The Use of Magnetoencephalography in the Diagnosis and Monitoring of Mild Traumatic Brain Injuries and Post-Concussion Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Studies Selection
2.3.1. Inclusion Criteria
- Population: Patients diagnosed with mTBI or PCS.
- Intervention: Use of MEG for assessing brain function, either at rest or during cognitive tasks.
- Outcomes: MEG-based biomarkers, such as abnormal oscillatory activity, functional connectivity disruptions, and slow-wave detection, in relation to diagnosing or monitoring mTBI or PCS.
- Study Design: Observational (e.g., case–control and cohort studies) and experimental (e.g., clinical trials) designs.
2.3.2. Exclusion Criteria
- Studies not using MEG as the primary neuroimaging tool.
- Studied not reporting results specific to mTBIs.
- Review articles, meta-analyses, editorials, or opinion papers.
2.4. Data Extraction
- Study characteristics: author names, publication year, study design, and sample size.
- Participant characteristics: age, sex, and specific diagnosis (mTBI, PCS).
- MEG protocols: frequency bands analyzed (e.g., delta, theta, alpha, beta, gamma), resting-state versus task-based MEG, and analysis methods (e.g., functional connectivity, source localization).
- Outcomes: key MEG findings, including abnormal brain oscillations, connectivity changes, and correlations with cognitive or behavioral measures.
2.5. Data Synthesis and Analysis
- MEG biomarkers for mTBI: Detection of abnormal oscillations (e.g., delta and theta waves) and their association with cognitive dysfunction.
- MEG functional connectivity analysis: Disruptions in neural networks, particularly in the default mode network (DMN) and thalamocortical circuitry.
- MEG for differentiating mTBI from PTSD: Studies that explored MEG’s ability to distinguish mTBI from PTSD based on distinct brain activity patterns.
- MEG as a tool for tracking recovery: The role of MEG in monitoring changes in brain function over time and its correlation with clinical outcomes.
2.6. Quality Assessment
2.6.1. Assessment of Observational Studies Using the Newcastle–Ottawa Scale (NOS)
- Selection of Participants. Most of the studies included in this review had well-defined inclusion criteria for patients with mTBI or PCS. Mainly participants from clinical settings were recruited, ensuring the representativeness of the target population. However, a few studies had small sample sizes, which may have affected the generalizability of their findings.
- Comparability of Groups. Several studies included control groups of healthy participants (healthy controls, HCs) or individuals with orthopedic injuries (orthopedic trauma controls, OTCs) to allow for the comparison of MEG results. The studies that matched HCs based on age, sex, and education were rated highly for comparability. Some studies failed to control for confounding factors, such as co-occurring conditions—i.e., post-traumatic stress disorder (PTSD)—which could have introduced bias into the results.
- Outcome Measurement. The outcome measurement for most studies was based on objective MEG biomarkers, such as abnormal oscillatory activity, functional connectivity, and slow-wave detection. Many studies used automated MEG analysis techniques, reducing the likelihood of observer bias. However, some studies relied on subjective cognitive assessments, which could have introduced variability in outcome reporting. A brief overview of the standard techniques for the analysis of MEG outcomes was previously addressed by multiple technical studies [15,18,19,20].
2.6.2. Assessment of Randomized Controlled Trials Using the Cochrane Risk of Bias Tool
- Selection Bias. Randomization methods were generally well reported in the included RCTs. The majority of studies used appropriate randomization procedures, such as computer-generated random sequences, to assign participants to different intervention groups (e.g., MEG assessment versus other diagnostic tools). However, a few studies did not adequately describe their randomization process, leading to an unclear risk of selection bias.
- Performance Bias. Blinding of participants and personnel was rarely implemented in the studies, particularly in those using MEG as a diagnostic tool. While blinding is challenging in diagnostic studies, the lack of blinding could have introduced performance bias in studies where subjective outcomes (such as cognitive performance) were measured alongside MEG data.
- Detection Bias. Detection bias was minimized in most studies by the use of objective MEG biomarkers (e.g., frequency band analysis and source localization). Automated MEG analysis tools reduced the likelihood of bias in outcome detection. However, in studies that used cognitive tests as secondary outcomes, there was a potential for detection bias, especially if assessors were not blinded to the intervention groups.
- Attrition Bias. Most studies reported low rates of participant dropout, and reasons for dropout were typically well documented. Studies with long follow-up periods, however, had higher rates of attrition, which could have influenced the results. The effect of attrition bias was generally low, as intention-to-treat analyses were applied in most cases.
- Reporting Bias. Selective reporting of outcomes was minimal in the included studies, as most trials were pre-registered and reported all pre-specified outcomes. However, a few studies failed to report secondary outcomes, raising the potential for reporting bias.
2.7. Statistical Considerations
3. Results
3.1. Summary of Quality Findings
3.1.1. Overall Quality
3.1.2. Key Strengths
- ○
- The use of MEG as an objective high-resolution functional neuroimaging tool minimized observer and detection biases in many studies.
- ○
- Studies that incorporated machine learning techniques in MEG analysis enhanced the precision of mTBI diagnosis and reduced subjective variability.
3.1.3. Key Limitations
- ○
- A lack of blinding in many studies and small sample sizes in some resulted in an increased risk of bias.
- ○
- Studies that did not control for co-occurring conditions, such as PTSD or psychiatric disorders, may have confounded the findings related to mTBI and MEG biomarkers.
3.2. MEG in mTBI Diagnosis and Biomarker Development
3.3. Functional Connectivity Changes in mTBI
3.4. MEG in Differentiating mTBI from PTSD
3.5. MEG as a Tool for Monitoring Recovery in mTBI
3.6. Cognitive and Behavioral Correlates of MEG Findings
3.7. MEG’s Role in Identifying Subtle Brain Injury
3.8. Applications of Machine Learning and Deep Learning in MEG
4. Discussion
4.1. MEG as a Diagnostic Tool for mTBI
4.2. Functional Connectivity and Network Disruptions
4.3. MEG as a Tool for Monitoring Recovery
4.4. Limitations of MEG in mTBI Diagnosis
4.5. Implications for Clinical Practice and Future Perspectives
4.6. Limitations of the Current Systematic Review
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jackson, W.T.; Starling, A.J. Concussion Evaluation and Management. Med. Clin. N. Am. 2019, 103, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Bielanin, J.P.; Metwally, S.A.H.; Paruchuri, S.S.; Sun, D. An overview of mild traumatic brain injuries and emerging therapeutic targets. Neurochem. Int. 2024, 172, 105655. [Google Scholar] [CrossRef] [PubMed]
- Bischof, G.N.; Cross, D.J. Brain Trauma Imaging. J. Nuclear Med. 2023, 64, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.R.; Huang, M. Magnetoencephalography in the diagnosis of concussion. Prog. Neurol. Surg. 2014, 28, 94–111. [Google Scholar] [CrossRef]
- Davenport, E.M.; Urban, J.E.; Vaughan, C.; DeSimone, J.C.; Wagner, B.; Espeland, M.A.; Powers, A.K.; Whitlow, C.T.; Stitzel, J.D.; Maldjian, J.A. MEG measured delta waves increase in adolescents after concussion. Brain Behav. 2022, 12, e2720. [Google Scholar] [CrossRef]
- Allen, C.M.; Halsey, L.; Topcu, G.; Rier, L.; Gascoyne, L.E.; Scadding, J.W.; Furlong, P.L.; Dunkley, B.T.; das Nair, R.; Brookes, M.J.; et al. Magnetoencephalography abnormalities in adult mild traumatic brain injury: A systematic review. NeuroImage Clin. 2021, 31, 102697. [Google Scholar] [CrossRef]
- Desjardins, M.; Drisdelle, B.L.; Lefebvre, C.; Gagnon, J.F.; De Beaumont, L.; Jolicoeur, P. Interhemispheric differences in P1 and N1 amplitude in EEG and MEG differ across older individuals with a concussion compared with age-matched controls. Psychophysiology 2021, 58, e13751. [Google Scholar] [CrossRef]
- Krieger, D.; Shepard, P.; Soose, R.; Puccio, A.; Beers, S.; Schneider, W.; Kontos, A.P.; Collins, M.W.; Okonkwo, D.O. MEG-Derived Symptom-Sensitive Biomarkers with Long-Term Test-Retest Reliability. Diagnostics 2021, 12, 84. [Google Scholar] [CrossRef]
- Suri, A.K.; Lipton, M.L. Neuroimaging of brain trauma in sports. Handb. Clin. Neurol. 2018, 158, 205–216. [Google Scholar] [CrossRef]
- Stein, M.B.; McAllister, T.W. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am. J. Psychiatry 2009, 166, 768–776. [Google Scholar] [CrossRef]
- Abhang, P.A.; Gawali, B.W.; Mehrotra, S.C. Technological Basics of EEG Recording and Operation of Apparatus. In Introduction to EEG- and Speech-Based Emotion Recognition; Elsevier: Amsterdam, The Netherlands, 2016; pp. 19–50. [Google Scholar] [CrossRef]
- Olaniyan, O.T.; Adetunji, C.O.; Dare, A.; Adeyomoye, O.; Adeniyi, M.J.; Enoch, A. Neural signaling and communication using machine learning. In Artificial Intelligence for Neurological Disorders; Chapter 15; Academic Press: Cambridge, MA, USA, 2023; pp. 245–260. [Google Scholar]
- Hoshi, H.; Hirata, Y.; Fukasawa, K.; Kobayashi, M.; Shigihara, Y. Oscillatory characteristics of resting-state magnetoencephalography reflect pathological and symptomatic conditions of cognitive impairment. Front. Aging Neurosci. 2024, 16, 1273738. [Google Scholar] [CrossRef] [PubMed]
- Afnan, J.; von Ellenrieder, N.; Lina, J.M.; Pellegrino, G.; Arcara, G.; Cai, Z.; Hedrich, T.; Abdallah, C.; Khajehpour, H.; Frauscher, B.; et al. Validating MEG source imaging of resting state oscillatory patterns with an intracranial EEG atlas. NeuroImage 2023, 274, 120158. [Google Scholar] [CrossRef] [PubMed]
- Fred, A.L.; Kumar, S.N.; Kumar Haridhas, A.; Ghosh, S.; Purushothaman Bhuvana, H.; Sim, W.K.; Vimalan, V.; Givo, F.A.; Jousmäki, V.; Padmanabhan, P.; et al. A Brief Introduction to Magnetoencephalography (MEG) and Its Clinical Applications. Brain Sci. 2022, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Gierisch, J.M.; Beadles, C.; Shapiro, A.; McDuffie, J.; Cunningham, N.; Bradford, D.; Strauss, J.; Callahan, M.; Chen, M.; Hemminger, A.; et al. Health Disparities in Quality Indicators of Healthcare Among Adults with Mental Illness [Internet]. Washington (DC): Department of Veterans Affairs (US); 2014 Oct. Appendix B, Newcastle-Ottawa Scale Coding Manual for Cohort Studies. Available online: https://www.ncbi.nlm.nih.gov/books/NBK299087/ (accessed on 4 December 2024).
- Higgins, J.P.T.; Altman, D.G.; Gatzsche, P.C.; Jani, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Gross, J.; Baillet, S.; Barnes, G.R.; Henson, R.N.; Hillebrand, A.; Jensen, O.; Jerbi, K.; Litvak, V.; Maess, B.; Oostenveld, R.; et al. Good practice for conducting and reporting MEG research. NeuroImage 2013, 65, 349–363. [Google Scholar] [CrossRef]
- Cichy, R.M.; Pantazis, D. Multivariate pattern analysis of MEG and EEG: A comparison of representational structure in time and space. NeuroImage 2017, 158, 441–454. [Google Scholar] [CrossRef]
- Singh, S.P. Magnetoencephalography: Basic principles. Ann. Indian. Acad. Neurol. 2014, 17 (Suppl. S1), S107–S112. [Google Scholar] [CrossRef]
- Huang, M.X.; Theilmann, R.J.; Robb, A.; Angeles, A.; Nichols, S.; Drake, A.; D’Andrea, J.; Levy, M.; Holland, M.; Song, T.; et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma 2009, 26, 1213–1226. [Google Scholar] [CrossRef]
- Huang, M.X.; Nichols, S.; Robb-Swan, A.; Angeles-Quinto, A.; Harrington, D.L.; Drake, A.; Huang, C.W.; Song, T.; Diwakar, M.; Risbrough, V.B.; et al. MEG Working Memory N-Back Task Reveals Functional Deficits in Combat-Related Mild Traumatic Brain Injury. Cereb. Cortex 2019, 29, 1953–1968. [Google Scholar] [CrossRef]
- Huang, M.X.; Robb Swan, A.; Angeles Quinto, A.; Huang, J.W.; De-la-Garza, B.G.; Huang, C.W.; Hesselink, J.R.; Bigler, E.D.; Wilde, E.A.; Max, J.E. Resting-State Magnetoencephalography Source Imaging Pilot Study in Children with Mild Traumatic Brain Injury. J. Neurotrauma 2020, 37, 994–1001. [Google Scholar] [CrossRef]
- Huang, M.X.; Huang, C.W.; Harrington, D.L.; Nichols, S.; Robb-Swan, A.; Angeles-Quinto, A.; Le, L.; Rimmele, C.; Drake, A.; Song, T.; et al. Marked Increases in Resting-State MEG Gamma-Band Activity in Combat-Related Mild Traumatic Brain Injury. Cereb. Cortex 2020, 30, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.X.; Nichols, S.; Robb, A.; Angeles, A.; Drake, A.; Holland, M.; Asmussen, S.; D’Andrea, J.; Chun, W.; Levy, M.; et al. An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. NeuroImage 2012, 61, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.X.; Angeles-Quinto, A.; Robb-Swan, A.; De-la-Garza, B.G.; Huang, C.W.; Cheng, C.K.; Hesselink, J.R.; Bigler, E.D.; Wilde, E.A.; Vaida, F.; et al. Assessing Pediatric Mild Traumatic Brain Injury and Its Recovery Using Resting-State Magnetoencephalography Source Magnitude Imaging and Machine Learning. J. Neurotrauma 2023, 40, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Alhourani, A.; Wozny, T.A.; Krishnaswamy, D.; Pathak, S.; Walls, S.A.; Ghuman, A.S.; Krieger, D.N.; Okonkwo, D.O.; Richardson, R.M.; Niranjan, A. Magnetoencephalography-based identification of functional connectivity network disruption following mild traumatic brain injury. J. Neurophysiol. 2016, 116, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.A.; Stapleton-Kotloski, J.R.; Alberto, G.E.; Rawley, J.A.; Kotloski, R.J.; Taber, K.H.; Godwin, D.W. Contrasting Effects of Posttraumatic Stress Disorder and Mild Traumatic Brain Injury on the Whole-Brain Resting-State Network: A Magnetoencephalography Study. Brain Connect. 2017, 7, 45–57. [Google Scholar] [CrossRef]
- Diwakar, M.; Harrington, D.L.; Maruta, J.; Ghajar, J.; El-Gabalawy, F.; Muzzatti, L.; Corbetta, M.; Huang, M.X.; Lee, R.R. Filling in the gaps: Anticipatory control of eye movements in chronic mild traumatic brain injury. NeuroImage Clin. 2015, 8, 210–223. [Google Scholar] [CrossRef]
- Robb Swan, A.; Nichols, S.; Drake, A.; Angeles, A.; Diwakar, M.; Song, T.; Lee, R.R.; Huang, M.X. Magnetoencephalography Slow-Wave Detection in Patients with Mild Traumatic Brain Injury and Ongoing Symptoms Correlated with Long-Term Neuropsychological Outcome. J. Neurotrauma 2015, 32, 1510–1521. [Google Scholar] [CrossRef]
- Zhang, J.; Safar, K.; Emami, Z.; Ibrahim, G.M.; Scratch, S.E.; da Costa, L.; Dunkley, B.T. Local and large-scale beta oscillatory dysfunction in males with mild traumatic brain injury. J. Neurophysiol. 2020, 124, 1948–1958. [Google Scholar] [CrossRef]
- Dunkley, B.T.; Da Costa, L.; Bethune, A.; Jetly, R.; Pang, E.W.; Taylor, M.J.; Doesburg, S.M. Low-frequency connectivity is associated with mild traumatic brain injury. NeuroImage Clin. 2015, 7, 611–621. [Google Scholar] [CrossRef]
- Zhang, J.; Emami, Z.; Safar, K.; McCunn, P.; Richardson, J.D.; Rhind, S.G.; da Costa, L.; Jetly, R.; Dunkley, B.T. Teasing apart trauma: Neural oscillations differentiate individual cases of mild traumatic brain injury from post-traumatic stress disorder even when symptoms overlap. Transl. Psychiatry 2021, 11, 345. [Google Scholar] [CrossRef]
- Li, L.; Arakaki, X.; Harrington, M.; Zouridakis, G. Source Connectivity Analysis Can Assess Recovery of Acute Mild Traumatic Brain Injury Patients. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 3165–3168. [Google Scholar] [CrossRef]
- Pang, E.W.; Dunkley, B.T.; Doesburg, S.M.; da Costa, L.; Taylor, M.J. Reduced brain connectivity and mental flexibility in mild traumatic brain injury. Ann. Clin. Transl. Neurol. 2015, 3, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Antonakakis, M.; Dimitriadis, S.I.; Zervakis, M.; Papanicolaou, A.C.; Zouridakis, G. Mining cross-frequency coupling microstates from resting state MEG: An application to mild traumatic brain injury. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5513–5516. [Google Scholar] [CrossRef]
- Lawton, T.; Huang, M.X. Dynamic cognitive remediation for a Traumatic Brain Injury (TBI) significantly improves attention, working memory, processing speed, and reading fluency. Restor. Neurol. Neurosci. 2019, 37, 71–86. [Google Scholar] [CrossRef]
- Shah-Basak, P.P.; Urbain, C.; Wong, S.; da Costa, L.; Pang, E.W.; Dunkley, B.T.; Taylor, M.J. Concussion Alters the Functional Brain Processes of Visual Attention and Working Memory. J. Neurotrauma 2018, 35, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Rier, L.; Zamyadi, R.; Zhang, J.; Emami, Z.; Seedat, Z.A.; Mocanu, S.; Gascoyne, L.E.; Allen, C.M.; Scadding, J.W.; Furlong, P.L.; et al. Mild traumatic brain injury impairs the coordination of intrinsic and motor-related neural dynamics. NeuroImage Clin. 2021, 32, 102841. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; Hughes, J.D.; Popescu, E.A.; Riedy, G.; Degraba, T.J. Reduced prefrontal MEG alpha-band power in mild traumatic brain injury with associated posttraumatic stress disorder symptoms. Clin. Neurophysiol. 2016, 127, 3075–3085. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; Vandewouw, M.; Emami, Z.; Bells, S.; Rudberg, N.; da Costa, L.; Dunkley, B.T. Memory retrieval brain-behavior disconnection in mild traumatic brain injury: A magnetoencephalography and diffusion tensor imaging study. Hum. Brain Mapp. 2022, 43, 5296–5309. [Google Scholar] [CrossRef]
- Kaltiainen, H.; Liljeström, M.; Helle, L.; Salo, A.; Hietanen, M.; Renvall, H.; Forss, N. Mild Traumatic Brain Injury Affects Cognitive Processing and Modifies Oscillatory Brain Activity during Attentional Tasks. J. Neurotrauma 2019, 36, 2222–2232. [Google Scholar] [CrossRef]
- Kaltiainen, H.; Helle, L.; Liljeström, M.; Renvall, H.; Forss, N. Theta-Band Oscillations as an Indicator of Mild Traumatic Brain Injury. Brain Topogr. 2018, 31, 1037–1046. [Google Scholar] [CrossRef]
- Itälinna, V.; Kaltiainen, H.; Forss, N.; Liljeström, M.; Parkkonen, L. Using normative modeling and machine learning for detecting mild traumatic brain injury from magnetoencephalography data. PLoS. Comput. Biol. 2023, 19, e1011613. [Google Scholar] [CrossRef]
- Vakorin, V.A.; Doesburg, S.M.; da Costa, L.; Jetly, R.; Pang, E.W.; Taylor, M.J. Detecting Mild Traumatic Brain Injury Using Resting State Magnetoencephalographic Connectivity. PLoS Comput. Biol. 2016, 12, e1004914. [Google Scholar] [CrossRef]
- Dimitriadis, S.I.; Zouridakis, G.; Rezaie, R.; Babajani-Feremi, A.; Papanicolaou, A.C. Functional connectivity changes detected with magnetoencephalography after mild traumatic brain injury. NeuroImage Clin. 2015, 9, 519–531. [Google Scholar] [CrossRef]
- Peitz, G.W.; Wilde, E.A.; Grandhi, R. Magnetoencephalography in the Detection and Characterization of Brain Abnormalities Associated with Traumatic Brain Injury: A Comprehensive Review. Med. Sci. 2021, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Antonakakis, M.; Schrader, S.; Wollbrink, A.; Oostenveld, R.; Rampp, S.; Haueisen, J.; Wolters, C.H. The effect of stimulation type, head modeling, and combined EEG and MEG on the source reconstruction of the somatosensory P20/N20 component. Hum. Brain Mapp. 2019, 40, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, D.R.; Engdahl, B.E.; Leuthold, A.; Georgopoulos, A.P. Assessing Recovery from Mild Traumatic Brain Injury (Mtbi) using Magnetoencephalography (MEG): An Application of the Synchronous Neural Interactions (SNI) Test. J. Neurol. Neuromed. 2020, 5, 28–34. [Google Scholar] [CrossRef]
- da Costa, L.; Robertson, A.; Bethune, A.; MacDonald, M.J.; Shek, P.N.; Taylor, M.J.; Pang, E.W. Delayed and disorganised brain activation detected with magnetoencephalography after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Proskovec, A.L.; Shah, B.R.; Yu, F.F.; Achilleos, M.; Maldjian, J.A.; Davenport, E.M. Magnetoencephalography and Mild Traumatic Brain Injury. Adv. Clin. Radiol. 2020, 2, 341–350. [Google Scholar] [CrossRef]
- Solar, K.G.; Ventresca, M.; Zamyadi, R.; Zhang, J.; Jetly, R.; Vartanian, O.; Rhind, S.G.; Dunkley, B.T. Repetitive subconcussion results in disrupted neural activity independent of concussion history. Brain Commun. 2024, 6, fcae348. [Google Scholar] [CrossRef]
- Aaltonen, J.; Heikkinen, V.; Kaltiainen, H.; Salmelin, R.; Renvall, H. Sensor-level MEG combined with machine learning yields robust classification of mild traumatic brain injury patients. Clin. Neurophysiol. 2023, 153, 79–87. [Google Scholar] [CrossRef]
- Safar, K.; Zhang, J.; Emami, Z.; Gharehgazlou, A.; Ibrahim, G.; Dunkley, B.T. Mild traumatic brain injury is associated with dysregulated neural network functioning in children and adolescents. Brain Commun. 2021, 3, fcab044. [Google Scholar] [CrossRef]
- Mardell, L.C.; Spedden, M.E.; O’Neill, G.C.; Tierney, T.M.; Timms, R.C.; Zich, C.; Barnes, G.R.; Bestmann, S. Concurrent spinal and brain imaging with optically pumped magnetometers. J. Neurosci. Methods 2024, 406, 110131. [Google Scholar] [CrossRef]
- Geerligs, L.; Renken, R.J.; Saliasi, E.; Maurits, N.M.; Lorist, M.M. A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cerebral. Cortex 2015, 25, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.A.; Erhardt, E.B.; Damaraju, E.; Gruner, W.; Segall, J.M.; Silva, R.F.; Havlicek, M.; Rachakonda, S.; Fries, J.; Kalyanam, R.; et al. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011, 5, 2. [Google Scholar] [CrossRef]
- Yener, G.; Kıyı, İ.; Düzenli-Öztürk, S.; Yerlikaya, D. Age-Related Aspects of Sex Differences in Event-Related Brain Oscillatory Responses: A Turkish Study. Brain Sci. 2024, 14, 567. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Cheung, V.C.K.; Chan, R.H.M. Aging amplifies sex differences in low alpha and low beta EEG oscillations. bioRxiv 2024, 603949. [Google Scholar] [CrossRef]
- Bittencourt-Villalpando, M.; van der Horn, H.J.; Maurits, N.M.; van der Naalt, J. Disentangling the effects of age and mild traumatic brain injury on brain network connectivity: A resting state fMRI study. NeuroImage Clin. 2021, 29, 102534. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.S.; Kim, J.H.; Yoon, S.H.; Lee, S.; Park, K.-J.; Ha, S.-K.; Choi, J.-G.; Jo, K.-W.; Kim, J.; Kang, S.H.; et al. Gender differences in adult traumatic brain injury according to the Glasgow coma scale: A multicenter descriptive study. Chin. J. Traumatol. 2021, 24, 333–343. [Google Scholar] [CrossRef]
- Wågberg, S.; Stålnacke, B.M.; Magnusson, B.M. Gender and Age Differences in Outcomes after Mild Traumatic Brain Injury. J. Clin. Med. 2023, 12, 4883. [Google Scholar] [CrossRef]
- Levin, H.S.; Temkin, N.R.; Barber, J.; Nelson, L.D.; Robertson, C.; Brennan, J.; Stein, M.B.; Yue, J.K.; Giacino, J.T.; McCrea, M.A.; et al. Association of Sex and Age with Mild Traumatic Brain Injury–Related Symptoms: A TRACK-TBI Study. JAMA Netw. Open 2021, 4, e213046. [Google Scholar] [CrossRef]
- Vakhtin, A.A.; Zhang, Y.; Wintermark, M.; Ashford, J.W.; Furst, A.J. Distant histories of mild traumatic brain injury exacerbate age-related differences in white matter properties. Neurobiol. Aging 2021, 107, 30–41. [Google Scholar] [CrossRef]
- Starkey, N.J.; Duffy, B.; Jones, K.; Theadom, A.; Barker-Collo, S.; Feigin, V.; BIONIC8 Research Group. Sex differences in outcomes from mild traumatic brain injury eight years post-injury. PLoS ONE 2022, 17, e0269101. [Google Scholar] [CrossRef]
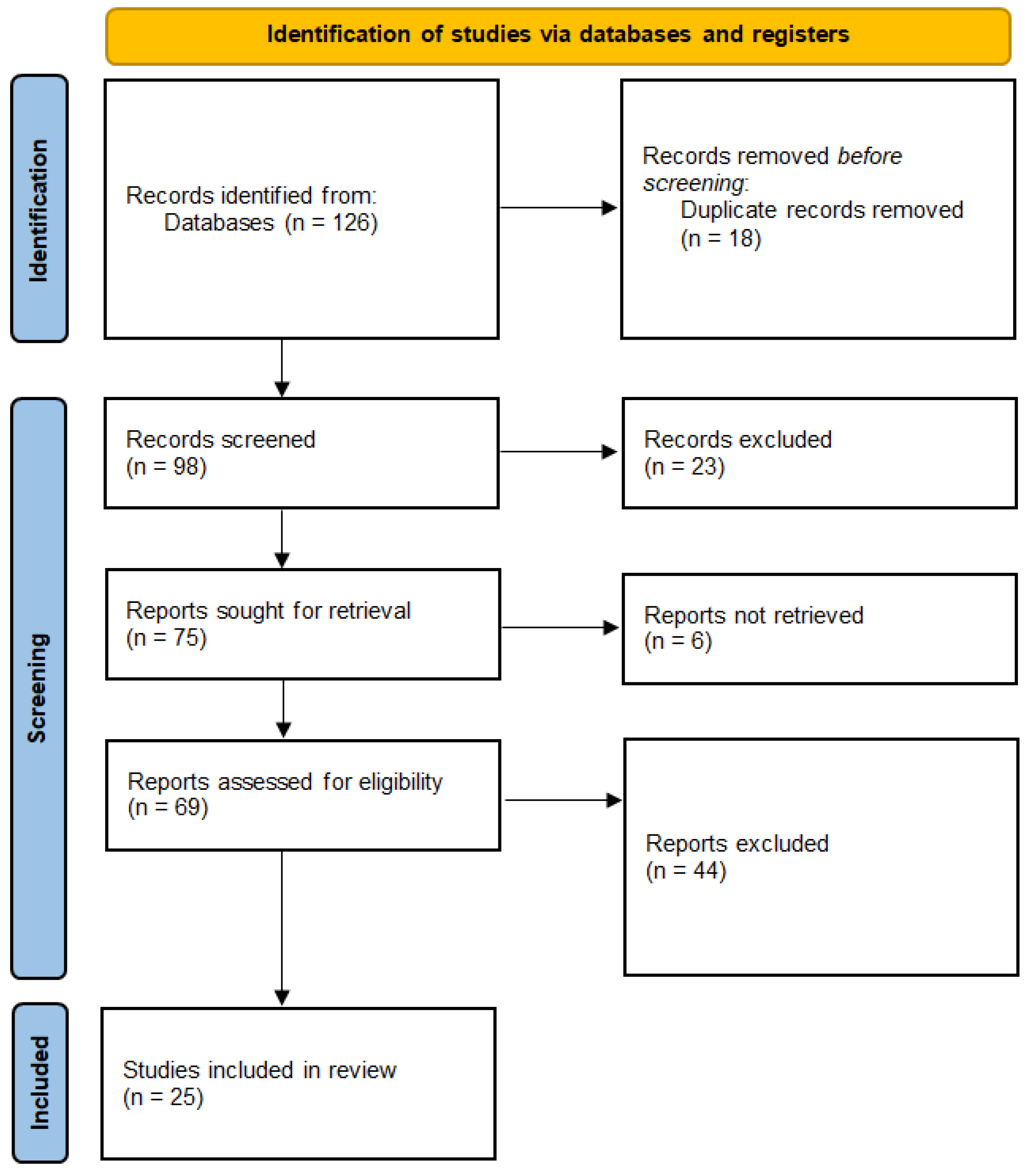
| Ref | Aim | Type | Design | Intervention | TIA | Outcomes | Conclusion |
|---|---|---|---|---|---|---|---|
| [21] | Evaluating the potential of MEG and DTI integrated imaging in detecting mTBI | OS | 10 post-acute mTBI (aged 25.0 ± 11.5, 1 female soccer player, 3 male sport players, 4 male veterans, 2 males who had suffered motor vehicle accidents); 14 HCs (aged 27.4 ± 15.2) | Resting-state MEG while awake, eyes opened; frequency bands: delta waves (1–4 Hz); analysis methods: functional connectivity, source localization with VESTAL. | 1–46 months | Subtle neuronal injury detected by MEG, not visible by CT or MRI; abnormal delta waves detected by MEG (in many brain areas, such as dorsal lateral prefrontal cortex, middle frontal gyrus, orbital frontal cortex, anterior cingulate cortex, and parietal–temporal junction regions), not seen by DTI. | MEG is more sensitive than traditional imaging techniques for diagnosing mTBI. |
| [22] | Evaluating task-related MEG in detecting functional deficits in mTBI | OS | 25 male veterans with history of mTBI due to blasts or military training; 20 male veterans (HCs) | Task-related MEG (N-back WM task); frequency bands: alpha (8–12 Hz), beta (15–30 Hz), gamma (30–90 Hz), and low-frequency (1–7 Hz); analysis methods: functional connectivity, source localization with fast-VESTAL. | ~10 months | Functional changes across several brain areas (increased signals in frequency bands across frontal pole, ventromedial prefrontal cortex, orbitofrontal cortex, and anterior dorsolateral prefrontal cortex; decreased signals in anterior cingulate cortex) correlated with slower reaction times in the working memory task. | Task-related MEG could evaluate functional deficits correlated with cognitive impairments. |
| [23] | Evaluating resting-state MEG as a potential diagnosis tool in pediatric mTBI patients | OS | 24 children aged 8–15 years: 12 mTBI due to closed head injury (83% males, 8 falls, 3 head collisions, 1 motor vehicle accident); 12 HC or OTC (92% males) | Resting-state MEG, 3 × 5′ eyes-closed sessions alternating with 3 × 5′ eyes opened sessions; frequency bands: alpha band (8–12 Hz), beta band (15–30 Hz), gamma band (30–80 Hz), high-gamma band (80–150 Hz), and—combined delta (1–4 Hz) and theta bands (4–7 Hz); analysis methods: functional connectivity, source localization with fast-VESTAL. | ~6 months | Changes in alpha (hyperactivation—prefrontal cortex, hypoactivation—parahippocampus and insula), beta, gamma (frontal, temporal, occipital, and cerebella regions), and low-frequency bands of mTBI patients during resting-state scanning; differences between adult and children mTBI scan patterns could occur. | Resting-state MEG could be used to discriminate between mTBI and non-mTBI children. |
| [24] | Evaluating resting-state MEG gamma bands as a potential biomarker for mTBI | OS | 60 males active/retired military personnel: 25 mTBI due to blasts (aged 28 ± 7.52 years); 35 HCs (aged 29 ± 5 years). | Resting-state MEG, 2 × 5′ sessions with eyes closed, 2 × 5′ sessions with eyes open; frequency bands: gamma waves (30–80 Hz); analysis methods: functional connectivity, source localization with fast-VESTAL. | 19.5 months | Significant changes in gamma band activity of mTBI patients, as compared to HC during resting-state scanning: hyperactivation in prefrontal cortex, supplementary motor area and right premotor cortex, posterior parietal areas, superior temporal gyri, occipital areas, right cerebellum. | Gamma waves activity evaluated by resting-state MEG could be a promising biomarker of mTBI. |
| [25] | Evaluating the potential of automated MEG in diagnosing mTBI | RCT | 55 mTBI patients: 23 male military personnel (blast injuries, aged 26.0 ± 5.3 years); 22 civilians (mTBIs diverse injuries, aged 29.1 ± 13.3 years, 68% males); 10 civilians (moderate TBI diverse injuries, aged 29.2 ± 13.2 years, 80% males); 44 HC (aged 26.5 ± 8.0 years, 84% males). | Resting-state MEG; Frequency bands: delta waves (1–4 Hz); analysis methods: functional connectivity, source localization with frequency domain VESTAL. | 4 weeks–3 years | Abnormal delta waves activity was found in 87% of mTBIs and 100% of moderate TBIs in 6 to 8 cortical regions; delta waves activity evaluated via resting-state MEG could discriminate between different sources of mTBI (i.e., blast versus non-blast); A correlation was established between abnormal slow-wave positive brain regions and PCS scores. | MEG low-frequency source imaging could be used to support the clinical diagnosis of TBI and PCS. MEG could perform significantly better in finding low-frequency waves changes in contrast to conventional neuroimaging approaches. |
| [26] | Evaluating the potential of automated resting-state MEG in diagnosing pediatric mTBI | LS | 59 mTBI pediatric patients, aged 12.05 ± 2.22 years, 66.1% males; 39 OTC pediatric patients (upper or lower limb fractures, aged 11.18 ± 1.88 years, 64.1% males) | Resting-state MEG, 2 × 5′ sessions, eyes closed; frequency bands: delta (1–4 Hz) and gamma (30–80 Hz) bands; analysis methods: functional connectivity, source localization with fast-VESTAL. | 3 weeks; 7 weeks; 12 weeks. | Changes were found for delta and gamma bands in frontal and temporal lobes: increased right and left frontal pole gamma band frequency—hypothesized mTBI imaging marker; resting state-MEG with machine learning successfully discriminated between mTBI and OTC. | Resting-state MEG could be used to support the clinical diagnosis of mTBI. |
| [27] | Detecting functional connectivity network disruption in mTBI | OS | 9 mTBI patients, 4 females, aged 14–62 years; 15 HCs | Resting-state MEG, 2–5′ sessions with eyes opened; frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz) bands; analysis methods: functional connectivity with phase locking value. | 3 months–3 years | Significant changes in brain activity were reported for delta, alpha, and beta frequency bands in superior parietal, cuneus, precuneus, inferior parietal, lateral occipital, and supramarginal lobules, with prominent beta phase locking reduction in the temporal lobes of mTBI patients. | MEG could detect brain activity changes in multiple frequency bands in mTBI. |
| [28] | Network disruption in mTBI | OS | 6 mTBI patients (male veterans, aged 39.0 ± 9.5 years); 10 HCs | Resting-state MEG, 8′ session eyes opened; frequency bands: delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), gamma (30–80 Hz), as well as DC-80 Hz bands; analysis methods: functional connectivity between nodes using weighted phase lag index. | 6.2 years | Resting-state MEG revealed that mTBI patients are not characterized by changes in alpha network metrics but by increased small worldness in the wideband network. | Graph-based analysis of resting-state MEG scans could be a valuable tool in diagnosing mTBI and co-morbidities. |
| [29] | Anticipatory control of eye movements in chronic mTBI | RCT | 25 mTBI + PCS patients (aged 32.7 ± 11.24 years, 84% males, 4 motor vehicle accidents, 13 sport-related injuries, 5 falls and head blows); 25 HCs (aged 31.8 ± 10.6, 68% males) | Task-related MEG, 3 eyes movement task sessions; frequency bands: alpha (8–13 Hz), beta (15–30 Hz), and gamma (30–100 Hz) bands; analysis methods: functional connectivity, source localization with fast-VESTAL. | ~32 months post-injury | Mild TBI patients performed poorer in the eye movement control task using gapped trajectories, as compared to HCs, and were correlated with beta band changes in ten brain regions. | Beta bands amplitude, as evaluated by MEG, could be used as a potent differentiator between mTBI and HCs. |
| [30] | Slow-wave detection in mTBI | RCT | 31 mTBI + PCS patients (veterans, aged 26.6 ± 6.1 years, 90.3% males); 33 HCs (veterans, aged 26.3 ± 8.3, 93.9% males) | Resting-state MEG, 3 × 4′ eyes closed sessions; frequency bands: delta bands (1–6 Hz); analysis methods: functional connectivity, source localization with fast-VESTAL. | ~3 months | Abnormal MEG slow-wave activity correlates with cognitive impairments (increased brain waves in prefrontal cortex and temporal areas; decreased brain waves in occipital area). | MEG provides objective evidence of brain injury in mTBI with PCS patients. |
| [31] | Exploring the beta waves changes in mTBI male patients | RCT | 27 mTBI male patients (aged 29.6 ± 6.7 years, 12 sports injuries, 7 motor vehicle accidents, 4 falls, 4 other injuries); 23 male HCs (aged 28.0 ± 5.6 years). | Resting-state MEG, 1 × 5′ eyes-open session; frequency bands: alpha (8–14 Hz), beta band (15–30 Hz); analysis methods: functional connectivity, Fitting Oscillations and One-Over F (FOOOF) algorithm. | <3 months | Thalamocortical dysconnectivity was identified in mTBI patients. Alpha peaks were seen in mTBI visual cortexes. Reduced beta band frequency was seen in bilateral frontal and temporal cortices of mTBI patients, with the lowest negative peak in the right middle frontal gyrus. Beta band functional connectivity could be used to classify biomarkers for mTBI, with better performance as compared to SCAT2. | Beta band functional connectivity assessed by MEG could be used to diagnose mTBI. |
| [32] | Exploring the functional connectivity status in mTBI | OS | 20 mTBI (males, 31.4 ± 6.85 years, 7 sports injuries, 7 motor vehicle accidents, 4 falls, 2 other injuries); 21 HCs (males, aged 27.0 ± 5 years) | Resting-state MEG, eyes opened; frequency bands: delta (1–4 Hz), theta (4–7 Hz), alpha (8–14 Hz), beta (15–30 Hz), low gamma (30–80 Hz) and high gamma (80–150 Hz); analysis methods: functional connectivity. | <3 months | Low-frequency functional connectivity affected in mTBI. Alpha bands reduced in parietal regions of mTBI affected brains; delta and theta band amplitudes increases were seen in the left hemisphere, left frontal, left temporal, and subcortical regions, and right posterior cingulate cortex of the mTBI-affected brains. | Low-frequency functional connectivity makers could be used in diagnosing mTBI. |
| [33] | Exploring the potential of neural oscillations to differentiate mTBI from PTSD | OS | 27 mTBI (military personnel, aged 29.6 ± 6.7 years; 23 HCs (civilians, aged 28.0 ± 5.6 years); | Resting-state MEG, 1 × 5′ eyes opened session, fixed point; frequency bands: delta (1–3 Hz), theta (4–7 Hz), alpha (8–14 Hz), beta (15–30 Hz), low gamma one (30–55 Hz), low gamma two (65–80 Hz), and high gamma (80–150 Hz); analysis methods: functional connectivity. | <3 months | The regional power from alpha and beta bands could be used as diagnosis markers for mTBI. | MEG could be used to detect subtle brain functional connectivity changes in mTBI. |
| [34] | Exploring the potential of MEG to assess recovery from mTBI | LS | 13 mTBI patients (aged 25.6 years, 54% males); 8 OTC (aged 27.2 years, 50% males). | Resting-state MEG, 1 × 5′ eyes-closed session; frequency bands: 0.1–4 Hz (delta), 4–8 Hz (theta), 8–10.5 Hz (alpha1), 10.5–13 Hz (alpha2), 13–20 Hz (beta1), 20–30 Hz (beta2), 30–40 Hz (gamma1), and 40–80 Hz (gamma2); analysis methods: source power analysis, BEM (boundary element method), functional connectivity. | Not reported | Significant right entorhinal and the left supramarginal gyrus in-flow connectivity in mTBI. Hyperactivity of delta band was seen in mTBI-affected brains (right isthmus cingulate, left pars triangularis, right postcentral, right precentral, and left precuneus). Stronger delta connections were seen in mTBI patients, as compared to OTC. | MEG could be used as a diagnosis tool for mTBI, with the delta band as a potential imagining biomarker. |
| [35] | Exploring the potential of MEG to detect brain connectivity alterations in mTBI | RCT | 16 mTBI male patients (civilians with motor vehicle and sports accidents, aged 31.0 ± 7.5 years); 16 male HCs (aged 27.7 ± 5.3 years) | Task-related MEG, tasked session of 10–30′; frequency bands: theta (4–7 Hz), alpha (8–14 Hz), beta (15–30 Hz), and gamma (30–80 Hz); analysis methods: functional connectivity. | <2 months | Brain connectivity was reduced in mTBI, as compared to HCs, when evaluated by task-related MEG. Lower connectivity was seen for alpha band in the brains of mTBI patients, in occipital cortices. | MEG could be used to detect brain connectivity issues in mTBI. |
| [36] | Exploring the potential of resting state MEG to predict the occurrence of mTBI | OS | 30 mTBI patients (aged 29.33 ± 9.2 years); 50 HCs (aged 29.25 ± 9.1 years). | Resting-state MEG, 1 × 3–5′ eyes-closed session; frequency bands: delta (0.5–4 Hz), beta (15–30 Hz) and gamma1 (30– 45 Hz); analysis methods: time-varying cross-frequency coupling. | <24 h | Dynamic network analysis showed microstate-related transitions in mTBI, as compared to HCs. | MEG could be employed to diagnose mTBI by identifying a dynamic connectome biomarker based on functional integration. |
| [37] | Exploring the potential of task-related MEG to detect cognitive remediation in mTBI | RCT | 4 mTBI male patients (aged 15, 50, 62, and 68 years) | Task-related MEG (N-back WM task); frequency bands: beta band (15–30 Hz); analysis methods: sensor-waveform covariance matrix, source localization with fast-VESTAL. | Not reported | MEG brain imaging successfully detected changes in activity of several brain networks. Signal increases were seen in the prefrontal cortex, orbitofrontal cortex, ventrolateral prefrontal cortex, medial posterior parietal cortex, premotor cortex, and dorsal cingulate/medial premotor cortex. | MEG brain imaging could be used to detect brain network activity mTBI. |
| [38] | Exploring the potential of MEG to detect functional connectivity alterations and correlations with visual attention and working memory in mTBI | RCT | 18 mTBI male patients (aged 30 ± 7.3 years, 6 sports injuries, 4 falls, 4 motor vehicle accidents, 3 other accidents, and 1 work-related injury); 19 male HCs (aged 27 ± 5.2 years) | Task-related MEG (visual working memory 1-back task); frequency bands: multiple (1–50 Hz); analysis methods: functional connectivity, source localization with fast-VESTAL. | 1.2 months | MEG was able to detect abnormal brain waves patterns in concussed patients (increased in left lingual gyrus and right hippocampus; decreased in right superior parietal, inferior parietal, and the left middle frontal cortices), correlated with visual working memory processing, attention, processing, and retrieval. The changes in frequency bands were correlated with symptom severity. | MEG could be used to discriminate between mTBI and HCs based on brain activity and cognitive tasks. |
| [39] | Evaluating the potential of MEG-related functional connectivity as a diagnosis tool for mTBI | OS | 29 male mTBI patients; 23 male HCs | Resting-state MEG, 1 × 5′ eyes-opened session and motor-task MEG; frequency bands: multiple; analysis methods: functional connectivity, source localization with hidden Markov model. | <3 months | By using MEG in resting state and during motor tasks, it was shown that mTBI disrupts the structural framework underlying neural synchrony and network functions, which are both very subtle consequences of head trauma. | Subtle changes in brain connectivity could be assessed by resting-state and task-related MEG. |
| [40] | Evaluating alpha-band power in mTBI with PTSD | OS | 32 male mTBI patients (active-duty service members) | Resting-state MEG, 1 × 5′ closed-eyes session; frequency bands: alpha band (8–13 Hz); analysis methods: functional connectivity, source localization. | 6 months to 11 years | Decreased alpha-band power was correlated with loss of consciousness and severity of aversive psychological traumatic stimuli. Decreased alpha band power was seen in mTBI patients more severely affected by PTSD in the superior frontal cortex, rostral middle frontal cortex, caudal middle frontal cortex, and frontal pole. | MEG could be used to detect PTSD associated with mTBI. |
| [41] | Evaluating the potential of MEG and DTI to detect memory retrieval brain–behavior disconnection in mTBI | OS | 23 male mTBI patients (aged 29.9 ± 6.9 years); 18 male HCs (aged 27.3 ± 5.3 years) | Task-related MEG (modified one-back visual working memory task); frequency bands: continuous scan for 1–50 Hz wavelengths; analysis methods: functional connectivity, source localization. | 2 weeks–3 months | MEG was able to detect functional disruptions in the brain activity of mTBI patients: limbic–prefrontal circuitry, thalamo-hippocampus process, amygdala activation. | MEG could be used to detect brain functional disruptions in mTBI. |
| [42] | Exploring the utility of MEG in detecting cognitive impairments in mTBI during cognitive tasks | LS | 25 mTBI patients (aged 42 ± 2 years, 56% males, 15 sports injuries, 6 motor vehicles accidents, 4 falls); 20 HCs (aged 39 ± 2 years, 60% males). | Resting-state MEG (1 × 10′ eyes-opened and 1 × 10′ eyes-closed sessions), task-related MEG (paced auditory serial addition test, vigilance test); frequency bands: alpha (8–14 Hz); analysis methods: source modeling of measured oscillatory brain activity. | <6 months | During cognitive tasks, mTBI patients registered decreased alpha band frequencies in task-relevant cortical regions (inferior parietal lobule and frontal cortex). MEG detected significant changes in oscillatory alpha activity in mTBI, as compared to HCs. | MEG could be used to discriminate between mTBI and HCs. |
| [43] | Exploring the potential of theta-band oscillations as diagnosis biomarkers for mTBI | LS | 26 mTBI patients (aged 41 ± 2 years, 57% males); 139 HCs (aged 31 ± 1 years, 26% males) | Resting-state MEG (1 × 10′ eyes opened and 1 × 10′ eyes closed sessions); frequency bands: low-frequency ranges (0.5–7 Hz); analysis methods: functional connectivity, source localization with cortically constrained L2 minimum-norm estimate. | 4 weeks and 6–7 months | Structural lesions in mTBI could be correlated with aberrant theta band activity (+ 2 standard deviations from mean). | Theta-band oscillations, as assessed by MEG, could be a valuable biomarker for brain dysfunctions in mTBI. |
| [44] | Evaluating the potential of machine learning-assisted MEG in detecting mTBI | LS | 25 mTBI patients (aged 41 ± 2 years, 57% males); 20 HCs (aged 39 years, 60% males); 621 HCs—as normative data. | Resting-state MEG (1 × 10′ eyes closed sessions); frequency bands: multiple bands; analysis methods: source modeling of measured oscillatory brain activity. | <6 months | Mild TBI patients’ brain oscillatory patterns were characterized by higher average activation at 10, 20, and 30 Hz. Theta-band activity was found to be a significant indicator of mTBI. | MEG is a potent tool in mTBI diagnosis. |
| [45] | Evaluating the potential of MEG in detecting mTBI-associated injuries | RCT | 20 male mTBI patients (aged 31 ± 7 years; 21 male HCs (aged 27 ± 5 years) | Resting-state MEG (1 × 5′ resting session); frequency bands: multiple; analysis methods: functional connectivity, source localization | <3 months | Delta, gamma, and alpha frequency ranges were associated with mTBI injuries. Based on MEG scans, alterations in brain connectivity were correlated with clinical symptom severity. | MEG could be used as a diagnosis tool in mTBI. |
| Ref | Outcomes | Conclusion |
|---|---|---|
| [27] | Disruption of functional connectivity in default mode network linked to cognitive deficits in mTBI patients. | MEG reveals network alterations that contribute to PCS-related cognitive issues. |
| [28] | Reduced local efficiency and functional connectivity in mTBI patients. | MEG provides detailed insight into network-level brain communication deficits after mTBI. |
| [29] | Functional connectivity was found to be affected by mTBI and correlated with cognitive tasks related to perception and motor control. | MEG could be used to detect functional connectivity changes in mTBI. |
| [31] | Functional connectivity patterns could be used in differentiating mTBI from PTSD. | MEG could be used to detect subtle brain functional connectivity changes in mTBI. |
| [32] | Low-frequency functional connectivity affected in mTBI. | Low-frequency functional connectivity makers could be used in diagnosing mTBI. |
| [36] | Brain connectivity was reduced in mTBI, as compared to HCs, when evaluated by task-related MEG. | MEG could be used to detect brain connectivity issues in mTBI. |
| [39] | Mild TBI disrupts the structural framework underlying neural synchrony and network functions, which are both very subtle consequences of head trauma. | Subtle changes in brain connectivity could be assessed by resting-state and task-related MEG. |
| [41] | Functional connectivity changes were seen in mTBI. | MEG could be used to detect functional connectivity issues in mTBI. |
| Ref | Outcomes | Conclusion |
|---|---|---|
| [28] | Using MEG scans, PTSD was characterized by lower values of network metrics that demonstrate decreased local connectivity and a hierarchically impaired network structure. | Graph-based analysis of resting-state MEG scans could be a valuable tool in diagnosing mTBI and potentially differentiate PTSD from mTBI. |
| [33] | The regional power from alpha and beta bands could be used as diagnosis markers for mTBI and differentiated diagnosis between mTBI and PTSD, exhibiting the best performance in classification. | MEG could be used to differentiate between mTBI and PTSD. |
| [40] | Alpha-band power was significantly altered in mTBI patients with severe PTSD. | MEG could be used to detect PTSD associated with mTBI. |
| Ref | Outcomes | Conclusion |
|---|---|---|
| [26] | Resting-state MEG with machine learning successfully predicted recovery of mTBI patients from PCS at 3 months. | Resting-state MEG could be used to predict recovery from PCS at 3 months. |
| [34] | The dynamic analysis of brain connectivity over time revealed the decrease in differences between mTBI and OTC. | MEG could be used to assess recovery from mTBI. |
| [36] | Dynamic network analysis showed microstate-related transitions in mTBI, as compared to HCs. | MEG could be employed to diagnose mTBI by identifying a dynamic connectome biomarker based on functional integration. |
| [37] | Vision and cognitive deficits were improved by intervention and MEG brain imaging; using the Fast-VESTAL procedure successfully detected that movement discrimination training improved the time-locked activity of several brain networks. | MEG brain imaging could be used to detect cognitive recovery from mTBI. |
| Ref | Outcomes | Conclusion |
|---|---|---|
| [22] | Functional changes across several brain areas correlated with slower reaction times in the working memory task. | Task-related MEG could evaluate functional deficits correlated with cognitive impairments. |
| [24] | Greater gamma activity was correlated with poorer cognitive performance. | MEG could be a promising biomarker of mTBI and PCS-associated cognitive impairment. |
| [27] | Disruption of functional connectivity in default mode network linked to cognitive deficits in mTBI patients. | MEG reveals network alterations that contribute to PCS-related cognitive issues. |
| [29] | Functional changes across several brain areas correlated with poorer eye movement control in correlation with beta band changes in ten brain regions. | Task-related MEG could evaluate functional deficits correlated with attention and coordination of movement/response. |
| [32] | Several differences in functional connectivity of the brain were observed in secondary symptoms of mTBI, such as lack of attention, anxiety, and depression. | Functional connectivity changes could reveal the occurrence of overlapping cognitive sequelae in mTBI. |
| [36] | Altered brain connectivity was correlated with decreased performance in mental flexibility tasks. | A correlation between brain connectivity, as evaluated by MEG, and cognitive performance was established. |
| [37] | MEG with source localization with fast-VESTAL detected that movement discrimination training improved time-locked activity of several brain networks. | MEG brain imaging could be used to detect cognitive recovery from mTBI. |
| [38] | The changes in frequency bands were correlated with cognitive symptom severity. | MEG could be used to correlate functional connectivity changes with cognitive task performance. |
| [41] | Functional and structural disruptions in brain–behavior interactions during working memory tasks were seen in mTBI. | MEG could be used to track brain–behavior interactions. |
| [42] | Oscillatory alpha activity was correlated with auditory performance. | MEG could be used to correlate cognitive impairments in mTBI patients. |
| Ref | Outcomes | Conclusion |
|---|---|---|
| [21] | Subtle neuronal injury detected by MEG, not visible by CT or MRI. | MEG is more sensitive than traditional imaging techniques for diagnosing mTBI. |
| [33] | The regional power from alpha and beta bands could be used as diagnosis markers for mTBI and differentiated diagnosis between mTBI and PTSD. | MEG could be used to detect subtle brain functional connectivity changes in mTBI. |
| [38] | MEG was able to detect subtle abnormal brain waves patterns in concussed patients. | MEG could be used to detect subtle brain wave changes. |
| [39] | Disrupted structural framework underlying neural synchrony and network functions were found in mTBI. | Subtle changes in brain connectivity could be assessed by resting-state and task-related MEG. |
| Ref | Outcomes | Conclusion |
|---|---|---|
| [24] | Machine learning was used to predict the differentiation between mTBI and HCs. | Machine learning could improve diagnosis sensitivity. |
| [26] | Machine learning was used to complement imaging markers across delta-band and gamma-band frequencies. | Machine learning could improve delta–gamma potential in clinical diagnosis and recovery prediction. |
| [31] | The machine learning-based downstream data analysis pipeline was used to increase discriminative performance. Machine learning offered more accurate predictions, as compared to standard analysis. | Machine learning could increase the performance of diagnosis procedure. |
| [33] | Machine learning was used to consistently improve the analysis of subtle brain connectivity changes and discrimination potential. Machine learning performed better in identifying subtle brain connectivity changes and discriminating between mTBI and PTSD, as compared to standard analysis. | MEG could be used to differentiate mTBI from PTSD, with the aid of machine learning. |
| [39] | Machine learning was used to show that dynamic coordination of neural network activity is impaired in mTBI. | Machine learning could be used in the data processing of MEG scans to detect neural network activity changes specific to mTBI. |
| [44] | Machine learning could be used to successfully assist MEG to discriminate mTBI patients from HCs. | Machine learning could assist MEG in mTBI diagnosis. |
| Limitation | Description |
|---|---|
| Limited accessibility and increased costs | MEG remains a costly and specialized neuroimaging technique, available in only a limited number of clinical centers, posing accessibility restrictions in current clinical practice. The infrastructure and expertise required to conduct and interpret MEG scans can also pose logistical challenges. |
| Lower spatial resolution, as compared to other imaging techniques | While excelling in temporal resolution, the spatial resolution offered by MEG is inferior to functional MRI, limiting the accuracy of localization of the identified functional abnormalities. Combining MEG with other imaging modalities, such as DTI, may improve diagnostic precision but also adds to complexity and cost. |
| Study design heterogeneity | The variability of study designs, MEG data-processing methods, and sample sizes posed the most significant challenge. Moreover, the variation in the frequency of the analyzed bands, the conditions in which MEG was administered to the participants (resting-state versus task-based), and the criteria used for patient selection prevented the drawing of uniform conclusions. |
| Small sample sizes | The generalizability of the findings could be affected by small sample sizes. Larger, multi-centered studies are needed to validate its diagnostic efficacy across diverse populations and injury types. |
| Lack of standardized MEG protocols | The lack of standardized protocols for MEG data acquisition, analysis, and interpretation in mTBI patients prevents its use as a common evaluation tool in clinical practice. The development of globally accepted guidelines could be essential for clinicians and researchers. |
| Aspects to Be Developed | Description |
|---|---|
| Standardized MEG protocols | Establishing standardized MEG protocols for mTBI assessment is crucial for ensuring consistency across studies and enabling wider clinical application. This would involve standardizing data acquisition, pre-processing, and analysis pipelines, as well as defining universally accepted biomarkers for mTBI. |
| Combining MEG with other neuroimaging techniques | The integration of other imaging techniques, such as DTI or functional MRI, could improve both the spatial and temporal resolution of MEG-based scans. Also, the multimodal approach could improve the sensitivity of detecting structural and functional abnormalities and provide a more comprehensive understanding of mTBI. |
| Further machine learning and artificial intelligence integration | The analysis of MEG data assisted by machine learning shows promise in improving diagnostic accuracy and predicting clinical outcomes. Further development of AI models that can integrate complex MEG datasets, clinical information, and patient outcomes will enhance the utility of MEG as a predictive tool for mTBI prognosis. |
| Focusing on longitudinal studies | Longitudinal studies are essential to understand the dynamics of MEG biomarkers and their correlation with clinical recovery. Furthermore, these studies could provide valuable insights about treatment efficiency and rehabilitation strategies, particularly seen in chronic cases of mTBI and PCS. |
| Cost-effective optimization | Making MEG more cost-effective could be crucial in improving accessibility and diagnostic potential. The costly and non-portable MEG systems and the lack of lower-cost hardware or cloud-based analysis platforms prevents its broader use in clinical settings. |
| Differentiated diagnosis | The potential of MEG to discriminate between mTBI and other neurological conditions, such as PTSD, was significantly reported. Further research could focus on developing specific MEG-based biomarkers with increased accuracy in distinguishing between overlapping or co-occurring conditions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroudis, I.; Kazis, D.; Petridis, F.E.; Balmus, I.-M.; Ciobica, A. The Use of Magnetoencephalography in the Diagnosis and Monitoring of Mild Traumatic Brain Injuries and Post-Concussion Syndrome. Brain Sci. 2025, 15, 154. https://doi.org/10.3390/brainsci15020154
Mavroudis I, Kazis D, Petridis FE, Balmus I-M, Ciobica A. The Use of Magnetoencephalography in the Diagnosis and Monitoring of Mild Traumatic Brain Injuries and Post-Concussion Syndrome. Brain Sciences. 2025; 15(2):154. https://doi.org/10.3390/brainsci15020154
Chicago/Turabian StyleMavroudis, Ioannis, Dimitrios Kazis, Foivos E. Petridis, Ioana-Miruna Balmus, and Alin Ciobica. 2025. "The Use of Magnetoencephalography in the Diagnosis and Monitoring of Mild Traumatic Brain Injuries and Post-Concussion Syndrome" Brain Sciences 15, no. 2: 154. https://doi.org/10.3390/brainsci15020154
APA StyleMavroudis, I., Kazis, D., Petridis, F. E., Balmus, I.-M., & Ciobica, A. (2025). The Use of Magnetoencephalography in the Diagnosis and Monitoring of Mild Traumatic Brain Injuries and Post-Concussion Syndrome. Brain Sciences, 15(2), 154. https://doi.org/10.3390/brainsci15020154







