation of the Gut Microbiome in Multiple Sclerosis Patients: A Pilot Study. Front. Immunol. 2017, 8, 1391. [Google Scholar] [CrossRef]
- Asghari, K.M.; Dolatkhah, N.; Ayromlou, H.; Mirnasiri, F.; Dadfar, T.; Hashemian, M. The Effect of Probiotic Supplementation on the Clinical and Para-Clinical Findings of Multiple Sclerosis: A Randomized Clinical Trial. Sci. Rep. 2023, 13, 18577. [Google Scholar] [CrossRef]
- Kouchaki, E.; Tamtaji, O.R.; Salami, M.; Bahmani, F.; Daneshvar Kakhaki, R.; Akbari, E.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Clinical and Metabolic Response to Probiotic Supplementation in Patients with Multiple Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2017, 36, 1245–1249. [Google Scholar] [CrossRef]
- Straus Farber, R.; Walker, E.L.; Diallo, F.; Onomichi, K.; Riley, C.; Zhang, L.; Zhu, W.; De Jager, P.L.; Xia, Z. A Randomized Cross-Over Trial of Prebiotics and Probiotics in Multiple Sclerosis: Trial Feasibility, Supplement Tolerability, and Symptom Abatement. Mult. Scler. Relat. Disord. 2024, 89, 105762. [Google Scholar] [CrossRef]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.W.; et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, M.; Prathiraja, O.; Kayani, A.M.A.; Jena, R.; Caldera, D.; Silva, M.S.; Singhal, M.; Pierre, J., Jr. The Role of Diet and Gut Microbiome in Multiple Sclerosis. Cureus 2022, 14, e28975. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Papiri, G.; D’Andreamatteo, G.; Cacchiò, G.; Alia, S.; Silvestrini, M.; Paci, C.; Luzzi, S.; Vignini, A. Multiple Sclerosis: Inflammatory and Neuroglial Aspects. Curr. Issues Mol. Biol. 2023, 45, 1443–1470. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, W.; Yang, C.; Wang, Z.; Zhang, J.; Xu, D.; Sun, X.; Sun, W. Emerging role of gut microbiota in autoimmune diseases. Front. Immunol. 2024, 15, 1365554. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Thirion, F.; Sellebjerg, F.; Fan, Y.; Lyu, L.; Hansen, T.H.; Pons, N.; Levenez, F.; Quinquis, B.; Stankevic, E.; Søndergaard, H.B.; et al. The Gut Microbiota in Multiple Sclerosis Varies with Disease Activity. Genome Med. 2023, 15, 1. [Google Scholar] [CrossRef]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple Sclerosis Patients Have a Distinct Gut Microbiota Compared to Healthy Controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef]
- Schoeps, V.A.; Zhou, X.; Horton, M.K.; Zhu, F.; McCauley, K.E.; Nasr, Z.; Virupakshaiah, A.; Gorman, M.P.; Benson, L.A.; Weinstock-Guttman, B.; et al. Short-Chain Fatty Acid Producers in the Gut Are Associated with Pediatric Multiple Sclerosis Onset. Ann. Clin. Transl. Neurol. 2024, 11, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Zhu, F.; Arnold, D.; Bar-Or, A.; Bernstein, C.N.; Bonner, C.; Forbes, J.D.; Graham, M.; Hart, J.; Knox, N.C.; et al. The gut microbiota in pediatric multiple sclerosis and demyelinating syndromes. Ann. Clin. Transl. Neurol. 2021, 8, 2252–2269. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.I.; Zhu, F.; Knox, N.; Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N.; Graham, M.; Marrie, R.A.; Hart, J.; Yeh, E.A.; et al. Metagenomic Analysis of the Pediatric-Onset Multiple Sclerosis Gut Microbiome. Neurology 2022, 98, e1050–e1063. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhao, Y.; Arnold, D.L.; Bar-Or, A.; Bernstein, C.N.; Bonner, C.; Graham, M.; Hart, J.; Knox, N.; Marrie, R.A.; et al. A Cross-Sectional Study of MRI Features and the Gut Microbiome in Pediatric-Onset Multiple Sclerosis. Ann. Clin. Transl. Neurol. 2024, 11, 486–496. [Google Scholar] [CrossRef]
- Matsuyama, M.; Gomez-Arango, L.F.; Fukuma, N.M.; Morrison, M.; Davies, P.S.W.; Hill, R.J. Breastfeeding: A key modulator of gut microbiota characteristics in late infancy. J. Dev. Orig. Health Dis. 2019, 10, 206–213. [Google Scholar] [CrossRef]
- van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef]
- Kirby, T.O.; Ochoa-Repáraz, J. The Gut Microbiome in Multiple Sclerosis: A Potential Therapeutic Avenue. Med. Sci. 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I Interferons and Microbial Metabolites of Tryptophan Modulate Astrocyte Activity and Central Nervous System Inflammation via the Aryl Hydrocarbon Receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef]
- Rothhammer, V.; Kenison, J.; Li, Z.; Tjon, E.; Takenaka, M.; Chao, C.; Alves de Lima, K.; Borucki, D.; Kaye, J.; Quintana, F. Aryl Hydrocarbon Re-Ceptor Activation in Astrocytes by Laquinimod Ameliorates Autoimmune Inflammation in the CNS. Neurol. Neuroimmunol. Neuroinflamm 2021, 8, e946. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Maged, M.; Hairul-Islam, M.I.; Osama, I.A.; Maha, H.; Manal, A.; Hamza, H. Activation of aryl hydrocarbon receptor signaling by a novel agonist ameliorates autoimmune encephalomyelitis. PLoS ONE 2019, 14, e0215981, Erratum in PLoS ONE 2019, 14, e0223429. [Google Scholar] [CrossRef]
- Sheu, M.L.; Pan, L.Y.; Yang, C.N.; Sheehan, J.; Pan, L.Y.; You, W.C.; Wang, C.C.; Pan, H.C. Thrombin-Induced Microglia Activation Modulated through Aryl Hydrocarbon Receptors. Int. J. Mol. Sci. 2023, 24, 1416. [Google Scholar] [CrossRef] [PubMed]
- Rahimlou, M.; Hosseini, S.A.; Majdinasab, N.; Haghighizadeh, M.H.; Husain, D. Effects of long-term administration of Multi-Strain Probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Nutr. Neurosci. 2022, 25, 411–422. [Google Scholar] [CrossRef]
- Hasaniani, N.; Ghasemi-Kasman, M.; Halaji, M.; Rostami-Mansoor, S. Bifidobacterium breve Probiotic Compared to Lactobacillus casei Causes a Better Reduction in Demyelination and Oxidative Stress in Cuprizone-Induced Demyelination Model of Rat. Mol. Neurobiol. 2024, 61, 498–509. [Google Scholar] [CrossRef]
- Mirashrafi, S.; Hejazi Taghanaki, S.Z.; Sarlak, F.; Moravejolahkami, A.R.; Hojjati Kermani, M.A.; Haratian, M. Effect of probiotics supplementation on disease progression, depression, general health, and anthropometric measurements in relapsing-remitting multiple sclerosis patients: A systematic review and meta-analysis of clinical trials. Int. J. Clin. Pract. 2021, 75, e14724. [Google Scholar] [CrossRef]
- Megur, A.; Daliri, E.B.; Baltriukienė, D.; Burokas, A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef]
- Sajedi, D.; Shabani, R.; Elmieh, A. The Effect of Aerobic Training With the Consumption of Probiotics on the Myelination of Nerve Fibers in Cuprizone-induced Demyelination Mouse Model of Multiple Sclerosis. Basic. Clin. Neurosci. 2023, 14, 73–86. [Google Scholar] [CrossRef]
- Guglielmetti, M.; Al-Qahtani, W.H.; Ferraris, C.; Grosso, G.; Fiorini, S.; Tavazzi, E.; Greco, G.; La Malfa, A.; Bergamaschi, R.; Tagliabue, A. Adherence to Mediterranean Diet Is Associated with Multiple Sclerosis Severity. Nutrients 2023, 15, 4009. [Google Scholar] [CrossRef] [PubMed]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Multiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef]
- Di Majo, D.; Cacciabaudo, F.; Accardi, G.; Gambino, G.; Giglia, G.; Ferraro, G.; Candore, G.; Sardo, P. Ketogenic and Modified Mediterranean Diet as a Tool to Counteract Neuroinflammation in Multiple Sclerosis: Nutritional Suggestions. Nutrients 2022, 14, 2384. [Google Scholar] [CrossRef]
- Carnovale, E.; Nutrizione, I.n.d.; Marletta, L. Tabelle di Composizione Degli Alimenti; Edra: Tuscany, Italy, 1997. [Google Scholar]
- Accardi, G.; Aiello, A.; Gargano, V.; Gambino, C.M.; Caracappa, S.; Marineo, S.; Vesco, G.; Carru, C.; Zinellu, A.; Zarcone, M.; et al. Nutraceutical effects of table green olives: A pilot study with Nocellara del Belice olives. Immun. Ageing 2016, 13, 11. [Google Scholar] [CrossRef]
- Esposito, S.; Sparaco, M.; Maniscalco, G.T.; Signoriello, E.; Lanzillo, R.; Russo, C.; Carmisciano, L.; Cepparulo, S.; Lavorgna, L.; Gallo, A.; et al. Lifestyle and Mediterranean diet adherence in a cohort of Southern Italian patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 47, 102636. [Google Scholar] [CrossRef]
- Bohlouli, J.; Namjoo, I.; Borzoo-Isfahani, M.; Poorbaferani, F.; Moravejolahkami, A.R.; Clark, C.C.T.; Hojjati Kermani, M.A. Modified Mediterranean diet v. traditional Iranian diet: Efficacy of dietary interventions on dietary inflammatory index score, fatigue severity and disability in multiple sclerosis patients. Br. J. Nutr. 2022, 128, 1274–1284. [Google Scholar] [CrossRef]
- Katz Sand, I.; Benn, E.K.T.; Fabian, M.; Fitzgerald, K.C.; Digga, E.; Deshpande, R.; Miller, A.; Gallo, S.; Arab, L. Randomized-controlled trial of a modified Mediterranean dietary program for multiple sclerosis: A pilot study. Mult. Scler. Relat. Disord. 2019, 36, 101403. [Google Scholar] [CrossRef]
- Mirza, A.; Zhu, F.; Knox, N.; Black, L.; Daly, A.; Bonner, C.; Domselaar, G.; Bernstein, C.; Marrie, R.; Hart, J.; et al. Mediterranean Diet and Associations with the Gut Microbiota and Pediatric-Onset Multiple Sclerosis: A Trivariate Analysis. 2023. Available online: https://pubmed.ncbi.nlm.nih.gov/39030379/ (accessed on 26 December 2024).
- Azary, S.; Schreiner, T.; Graves, J.; Waldman, A.; Belman, A.; Guttman, B.W.; Aaen, G.; Tillema, J.M.; Mar, S.; Hart, J.; et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 28–33. [Google Scholar] [CrossRef]
- Cantoni, C.; Lin, Q.; Dorsett, Y.; Ghezzi, L.; Liu, Z.; Pan, Y.; Chen, K.; Han, Y.; Li, Z.; Xiao, H.; et al. Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine 2022, 76, 103798. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Mendozzi, L.; D’Amico, F.; Saresella, M.; Rampelli, S.; Piancone, F.; La Rosa, F.; Marventano, I.; Clerici, M.; d’Arma, A.; et al. Influence of a High-Impact Multidimensional Rehabilitation Program on the Gut Microbiota of Patients with Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 7173. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.; Karber, M.; Kuhn, H. Ketogenic diets attenuate cyclooxygenase and lipoxygenase gene expression in multiple sclerosis. EBioMedicine 2018, 36, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Göktas, Ö.; Reißhauer, A.; Neuhaus, J.; Weylandt, K.H.; Guschin, A.; Bock, M. Reduced Mass and Diversity of the Colonic Microbiome in Patients with Multiple Sclerosis and Their Improvement with Ketogenic Diet. Front. Microbiol. 2017, 8, 1141. [Google Scholar] [CrossRef]
- Esquifino, A.I.; Cano, P.; Jimenez-Ortega, V.; Fernández-Mateos, M.P.; Cardinali, D.P. Immune response after experimental allergic encephalomyelitis in rats subjected to calorie restriction. J. Neuroinflamm. 2007, 4, 6. [Google Scholar] [CrossRef]
- Piccio, L.; Stark, J.L.; Cross, A.H. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J. Leukoc. Biol. 2008, 84, 940–948. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e1226. [Google Scholar] [CrossRef]
- Bai, M.; Wang, Y.; Han, R.; Xu, L.; Huang, M.; Zhao, J.; Lin, Y.; Song, S.; Chen, Y. Intermittent caloric restriction with a modified fasting-mimicking diet ameliorates autoimmunity and promotes recovery in a mouse model of multiple sclerosis. J. Nutr. Biochem. 2021, 87, 108493. [Google Scholar] [CrossRef]
- Razeghi Jahromi, S.; Ghaemi, A.; Alizadeh, A.; Sabetghadam, F.; Moradi Tabriz, H.; Togha, M. Effects of Intermittent Fasting on Experimental Autoimune Encephalomyelitis in C57BL/6 Mice. Iran. J. Allergy Asthma Immunol. 2016, 15, 212–219. [Google Scholar] [PubMed]
- Kafami, L.; Raza, M.; Razavi, A.; Mirshafiey, A.; Movahedian, M.; Khorramizadeh, M.R. Intermittent feeding attenuates clinical course of experimental autoimmune encephalomyelitis in C57BL/6 mice. Avicenna J. Med. Biotechnol. 2010, 2, 47–52. [Google Scholar] [PubMed]
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019, 178, 1102–1114.e1117. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Bhargava, P.; Smith, M.D.; Vizthum, D.; Henry-Barron, B.; Kornberg, M.D.; Cassard, S.D.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Intermittent calorie restriction alters T cell subsets and metabolic markers in people with multiple sclerosis. EBioMedicine 2022, 82, 104124. [Google Scholar] [CrossRef]
- Yi, B.; Titze, J.; Rykova, M.; Feuerecker, M.; Vassilieva, G.; Nichiporuk, I.; Schelling, G.; Morukov, B.; Choukèr, A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: A longitudinal study. Transl. Res. 2015, 166, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Quach, A.; Zhang, H.; Terrera, M.; Jackemeyer, D.; Xian, X.; Tsow, F.; Tao, N.; Forzani, E.S. Acetone as biomarker for ketosis buildup capability--a study in healthy individuals under combined high fat and starvation diets. Nutr. J. 2015, 14, 41. [Google Scholar] [CrossRef]
- Sourbron, J.; Klinkenberg, S.; van Kuijk, S.M.J.; Lagae, L.; Lambrechts, D.; Braakman, H.M.H.; Majoie, M. Ketogenic diet for the treatment of pediatric epilepsy: Review and meta-analysis. Childs Nerv. Syst. 2020, 36, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Gough, S.M.; Casella, A.; Ortega, K.J.; Hackam, A.S. Neuroprotection by the Ketogenic Diet: Evidence and Controversies. Front. Nutr. 2021, 8, 782657. [Google Scholar] [CrossRef]
- Koh, S.; Dupuis, N.; Auvin, S. Ketogenic diet and Neuroinflammation. Epilepsy Res. 2020, 167, 106454. [Google Scholar] [CrossRef]
- Yao, A.; Li, Z.; Lyu, J.; Yu, L.; Wei, S.; Xue, L.; Wang, H.; Chen, G.Q. On the nutritional and therapeutic effects of ketone body D-β-hydroxybutyrate. Appl. Microbiol. Biotechnol. 2021, 105, 6229–6243. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Yamanashi, T.; Iwata, M.; Kamiya, N.; Tsunetomi, K.; Kajitani, N.; Wada, N.; Iitsuka, T.; Yamauchi, T.; Miura, A.; Pu, S.; et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci. Rep. 2017, 7, 7677. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, N.; Zhang, R.; Jin, L.; Petridis, A.K.; Loers, G.; Zheng, X.; Wang, Z.; Siebert, H.C. Cuprizone-Induced Demyelination in Mouse Hippocampus Is Alleviated by Ketogenic Diet. J. Agric. Food Chem. 2020, 68, 11215–11228. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.; Steffen, F.; Zipp, F.; Bittner, S. Impact of Dietary Intervention on Serum Neurofilament Light Chain in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm 2022, 9, 1102. [Google Scholar] [CrossRef]
- O’Neill, B.; Raggi, P. The ketogenic diet: Pros and cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, L.G.; Cheek, J.J.; Fox, S.S.; Healy, H.S.; Schweizer, M.L.; Bao, W.; Kamholz, J.; Titcomb, T.J. Efficacy of Diet on Fatigue and Quality of Life in Multiple Sclerosis: A Systematic Review and Network Meta-analysis of Randomized Trials. Neurology 2023, 100, e357–e366. [Google Scholar] [CrossRef]
- Cantoni, C.; Dorsett, Y.; Fontana, L.; Zhou, Y.; Piccio, L. Effects of dietary restriction on gut microbiota and CNS autoimmunity. Clin. Immunol. 2022, 235, 108575. [Google Scholar] [CrossRef]
- Templeman, I.; Gonzalez, J.T.; Thompson, D.; Betts, J.A. The role of intermittent fasting and meal timing in weight management and metabolic health. Proc. Nutr. Soc. 2020, 79, 76–87. [Google Scholar] [CrossRef]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Vizthum, D.; Henry-Barron, B.; Schweitzer, A.; Cassard, S.D.; Kossoff, E.; Hartman, A.L.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 23, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wingo, B.C.; Rinker, J.R., 2nd; Green, K.; Peterson, C.M. Feasibility and acceptability of time-restricted eating in a group of adults with multiple sclerosis. Front. Neurol. 2022, 13, 1087126. [Google Scholar] [CrossRef]
- Spain, R.I.; Piccio, L.; Langer-Gould, A.M. The Role of Diet in Multiple Sclerosis: Food for Thought. Neurology 2023, 100, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, S.; Gao, Y. The effects of intermittent fasting for patients with multiple sclerosis (MS): A systematic review. Front. Nutr. 2023, 10, 1328426. [Google Scholar] [CrossRef] [PubMed]
- Hucke, S.; Wiendl, H.; Klotz, L. Implications of dietary salt intake for multiple sclerosis pathogenesis. Mult. Scler. 2016, 22, 133–139. [Google Scholar] [CrossRef]
- Probst, Y.; Mowbray, E.; Svensen, E.; Thompson, K. A Systematic Review of the Impact of Dietary Sodium on Autoimmunity and Inflammation Related to Multiple Sclerosis. Adv. Nutr. 2019, 10, 902–910. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef]
- Krementsov, D.N.; Case, L.K.; Hickey, W.F.; Teuscher, C. Exacerbation of autoimmune neuroinflammation by dietary sodium is genetically controlled and sex specific. Faseb J. 2015, 29, 3446–3457. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, L.; Ji, W.J.; Yuan, F.; Guo, Z.Z.; Pang, B.; Luo, T.; Liu, X.; Zhang, W.C.; Jiang, T.M.; et al. Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyte-platelet aggregates in humans: Implications in end organ inflammation. PLoS ONE 2013, 8, e60332. [Google Scholar] [CrossRef]
- Luo, T.; Ji, W.J.; Yuan, F.; Guo, Z.Z.; Li, Y.X.; Dong, Y.; Ma, Y.Q.; Zhou, X.; Li, Y.M. Th17/Treg Imbalance Induced by Dietary Salt Variation Indicates Inflammation of Target Organs in Humans. Sci. Rep. 2016, 6, 26767. [Google Scholar] [CrossRef]
- Stegbauer, J.; Lee, D.H.; Seubert, S.; Ellrichmann, G.; Manzel, A.; Kvakan, H.; Muller, D.N.; Gaupp, S.; Rump, L.C.; Gold, R.; et al. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc. Natl. Acad. Sci. USA 2009, 106, 14942–14947. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.; Seshadri, S.; Beiser, A.; Himali, J.J.; Au, R.; Fletcher, E.; Carmichael, O.; Wolf, P.A.; DeCarli, C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: A cross-sectional study. Lancet Neurol. 2012, 11, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, B.; Graves, J.; Casper, T.C.; Lulu, S.; Waldman, A.; Belman, A.; Greenberg, B.; Weinstock-Guttman, B.; Aaen, G.; Tillema, J.M.; et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1350–1353. [Google Scholar] [CrossRef]
- Cortese, M.; Yuan, C.; Chitnis, T.; Ascherio, A.; Munger, K.L. No association between dietary sodium intake and the risk of multiple sclerosis. Neurology 2017, 89, 1322–1329. [Google Scholar] [CrossRef]
- McDonald, J.; Graves, J.; Waldman, A.; Lotze, T.; Schreiner, T.; Belman, A.; Greenberg, B.; Weinstock-Guttman, B.; Aaen, G.; Tillema, J.M.; et al. A case-control study of dietary salt intake in pediatric-onset multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 6, 87–92. [Google Scholar] [CrossRef]
- Farez, M.F.; Fiol, M.P.; Gaitán, M.I.; Quintana, F.J.; Correale, J. Sodium intake is associated with increased disease activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Munger, K.L.; Hartung, H.P.; Freedman, M.S.; Montalbán, X.; Edan, G.; Wicklein, E.M.; Radue, E.W.; Kappos, L.; Pohl, C.; et al. Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann. Neurol. 2017, 82, 20–29. [Google Scholar] [CrossRef]
- Zostawa, J.; Adamczyk, J.; Sowa, P.; Adamczyk-Sowa, M. The influence of sodium on pathophysiology of multiple sclerosis. Neurol. Sci. 2017, 38, 389–398. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Valizadeh, S.; Majdi Seghinsara, A.; Maleki Chollou, K.; Bahadori, A.; Abbaszadeh, S.; Taghdir, M.; Behniafar, H.; Riahi, S.M. The efficacy of probiotics in experimental autoimmune encephalomyelitis (an animal model for MS): A systematic review and meta-analysis. Lett. Appl. Microbiol. 2021, 73, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Kap, Y.S.; Bus-Spoor, C.; van Driel, N.; Dubbelaar, M.L.; Grit, C.; Kooistra, S.M.; Fagrouch, Z.C.; Verschoor, E.J.; Bauer, J.; Eggen, B.J.L.; et al. Targeted Diet Modification Reduces Multiple Sclerosis-like Disease in Adult Marmoset Monkeys from an Outbred Colony. J. Immunol. 2018, 201, 3229–3243. [Google Scholar] [CrossRef] [PubMed]
- Mestre, L.; Carrillo-Salinas, F.J.; Feliú, A.; Mecha, M.; Alonso, G.; Espejo, C.; Calvo-Barreiro, L.; Luque-García, J.L.; Estevez, H.; Villar, L.M.; et al. How oral probiotics affect the severity of an experimental model of progressive multiple sclerosis? Bringing commensal bacteria into the neurodegenerative process. Gut Microbes 2020, 12, 1813532. [Google Scholar] [CrossRef]
- Morshedi, M.; Hashemi, R.; Moazzen, S.; Sahebkar, A.; Hosseinifard, E.-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: A systematic review. J. Neuroinflamm. 2019, 16, 231. [Google Scholar] [CrossRef]
- Jiang, J.; Chu, C.; Wu, C.; Wang, C.; Zhang, C.; Li, T.; Zhai, Q.; Yu, L.; Tian, F.; Chen, W. Efficacy of probiotics in multiple sclerosis: A systematic review of preclinical trials and meta-analysis of randomized controlled trials. Food Funct. 2021, 12, 2354–2377. [Google Scholar] [CrossRef]
- Patterson, E.; Tan, H.T.T.; Groeger, D.; Andrews, M.; Buckley, M.; Murphy, E.F.; Groeger, J.A. Bifidobacterium longum 1714 improves sleep quality and aspects of well-being in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Sci. Rep. 2024, 14, 3725. [Google Scholar] [CrossRef]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Tjon, E.; Laghi, L.; Cox, L.M.; Kivisäkk, P.; Pierre, I.V.; Hrishikesh, L.; Gandhi, R.; et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann. Neurol. 2018, 83, 1147–1161. [Google Scholar] [CrossRef]
- Lavasani, S.; Dzhambazov, B.; Nouri, M.; Fåk, F.; Buske, S.; Molin, G.; Thorlacius, H.; Alenfall, J.; Jeppsson, B.; Weström, B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 2010, 5, e9009. [Google Scholar] [CrossRef]
- Gharehkhani Digehsara, S.; Name, N.; Esfandiari, B.; Karim, E.; Taheri, S.; Tajabadi-Ebrahimi, M.; Arasteh, J. Effects of Lactobacillus casei Strain T2 (IBRC-M10783) on the Modulation of Th17/Treg and Evaluation of miR-155, miR-25, and IDO-1 Expression in a Cuprizone-Induced C57BL/6 Mouse Model of Demyelination. Inflammation 2021, 44, 334–343. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Enam, F.; Mansell, T.J. Prebiotics: Tools to manipulate the gut microbiome and metabolome. J. Ind. Microbiol. Biotechnol. 2019, 46, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Fransen, F.; Sahasrabudhe, N.; Elderman, M.; Bosveld, M.; El Aidy, S.; Hugenholtz, F.; Borghuis, T.; Kousemaker, B.; Winkel, S.; Jongh, C.; et al. β2→1-Fructans Modulate the Immune System In Vivo in a Microbiota-Dependent and -Independent Fashion. Front. Immunol. 2017, 8, 154. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Mallet, J.F.; Graham, E.; Matar, C. Role of probiotics and prebiotics in immunomodulation. Curr. Opin. Food Sci. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Moravejolahkami, A.R.; Paknahad, Z.; Chitsaz, A. Dietary intake of energy and fiber in MS patients; an approach to prebiotics role. Nutr. Food Sci. 2019, 49, 1039–1050. [Google Scholar] [CrossRef]
- Lu, X.Y.; Han, B.; Deng, X.; Deng, S.Y.; Zhang, Y.Y.; Shen, P.X.; Hui, T.; Chen, R.H.; Li, X.; Zhang, Y. Pomegranate peel extract ameliorates the severity of experimental autoimmune encephalomyelitis via modulation of gut microbiota. Gut Microbes 2020, 12, 1857515. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Li, G.; Xie, F.; Yan, S.; Hu, X.; Jin, B.; Wang, J.; Wu, J.; Yin, D.; Xie, Q. Subhealth: Definition, criteria for diagnosis and potential prevalence in the central region of China. BMC Public. Health 2013, 13, 446. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; d’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Levi, I.; Gurevich, M.; Perlman, G.; Magalashvili, D.; Menascu, S.; Bar, N.; Godneva, A.; Zahavi, L.; Chermon, D.; Kosower, N.; et al. Potential role of indolelactate and butyrate in multiple sclerosis revealed by integrated microbiome-metabolome analysis. Cell Rep. Med. 2021, 2, 100246. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Melbye, P.; Olsson, A.; Hansen, T.H.; Søndergaard, H.B.; Bang Oturai, A. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol. Scand. 2019, 139, 208–219. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Kespohl, M.; Vachharajani, N.; Luu, M.; Harb, H.; Pautz, S.; Wolff, S.; Sillner, N.; Walker, A.; Schmitt-Kopplin, P.; Boettger, T.; et al. The Microbial Metabolite Butyrate Induces Expression of Th1-Associated Factors in CD4(+) T Cells. Front. Immunol. 2017, 8, 1036. [Google Scholar] [CrossRef]
- Mizuno, M.; Noto, D.; Kaga, N.; Chiba, A.; Miyake, S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS ONE 2017, 12, e0173032. [Google Scholar] [CrossRef]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Trend, S.; Leffler, J.; Jones, A.P.; Cha, L.; Gorman, S.; Brown, D.A.; Breit, S.N.; Kermode, A.G.; French, M.A.; Ward, N.C.; et al. Associations of serum short-chain fatty acids with circulating immune cells and serum biomarkers in patients with multiple sclerosis. Sci. Rep. 2021, 11, 5244. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, S.; Domínguez-Mozo, M.I.; Alonso-Gómez, A.; Medina, S.; Villarrubia, N.; Fernández-Velasco, J.I.; García-Martínez, M.; García-Calvo, E.; Estévez, H.; Costa-Frossard, L.; et al. Acetate correlates with disability and immune response in multiple sclerosis. PeerJ 2020, 8, e10220. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Gustavsen, S.; Nguyen, T.D.; Nyman, M.; Langkilde, A.R.; Hansen, T.H.; Sellebjerg, F.; Oturai, A.B.; Bach Søndergaard, H. Serum Short-Chain Fatty Acids and Associations with Inflammation in Newly Diagnosed Patients with Multiple Sclerosis and Healthy Controls. Front. Immunol. 2021, 12, 661493. [Google Scholar] [CrossRef]
- Calvo-Barreiro, L.; Eixarch, H.; Cornejo, T.; Costa, C.; Castillo, M.; Mestre, L.; Guaza, C.; Martínez-Cuesta, M.D.C.; Tanoue, T.; Honda, K.; et al. Selected Clostridia Strains from The Human Microbiota and their Metabolite, Butyrate, Improve Experimental Autoimmune Encephalomyelitis. Neurotherapeutics 2021, 18, 920–937. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Xie, L.; Saimaier, K.; Zhuang, W.; Han, M.; Liu, G.; Lv, J.; Shi, G.; Li, N.; et al. Methyl Butyrate Alleviates Experimental Autoimmune Encephalomyelitis and Regulates the Balance of Effector T Cells and Regulatory T Cells. Inflammation 2022, 45, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef]
- Haase, S.; Mäurer, J.; Duscha, A.; Lee, D.H.; Balogh, A.; Gold, R.; Müller, D.N.; Haghikia, A.; Linker, R.A. Propionic Acid Rescues High-Fat Diet Enhanced Immunopathology in Autoimmunity via Effects on Th17 Responses. Front. Immunol. 2021, 12, 701626. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Brara, S.M.; Beaber, B.E.; Koebnick, C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology 2013, 80, 548–552. [Google Scholar] [CrossRef]


| Diet | Effect | Immune System | Gut Microbiota | References |
|---|---|---|---|---|
| Mediterranean diet | ↑ | PD-L1+ monocytes Gut Treg suppression Th2 cells |
| [57,104,105] |
| ↓ | IL-17+, PD-1+ T cells Th17 cells |
| ||
| Ketogenic diet | ↑ | Inhibition of microglia activation (EAE) Peripheral lymphocytes count (EAE) Enzymes COX1, COX2 and ALOX5 |
| [106,107] |
| Caloric restriction | ↑ | Corticosterone, adiponectin (EAE) Tregs number Naïve T cells BDNF |
| [108,109,110,111,112,113,114,115,116] |
| ↓ | IL-6, leptin (EAE) T cells, B cells and INF–γ (EAE) Total CD4+ T cells Pro-inflammatory cytokines (EAE) Th1 and Th17 cells (EAE) TNFα, IL-1β, CXCL2 and CXCL10 (EAE) Memory T cell effector memory reductions in Th1 |
| ||
| Low-salt diet | ↑ | IL-10 Treg cells | n/d | [117] |
| ↓ | Th17 cells - IL-6, IL-23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Džidić Krivić, A.; Begagić, E.; Hadžić, S.; Bećirović, A.; Bećirović, E.; Hibić, H.; Tandir Lihić, L.; Kadić Vukas, S.; Bečulić, H.; Kasapović, T.; et al. Unveiling the Important Role of Gut Microbiota and Diet in Multiple Sclerosis. Brain Sci. 2025, 15, 253. https://doi.org/10.3390/brainsci15030253
Džidić Krivić A, Begagić E, Hadžić S, Bećirović A, Bećirović E, Hibić H, Tandir Lihić L, Kadić Vukas S, Bečulić H, Kasapović T, et al. Unveiling the Important Role of Gut Microbiota and Diet in Multiple Sclerosis. Brain Sciences. 2025; 15(3):253. https://doi.org/10.3390/brainsci15030253
Chicago/Turabian StyleDžidić Krivić, Amina, Emir Begagić, Semir Hadžić, Amir Bećirović, Emir Bećirović, Harisa Hibić, Lejla Tandir Lihić, Samra Kadić Vukas, Hakija Bečulić, Tarik Kasapović, and et al. 2025. "Unveiling the Important Role of Gut Microbiota and Diet in Multiple Sclerosis" Brain Sciences 15, no. 3: 253. https://doi.org/10.3390/brainsci15030253
APA StyleDžidić Krivić, A., Begagić, E., Hadžić, S., Bećirović, A., Bećirović, E., Hibić, H., Tandir Lihić, L., Kadić Vukas, S., Bečulić, H., Kasapović, T., & Pojskić, M. (2025). Unveiling the Important Role of Gut Microbiota and Diet in Multiple Sclerosis. Brain Sciences, 15(3), 253. https://doi.org/10.3390/brainsci15030253








