The Relation of Alpha Asymmetry to Physical Activity Duration and Intensity
Abstract
1. Introduction
2. Materials and Methods
2.1. Post Hoc Power Analysis
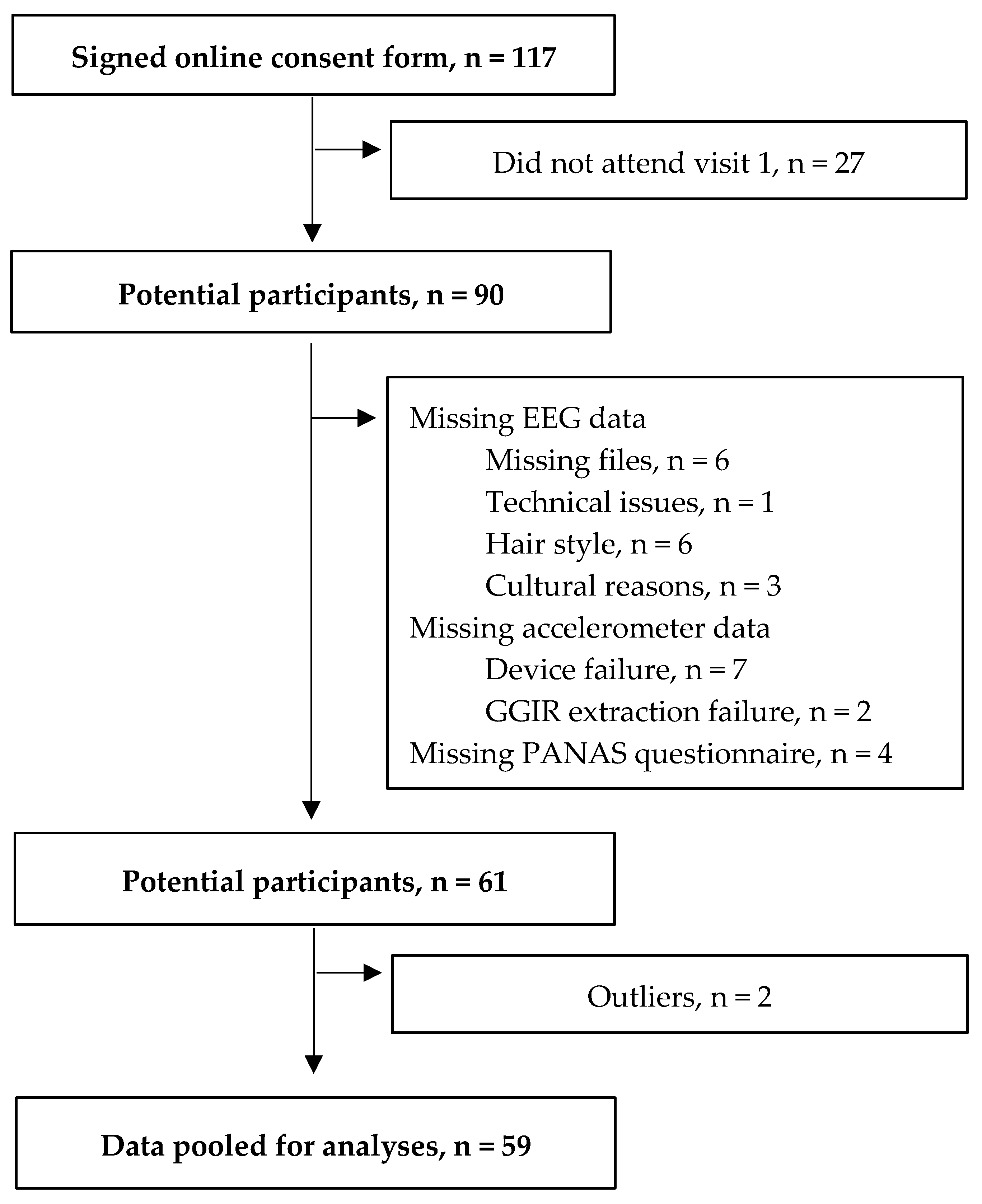
2.2. Participants
2.3. Measures
2.3.1. Accelerometry
2.3.2. EEG
Recording
Processing
AA
2.3.3. The Positive and Negative Affect Schedule (PANAS)
2.4. Procedure
2.5. Statistical Analysis
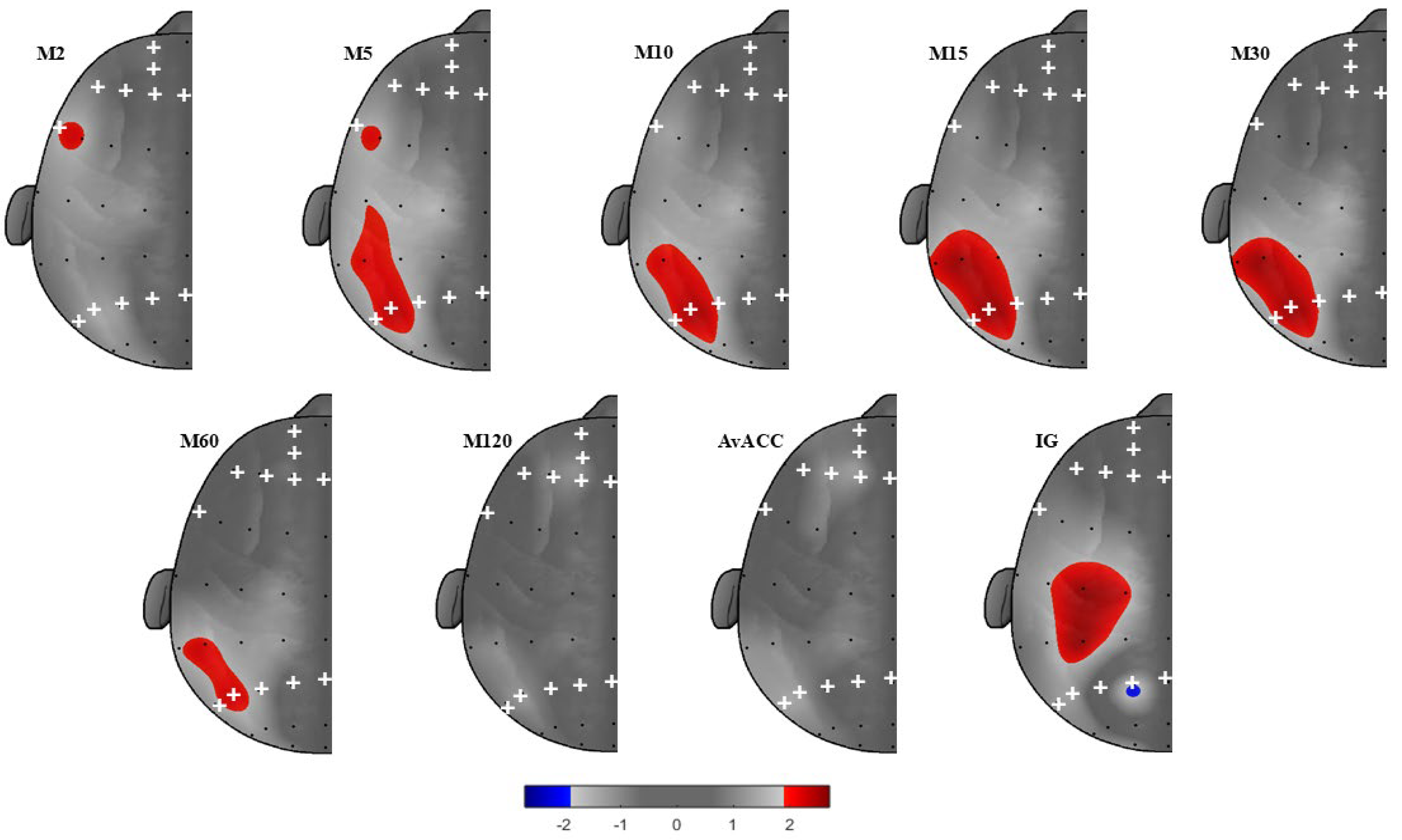
3. Results
Frontal and Parietal AA as Predictors of PA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018; p. 118.
- Matthews, C.E.; Carlson, S.A.; Saint-Maurice, P.F.; Patel, S.; Salerno, E.A.; Loftfield, E.; Troiano, R.P.; Fulton, J.E.; Sampson, J.N.; Tribby, C.; et al. Sedentary Behavior in U.S. Adults: Fall 2019. Med. Sci. Sports Exerc. 2021, 53, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, C.; Kantor, E.D.; Nguyen, L.H.; Zheng, X.; Park, Y.; Giovannucci, E.L.; Matthews, C.E.; Colditz, G.A.; Cao, Y.; et al. Trends in Sedentary Behavior Among the US Population, 2001–2016. JAMA 2019, 321, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Difrancesco, S.; Penninx, B.W.J.H.; Merikangas, K.R.; van Hemert, A.M.; Riese, H.; Lamers, F. Within-day bidirectional associations between physical activity and affect: A real-time ambulatory study in persons with and without depressive and anxiety disorders. Depression Anxiety 2022, 39, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Kekäläinen, T.; Luchetti, M.; Terracciano, A.; Gamaldo, A.A.; Sliwinski, M.J.; Sutin, A.R. Momentary Associations Between Physical Activity, Affect, and Purpose in Life. Ann. Behav. Med. 2024, 58, 752–762. [Google Scholar] [CrossRef]
- Pham, L.T.; Hernandez, R.; Spruijt-Metz, D.; Gonzalez, J.S.; Pyatak, E.A. Movement matters: Short-term impacts of physical activity on mood and well-being. J. Behav. Med. 2023, 46, 781–790. [Google Scholar] [CrossRef]
- Morris, T.P.; Burzynska, A.; Voss, M.; Fanning, J.; Salerno, E.A.; Prakash, R.; Gothe, N.P.; Whitfield-Gabrieli, S.; Hillman, C.H.; Mcauley, E.; et al. Brain Structure and Function Predict Adherence to an Exercise Intervention in Older Adults. Med. Sci. Sports Exerc. 2022, 54, 1483–1492. [Google Scholar] [CrossRef]
- Rodriguez-Ayllon, M.; Neumann, A.; Hofman, A.; Vernooij, M.W.; Neitzel, J. The bidirectional relationship between brain structure and physical activity: A longitudinal analysis in the UK Biobank. Neurobiol. Aging 2024, 138, 1–9. [Google Scholar] [CrossRef]
- Williams, D.M.; Dunsiger, S.; Ciccolo, J.T.; Lewis, B.A.; Albrecht, A.E.; Marcus, B.H. Acute affective response to a moderate-intensity exercise stimulus predicts physical activity participation 6 and 12 months later. Psychol. Sport Exerc. 2008, 9, 231–245. [Google Scholar] [CrossRef]
- Davidson, R.J. What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biol. Psychol. 2004, 67, 219–234. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Gable, P.A.; Peterson, C.K. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biol. Psychol. 2010, 84, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E.; Gable, P.A. On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology 2018, 55, e12879. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jia, S.; Wang, X.; Wang, X.; Wang, X. The impact of single sessions of aerobic exercise at varying intensities on depressive symptoms in college students: Evidence from resting-state EEG in the parietal region. BMC Psychiatry 2024, 24, 928. [Google Scholar] [CrossRef] [PubMed]
- Palmiero, M.; Piccardi, L. Frontal EEG Asymmetry of Mood: A Mini-Review. Front. Behav. Neurosci. 2017, 11, 224. [Google Scholar] [CrossRef]
- Silveira, R.; Prado, R.C.R.; Brietzke, C.; Coelho-Júnior, H.J.; Santos, T.M.; Pires, F.O.; Asano, R.Y. Prefrontal cortex asymmetry and psychological responses to exercise: A systematic review. Physiol. Behav. 2019, 208, 112580. [Google Scholar] [CrossRef]
- Laufs, H.; Kleinschmidt, A.; Beyerle, A.; Eger, E.; Salek-Haddadi, A.; Preibisch, C.; Krakow, K. EEG-correlated fMRI of human alpha activity. NeuroImage 2003, 19, 1463–1476. [Google Scholar] [CrossRef]
- Petruzzello, S.J.; Hall, E.E.; Ekkekakis, P. Regional brain activation as a biological marker of affective responsivity to acute exercise: Influence of fitness. Psychophysiology 2001, 38, 99–106. [Google Scholar] [CrossRef]
- Petruzzello, S.J.; Landers, D.M. State anxiety reduction and exercise: Does hemispheric activation reflect such changes? Med. Sci. Sports Exerc. 1994, 26, 1028–1035. [Google Scholar] [CrossRef]
- Petruzzello, S.J.; Tate, A.K. Brain activation, affect, and aerobic exercise: An examination of both state-independent and state-dependent relationships. Psychophysiology 1997, 34, 527–533. [Google Scholar] [CrossRef]
- Schneider, M.; Graham, D.; Grant, A.; King, P.; Cooper, D. Regional brain activation and affective response to physical activity among healthy adolescents. Biol. Psychol. 2009, 82, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Kim, S.; Kim, J.; Petruzzello, S.J.; Hatfield, B.D. The Influence of Exercise Intensity on Frontal Electroencephalographic Asymmetry and Self-Reported Affect. Res. Q. Exerc. Sport 2010, 81, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.E.; Ekkekakis, P.; Petruzzello, S.J. Regional brain activity and strenuous exercise: Predicting affective responses using EEG asymmetry. Biol. Psychol. 2007, 75, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.E.; Ekkekakis, P.; Petruzzello, S.J. Predicting affective responses to exercise using resting EEG frontal asymmetry: Does intensity matter? Biol. Psychol. 2010, 83, 201–206. [Google Scholar] [CrossRef]
- Woo, M.; Kim, S.; Kim, J.; Petruzzello, S.J.; Hatfield, B.D. Examining the exercise-affect dose–response relationship: Does duration influence frontal EEG asymmetry? Int. J. Psychophysiol. 2009, 72, 166–172. [Google Scholar] [CrossRef]
- Chesbro, G.A.; Owens, C.; Reese, M.; DE Stefano, L.; Kellawan, J.M.; Larson, D.J.; Wenger, M.J.; Larson, R.D. Changes in Brain Activity Immediately Post-Exercise Indicate a Role for Central Fatigue in the Volitional Termination of Exercise. Int. J. Exerc. Sci. 2024, 17, 220–234. [Google Scholar] [CrossRef]
- Crabbe, J.B.; Dishman, R.K. Brain electrocortical activity during and after exercise: A quantitative synthesis. Psychophysiologia 2004, 41, 563–574. [Google Scholar] [CrossRef]
- Heller, W.; Nitschke, J.B.; Etienne, M.A.; Miller, G.A. Patterns of regional brain activity differentiate types of anxiety. J. Abnorm. Psychol. 1997, 106, 376–385. [Google Scholar] [CrossRef]
- Heller, W.; Nitschke, J.B.; Miller, G.A. Lateralization in emotion and emotional disorders. Curr. Dir. Psychol. Sci. 1998, 7, 26–32. [Google Scholar] [CrossRef]
- Hosang, L.; Mouchlianitis, E.; Guérin, S.M.R.; Karageorghis, C.I. Effects of exercise on electroencephalography-recorded neural oscillations: A systematic review. Int. Rev. Sport Exerc. Psychol. 2022, 17, 926–979. [Google Scholar] [CrossRef]
- Threadgill, A.H.; Wilhelm, R.A.; Zagdsuren, B.; MacDonald, H.V.; Richardson, M.T.; Gable, P.A. Frontal asymmetry: A novel biomarker for physical activity and sedentary behavior. Psychophysiology 2020, 57, e13633. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, R.A.; Lacey, M.F.; Masters, S.L.; Breeden, C.J.; Mann, E.; MacDonald, H.V.; Gable, P.A.; White, E.J.; Stewart, J.L. Greater weekly physical activity linked to left resting frontal alpha asymmetry in women: A study on gender differences in highly active young adults. Psychol. Sport Exerc. 2024, 74, 102679. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Gorber, S.C.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Cardilli, L.; Reed, J.L.; Saunders, T.J.; Kite, C.; Douillette, K.; Fournier, K.; Buckley, J.P. A comparison of self-reported and device measured sedentary behaviour in adults: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 31. [Google Scholar] [CrossRef]
- Li, W.; Yang, P.; Ngetich, R.K.; Zhang, J.; Jin, Z.; Li, L. Differential involvement of frontoparietal network and insula cortex in emotion regulation. Neuropsychologia 2021, 161, 107991. [Google Scholar] [CrossRef]
- Phan, K.; Wager, T.; Taylor, S.F.; Liberzon, I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage 2002, 16, 331–348. [Google Scholar] [CrossRef]
- Reiman, E.M.; Lane, R.D.; Ahern, G.L.; Schwartz, G.E.; Davidson, R.J.; Friston, K.J.; Yun, L.S.; Chen, K. Neuroanatomical correlates of externally and internally generated human emotion. Am. J. Psychiatry 1997, 154, 918–925. [Google Scholar] [CrossRef]
- Greene, C.M.; Flannery, O.; Soto, D. Distinct parietal sites mediate the influences of mood, arousal, and their interaction on human recognition memory. Cogn. Affect. Behav. Neurosci. 2014, 14, 1327–1339. [Google Scholar] [CrossRef]
- Plasqui, G.; Westerterp, K.R. Physical Activity Assessment with Accelerometers: An Evaluation Against Doubly Labeled Water. Obesity 2007, 15, 2371–2379. [Google Scholar] [CrossRef]
- Doherty, A.; Jackson, D.; Hammerla, N.; Plötz, T.; Olivier, P.; Granat, M.H.; White, T.; van Hees, V.T.; Trenell, M.I.; Owen, C.G.; et al. Large Scale Population Assessment of Physical Activity Using Wrist Worn Accelerometers: The UK Biobank Study. PLoS ONE 2017, 12, e0169649. [Google Scholar] [CrossRef]
- Lee, D.H.; Rezende, L.F.; Joh, H.-K.; Keum, N.; Ferrari, G.; Rey-Lopez, J.P.; Rimm, E.B.; Tabung, F.K.; Giovannucci, E.L. Long-Term Leisure-Time Physical Activity Intensity and All-Cause and Cause-Specific Mortality: A Prospective Cohort of US Adults. Circulation 2022, 146, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; van Hees, V.T.; Stein, M.J.; Gastell, S.; Steindorf, K.; Herbolsheimer, F.; Ostrzinski, S.; Pischon, T.; Brandes, M.; Krist, L.; et al. Large-scale assessment of physical activity in a population using high-resolution hip-worn accelerometry: The German National Cohort (NAKO). Sci. Rep. 2024, 14, 7927. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, A.V. Moving Forward with Accelerometer-Assessed Physical Activity: Two Strategies to Ensure Meaningful, Interpretable, and Comparable Measures. Pediatr. Exerc. Sci. 2018, 30, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, N.P.; Yates, T.; Edwardson, C.L.; Maylor, B.; Davies, M.J.; Dunstan, D.; Highton, P.J.; Herring, L.Y.; Khunti, K.; Rowlands, A.V. Comparing 24 h physical activity profiles: Office workers, women with a history of gestational diabetes and people with chronic disease condition(s). J. Sports Sci. 2021, 39, 219–226. [Google Scholar] [CrossRef]
- Dygrýn, J.; Medrano, M.; Molina-Garcia, P.; Rubín, L.; Jakubec, L.; Janda, D.; Gába, A. Associations of novel 24-h accelerometer-derived metrics with adiposity in children and adolescents. Environ. Health Prev. Med. 2021, 26, 66. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Carson, V.; Chaput, J.-P.; Gorber, S.C.; Dinh, T.; Duggan, M.; Faulkner, G.; Gray, C.E.; Gruber, R.; Janson, K.; et al. Canadian 24-Hour Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. Appl. Physiol. Nutr. Metab. 2016, 41, S311–S327. [Google Scholar] [CrossRef]
- Fairclough, S.J.; Rowlands, A.V.; Cruz, B.d.P.; Crotti, M.; Foweather, L.; Graves, L.E.F.; Hurter, L.; Jones, O.; MacDonald, M.; McCann, D.A.; et al. Reference values for wrist-worn accelerometer physical activity metrics in England children and adolescents. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 35. [Google Scholar] [CrossRef]
- Rowlands, A.V.; Edwardson, C.L.; Davies, M.J.; Khunti, K.; Harrington, D.M.; Yates, T. Beyond Cut Points: Accelerometer Metrics that Capture the Physical Activity Profile. Med. Sci. Sports Exerc. 2018, 50, 1323–1332. [Google Scholar] [CrossRef]
- Rowlands, A.V.; Dawkins, N.P.; Maylor, B.; Edwardson, C.L.; Fairclough, S.J.; Davies, M.J.; Harrington, D.M.; Khunti, K.; Yates, T. Enhancing the value of accelerometer-assessed physical activity: Meaningful visual comparisons of data-driven translational accelerometer metrics. Sports Med.-Open 2019, 5, 47. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- RStudio Team. RStudio Desktop. Available online: https://posit.co/download/rstudio-desktop/ (accessed on 18 February 2025).
- van Hees, V.T.; Migueles, J.H.; Sabia, S.; Patterson, M.R.; Fang, Z.; Heywood, J.; Pujol, J.C.; Kushleyeva, L.; Chen, M.; Yerramalla, M.; et al. GGIR: Raw Accelerometer Data Analysis. 2024. Available online: https://CRAN.R-project.org/package=GGIR (accessed on 29 December 2022).
- Van Hees, V.T.; Fang, Z.; Langford, J.; Assah, F.; Mohammad, A.; Da Silva, I.C.M.; Trenell, M.I.; White, T.; Wareham, N.J.; Brage, S. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: An evaluation on four continents. J. Appl. Physiol. 2014, 117, 738–744. [Google Scholar] [CrossRef] [PubMed]
- van Hees, V.T.; Gorzelniak, L.; Dean León, E.C.; Eder, M.; Pias, M.; Taherian, S.; Ekelund, U.; Renström, F.; Franks, P.W.; Horsch, A.; et al. Separating Movement and Gravity Components in an Acceleration Signal and Implications for the Assessment of Human Daily Physical Activity. PLoS ONE 2013, 8, e61691. [Google Scholar] [CrossRef] [PubMed]
- Buchan, D.S.; Maylor, B.D. Comparison of physical activity metrics from two research-grade accelerometers worn on the non-dominant wrist and thigh in children. J. Sports Sci. 2023, 41, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Kaplan, E.R.; Rueschman, M.; Cailler, M.; Buxton, O.M.; Redline, S. Practical considerations in using accelerometers to assess physical activity, sedentary behavior, and sleep. Sleep Health 2015, 1, 275–284. [Google Scholar] [CrossRef]
- Hildebrand, M.; VAN Hees, V.T.; Hansen, B.H.; Ekelund, U. Age Group Comparability of Raw Accelerometer Output from Wrist- and Hip-Worn Monitors. Med. Sci. Sports Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef]
- Hildebrand, M.; Hansen, B.H.; van Hees, V.T.; Ekelund, U. Evaluation of raw acceleration sedentary thresholds in children and adults. Scand. J. Med. Sci. Sports 2017, 27, 1814–1823. [Google Scholar] [CrossRef]
- Chatrian, G.E.; Lettich, E.; Nelson, P.L. Ten Percent Electrode System for Topographic Studies of Spontaneous and Evoked EEG Activities. Am. J. EEG Technol. 1985, 25, 83–92. [Google Scholar] [CrossRef]
- Metzen, D.; Genç, E.; Getzmann, S.; Larra, M.F.; Wascher, E.; Ocklenburg, S. Frontal and parietal EEG alpha asymmetry: A large-scale investigation of short-term reliability on distinct EEG systems. Anat. Embryol. 2022, 227, 725–740. [Google Scholar] [CrossRef]
- Tomarken, A.J.; Davidson, R.J.; Henriques, J.B. Resting frontal brain asymmetry predicts affective responses to films. J. Pers. Soc. Psychol. 1990, 59, 791–801. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Lopez-Calderon, J.; Luck, S.J. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Hsu, S.-H.; Pion-Tonachini, L.; Jung, T.-P. Evaluation of Artifact Subspace Reconstruction for Automatic Artifact Components Removal in Multi-Channel EEG Recordings. IEEE Trans. Biomed. Eng. 2020, 67, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Kothe, C.A.; Makeig, S. BCILAB: A platform for brain–computer interface development. J. Neural Eng. 2013, 10, 056014. [Google Scholar] [CrossRef] [PubMed]
- Callan, D.E.; Torre–Tresols, J.J.; Laguerta, J.; Ishii, S. Shredding artifacts: Extracting brain activity in EEG from extreme artifacts during skateboarding using ASR and ICA. Front. Neuroergon 2024, 5, 1358660. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; Miskovic, V.; Laszlo, S. Evaluating the efficacy of fully automated approaches for the selection of eyeblink ICA components. Psychophysiology 2017, 54, 780–791. [Google Scholar] [CrossRef]
- Allen, J.J.B.; Urry, H.L.; Hitt, S.K.; Coan, J.A. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology 2004, 41, 269–280. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Niermann, C.Y.N.; Herrmann, C.; von Haaren, B.; van Kann, D.; Woll, A. Affect and Subsequent Physical Activity: An Ambulatory Assessment Study Examining the Affect-Activity Association in a Real-Life Context. Front. Psychol. 2016, 7, 677. [Google Scholar] [CrossRef]
- Pressman, S.D.; Petrie, K.J.; Sivertsen, B. How Strongly Connected Are Positive Affect and Physical Exercise? Results from a Large General Population Study of Young Adults. Clin. Psychol. Eur. 2020, 2, e3103. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Drollette, E.S.; Pasupathi, P.A.; Slutsky-Ganesh, A.B.; Etnier, J.L. Take a Break for Memory Sake! Effects of Short Physical Activity Breaks on Inhibitory Control, Episodic Memory, and Event-Related Potentials in Children. Brain Sci. 2024, 14, 626. [Google Scholar] [CrossRef]
- Haehl, W.; Mirifar, A.; Beckmann, J. Regulate to facilitate: A scoping review of prefrontal asymmetry in sport and exercise. Psychol. Sport Exerc. 2022, 60, 102143. [Google Scholar] [CrossRef]
- Ku, P.-W.; Steptoe, A.; Liao, Y.; Hsueh, M.-C.; Chen, L.-J. A cut-off of daily sedentary time and all-cause mortality in adults: A meta-regression analysis involving more than 1 million participants. BMC Med. 2018, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.A.; Hall, P.A.; Staines, W.R.; McIlroy, W.E. Frontal alpha asymmetry and aerobic exercise: Are changes due to cardiovascular demand or bilateral rhythmic movement? Biol. Psychol. 2018, 132, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T.; Schneider, S.; Brümmer, V.; Strüder, H.K. Frontal EEG asymmetry: The effects of sustained walking in the elderly. Neurosci. Lett. 2010, 485, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Liu, X.; Van Dam, N.T.; Hof, P.R.; Fan, J. Cognition–emotion integration in the anterior insular cortex. Cereb. Cortex 2013, 23, 20–27. [Google Scholar] [CrossRef]
- van Wijk, B.C.M.; Beek, P.J.; Daffertshofer, A. Neural synchrony within the motor system: What have we learned so far? Front. Hum. Neurosci. 2012, 6, 252. [Google Scholar] [CrossRef]
- Van Der Werf, J.; Buchholz, V.; Jensen, O.; Medendorp, W. Neuronal synchronization in human parietal cortex during saccade planning. Behav. Brain Res. 2009, 205, 329–335. [Google Scholar] [CrossRef]
- Culham, J.C.; Valyear, K.F. Human parietal cortex in action. Curr. Opin. Neurobiol. 2006, 16, 205–212. [Google Scholar] [CrossRef]
- Cefis, M.; Chaney, R.; Wirtz, J.; Méloux, A.; Quirié, A.; Leger, C.; Prigent-Tessier, A.; Garnier, P. Molecular mechanisms underlying physical exercise-induced brain BDNF overproduction. Front. Mol. Neurosci. 2023, 16, 1275924. [Google Scholar] [CrossRef]
- Oakes, T.R.; Pizzagalli, D.A.; Hendrick, A.M.; Horras, K.A.; Larson, C.L.; Abercrombie, H.C.; Schaefer, S.M.; Koger, J.V.; Davidson, R.J. Functional coupling of simultaneous electrical and metabolic activity in the human brain. Hum. Brain Mapp. 2004, 21, 257–270. [Google Scholar] [CrossRef]
- Dietrich, A. Functional neuroanatomy of altered states of consciousness: The transient hypofrontality hypothesis. Conscious. Cogn. 2003, 12, 231–256. [Google Scholar] [CrossRef]
- Ide, K.; Secher, N.H. Cerebral blood flow and metabolism during exercise. Prog. Neurobiol. 2000, 61, 397–414. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Coan, J.A.; Allen, J.J. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol. Psychol. 2004, 67, 7–50. [Google Scholar] [CrossRef]
- Marent, P.-J.; Cardon, G.; Dumuid, D.; Albouy, G.; van Uffelen, J. 24-hour movement behaviours are cross-sectionally associated with cognitive function in healthy adults aged 55 years and older. Sci. Rep. 2025, 15, 38619. [Google Scholar] [CrossRef]
- Rollo, S.; Antsygina, O.; Tremblay, M.S. The whole day matters: Understanding 24-hour movement guideline adherence and relationships with health indicators across the lifespan. J. Sport Health Sci. 2020, 9, 493–510. [Google Scholar] [CrossRef] [PubMed]
| Measure | Mean (±SD) |
|---|---|
| Age (years) | 21.76 (2.92) |
| Height (cm) | 170.49 (10.50) |
| Weight (kg) | 76.36 (15.92) |
| BMI (kg/m2) | 26.18 (5.00) |
| Gender (n [%]) | |
| Male | 17 [29%] |
| Female | 42 [71%] |
| Ethnicity (n [%]) | |
| Non-Hispanic | 43 [73%] |
| Hispanic | 12 [20%] |
| No response | 4 [7%] |
| ST (min/day) | 743.32 (95.91) |
| LPA (min/day) | 142.00 (35.36) |
| MVPA (min/day) | 109.34 (36.52) |
| IG (mg) | −2.48 (0.16) |
| AvAcc (mg) | 28.82 (6.94) |
| M120 (mg) | 69.78 (21.32) |
| M60 (mg) | 89.36 (30.49) |
| M30 (mg) | 110.78 (40.75) |
| M15 (mg) | 135.72 (52.34) |
| M10 (mg) | 152.76 (63.26) |
| M5 (mg) | 183.50 (81.80) |
| M2 (mg) | 235.41 (136.81) |
| a Positive affect | 24.90 (9.62) |
| a Negative affect | 12.98 (3.09) |
| Overall Model for Sex, Affect, and EEG | Predictor | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | F | p | B | Berror | β | t | p | CI | |
| IG | |||||||||
| P2 − P1 | 0.264 | 6.573 | 0.001 | −0.403 | 0.185 | −0.264 | −2.180 | 0.034 * | −0.774, −0.033 |
| M60 | |||||||||
| P6 − P5 | 0.234 | 5.614 | 0.002 | 34.700 | 14.600 | 0.292 | 2.377 | 0.021 * | 5.441, 63.960 |
| M30 | |||||||||
| P6 − P5 | 0.295 | 7.669 | 0.000 | 48.924 | 18.724 | 0.308 | 2.613 | 0.012 * | 11.401, 86.448 |
| P4 − P3 | 0.276 | 6.976 | 0.000 | 60.759 | 26.699 | 0.268 | 2.276 | 0.027 * | 7.254, 114.264 |
| M15 | |||||||||
| P6 − P5 | 0.297 | 7.761 | 0.000 | 62.783 | 24.009 | 0.308 | 2.615 | 0.011 * | 14.668, 110.897 |
| P4 − P3 | 0.278 | 7.076 | 0.000 | 78.156 | 34.227 | 0.269 | 2.283 | 0.026 * | 9.563, 146.748 |
| M10 | |||||||||
| P6 − P5 | 0.265 | 6.606 | 0.001 | 73.913 | 29.679 | 0.300 | 2.490 | 0.016 * | 14.434, 133.391 |
| P4 − P3 | 0.250 | 6.105 | 0.001 | 94.063 | 42.178 | 0.268 | 2.230 | 0.030 * | 9.538, 178.589 |
| M5 | |||||||||
| P6 − P5 | 0.260 | 6.440 | 0.001 | 91.054 | 38.508 | 0.286 | 2.365 | 0.022 * | 13.882, 168.227 |
| P4 − P3 | 0.244 | 5.918 | 0.001 | 113.726 | 54.752 | 0.250 | 2.077 | 0.042 * | 4.000, 223.453 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero-Herrera, B.; O’Brokta, M.M.; Pasupathi, P.A.; Drollette, E.S. The Relation of Alpha Asymmetry to Physical Activity Duration and Intensity. Brain Sci. 2025, 15, 1322. https://doi.org/10.3390/brainsci15121322
Montero-Herrera B, O’Brokta MM, Pasupathi PA, Drollette ES. The Relation of Alpha Asymmetry to Physical Activity Duration and Intensity. Brain Sciences. 2025; 15(12):1322. https://doi.org/10.3390/brainsci15121322
Chicago/Turabian StyleMontero-Herrera, Bryan, Megan M. O’Brokta, Praveen A. Pasupathi, and Eric S. Drollette. 2025. "The Relation of Alpha Asymmetry to Physical Activity Duration and Intensity" Brain Sciences 15, no. 12: 1322. https://doi.org/10.3390/brainsci15121322
APA StyleMontero-Herrera, B., O’Brokta, M. M., Pasupathi, P. A., & Drollette, E. S. (2025). The Relation of Alpha Asymmetry to Physical Activity Duration and Intensity. Brain Sciences, 15(12), 1322. https://doi.org/10.3390/brainsci15121322







