Routine Hair Testing Unmasks Hidden Synthetic Cannabinoid Use in Forensic Psychiatric Patients: A 10-Year Comparative Study in Two Bavarian Clinics
Abstract
1. Introduction
2. Methods
2.1. Sample
2.2. Procedure
2.3. Statistical Analysis
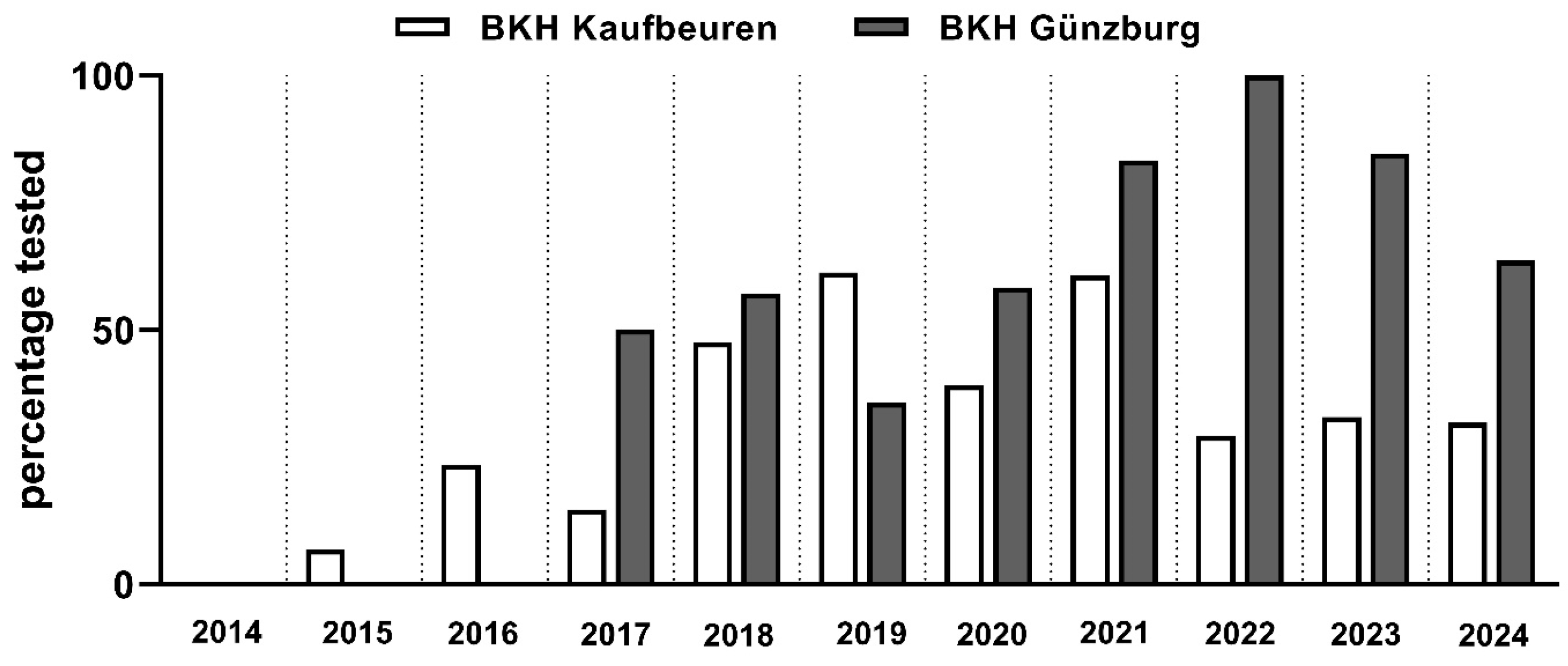
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arboleda-Flórez, J. Forensic psychiatry: Contemporary scope, challenges and controversies. World Psychiatry Off. J. World Psychiatr. Assoc. (WPA) 2006, 5, 87–91. [Google Scholar]
- Velinov, V.T.; Marinov, P.M. Forensic psychiatric practice: Worldwide similarities and differences. World Psychiatry Off. J. World Psychiatr. Assoc. (WPA) 2006, 5, 98–99. [Google Scholar]
- Salize, H.; Dressing, H. Die forensisch-psychiatrische Versorgung in Mitgliedstaaten der Europäischen Union—Versorgungskonzepte und Kapazitäten. Psychiatrische Praxis 2007, 34, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Bezzel, A. Therapie im Maßregelvollzug-und Dann? Eine Verlaufsuntersuchung an Forensischen Patienten (§§ 63 und 64 StGB). Ph.D. Thesis, Universität Regensburg, Regensburg, Germany, 2009. [Google Scholar] [CrossRef]
- Leygraf, N. Alkoholabhängige Straftäter: Zur Problematik der Unterbringung nach § 64 StGB. Fortschr. Neurol. Psychiatr. 1987, 55, 231–237. [Google Scholar] [CrossRef]
- Mayer, J.; Streb, J.; Steiner, I.; Wolf, V.; Klein, V.; Dudeck, M.; Franke, I. Alcohol use disorder as a risk factor for violent offending and reoffending in delinquent women with substance use disorders. Arch. Women’s Ment. Health 2023, 26, 331–339. [Google Scholar] [CrossRef]
- Pierce, M.; Hayhurst, K.; Bird, S.M.; Hickman, M.; Seddon, T.; Dunn, G.; Millar, T. Insights into the link between drug use and criminality: Lifetime offending of criminally-active opiate users. Drug Alcohol Depend. 2017, 179, 309–316. [Google Scholar] [CrossRef]
- Miller, N.S.; Gold, M.S.; Mahler, J.C. Violent Behaviors Associated with Cocaine Use: Possible Pharmacological Mechanisms. Int. J. Addict. 1991, 26, 1077–1088. [Google Scholar] [CrossRef]
- Zhong, S.; Yu, R.; Fazel, S. Drug Use Disorders and Violence: Associations With Individual Drug Categories. Epidemiol. Rev. 2020, 42, 103–116. [Google Scholar] [CrossRef]
- Dessecker, A.; Egg, R.; Kriminologische Zentralstelle, K. (Eds.) Die Strafrechtliche Unterbringung in Einer Entziehungsanstalt: Rechtliche, Empirische und Praktische Aspekte; Kriminologische Zentralstelle: Wiesbaden, Germany, 1995. [Google Scholar]
- Kröber, H.-L.; Dölling, D.; Leygraf, N.; Sass, H. (Eds.) Handbuch der Forensischen Psychiatrie; Steinkopff: Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Schalast, N.; Leygraf, N. Unterbringung und Behandlung im Maßregelvollzug gemäß § 64 StGB. In Alkohol und Schuldfähigkeit; Schneider, F., Frister, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 181–201. [Google Scholar] [CrossRef]
- Fritz, M.; Montiel, F.; Al Dirany, A.; Dudeck, M.; Streb, J. Unraveling Relapse in Male Forensic Psychiatric Patients with Substance Use Disorders—The Impact of Social, Psychiatric, and Personality Factors Post Long-Term Remission. Int. J. Ment. Health Addiction. 2024, 1–16. [Google Scholar] [CrossRef]
- Melemis, S.M. Relapse Prevention and the Five Rules of Recovery. Yale J. Biol. Med. 2015, 88, 325–332. [Google Scholar]
- Ninnemann, A.L.; Lechner, W.V.; Borges, A.; Lejuez, C.W. Synthetic cannabinoids to avoid urine drug screens: Implications for contingency management and other treatments for drug dependence. Addict. Behav. 2016, 63, 72–73. [Google Scholar] [CrossRef]
- Castaneto, M.S.; Scheidweiler, K.B.; Gandhi, A.; Wohlfarth, A.; Klette, K.L.; Martin, T.M.; Huestis, M.A. Quantitative urine confirmatory testing for synthetic cannabinoids in randomly collected urine specimens. Drug Test. Anal. 2015, 7, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Palamar, J.J.; Abukahok, N.; Le, A. Synthetic cannabinoid use among noninstitutionalized individuals in the United States, 2021–2023. Drug Alcohol Depend. 2025, 270, 112603. [Google Scholar] [CrossRef] [PubMed]
- Rauschert, C.; Möckl, J.; Seitz, N.N.; Wilms, N.; Olderbak, S.; Kraus, L. The use of psychoactive substances in Germany—Findings from the Epidemiological Survey of Substance Abuse 2021. Dtsch. Arztebl. Int. 2022, 119, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Austin, A.; Blagborough, I.; Sunderland, P.; Pudney, C.; Freeman, T. Synthetic Cannabinoids Use among Inmates in an English Prison. Emerg. Trends Drugs Addict. Health 2024, 4, 100082. [Google Scholar] [CrossRef]
- Hobbs, M.; Patel, R.; Morrison, P.D.; Kalk, N.; Stone, J.M. Synthetic cannabinoid use in psychiatric patients and relationship to hospitalisation: A retrospective electronic case register study. J. Psychopharmacol. 2020, 34, 648–653. [Google Scholar] [CrossRef]
- Brown, K.R. The Need to Distinguish between “Lapse” and “Relapse”. Perspect. Behav. Sci. 2025, 48, 115–132. [Google Scholar] [CrossRef]
- Tai, S.; Fantegrossi, W.E. Synthetic Cannabinoids: Pharmacology, Behavioral Effects, and Abuse Potential. Curr. Addict. Rep. 2014, 1, 129–136. [Google Scholar] [CrossRef]
- Diao, X.; Huestis, M. Approaches, Challenges, and Advances in Metabolism of New Synthetic Cannabinoids and Identification of Optimal Urinary Marker Metabolites. Clin. Pharmacol. Ther. 2017, 101, 239–253. [Google Scholar] [CrossRef]
- AlKhelb, D.; Burke, E.L.; Zvonok, A.; Iliopoulos-Tsoutsouvas, C.; Georgiadis, M.-O.; Jiang, S.; Ho, T.C.; Nikas, S.P.; Makriyannis, A.; Desai, R.I. Effects of cannabinoid agonists and antagonists in male rats discriminating the synthetic cannabinoid AM2201. Eur. J. Pharmacol. 2023, 960, 176168. [Google Scholar] [CrossRef]
- Hur, K.-H.; Ma, S.-X.; Lee, B.-R.; Ko, Y.-H.; Seo, J.-Y.; Ryu, H.W.; Kim, H.J.; Yoon, S.; Lee, Y.-S.; Lee, S.-Y.; et al. Abuse Potential of Synthetic Cannabinoids: AM-1248, CB-13, and PB-22. Biomol. Ther. 2021, 29, 384–391. [Google Scholar] [CrossRef]
- Hyatt, W.S.; Fantegrossi, W.E. Δ9-THC exposure attenuates aversive effects and reveals appetitive effects of K2/‘Spice’ constituent JWH-018 in mice. Behav. Pharmacol. 2014, 25, 253–257. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, Q.; Qian, Z.; Wang, H.; Liu, Z.; Ren, W.; Zhang, X.; Han, J. Cannabinoid-Elicited Conditioned Place Preference in a Modified Behavioral Paradigm. Biol. Pharm. Bull. 2016, 39, 747–753. [Google Scholar] [CrossRef]
- Xu, D.; Ji, J.; Xiang, P.; Yan, H.; Zhang, W.; Shen, M. Determination of 5 synthetic cannabinoids in hair by Segmental analysis using UHPLC-MS/MS and its application to eight polydrug abuse cases. Forensic Sci. Int. 2023, 346, 111611. [Google Scholar] [CrossRef] [PubMed]
- Covey, J.; Maggitti, A.; Fatigante, W.; Espourteille, F.; Lombardo, H.; Bates, T. Rapid quantitative screening of 15 synthetic cannabinoids in urine using direct analysis in real time tandem mass spectrometry: Screening method validation and cross-correlation study with liquid chromatography tandem mass spectrometry. Green Anal. Chem. 2025, 13, 100254. [Google Scholar] [CrossRef]
- Cherek, D.R.; Thompson, T.; Kelly, T. Chronic Δ9-tetrahydrocannabinol administration and schedule-induced aggression. Pharmacol. Biochem. Behav. 1980, 12, 305–309. [Google Scholar] [CrossRef]
- Miczek, K.A. Δ9-Tetrahydrocannabinol: Antiaggressive Effects in Mice, Rats, and Squirrel Monkeys. Science 1978, 199, 1459–1461. [Google Scholar] [CrossRef]
- Kolla, N.J.; Mishra, A. The Endocannabinoid System, Aggression, and the Violence of Synthetic Cannabinoid Use, Borderline Personality Disorder, Antisocial Personality Disorder, and Other Psychiatric Disorders. Front. Behav. Neurosci. 2018, 12, 41. [Google Scholar] [CrossRef]
- Van Amsterdam, J.; Brunt, T.; Van Den Brink, W. The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J. Psychopharmacol. 2015, 29, 254–263. [Google Scholar] [CrossRef]
- Iseger, T.A.; Bossong, M.G. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr. Res. 2015, 162, 153–161. [Google Scholar] [CrossRef]
- Fattore, L. Synthetic Cannabinoids—Further Evidence Supporting the Relationship Between Cannabinoids and Psychosis. Biol. Psychiatry 2016, 79, 539–548. [Google Scholar] [CrossRef]
- Meijer, K.A.; Russo, R.R.; Adhvaryu, D.V. Smoking Synthetic Marijuana Leads to Self-Mutilation Requiring Bilateral Amputations. Orthopedics 2014, 37, e391–e394. [Google Scholar] [CrossRef]
- Clayton, H.B.; Lowry, R.; Ashley, C.; Wolkin, A.; Grant, A.M. Health Risk Behaviors with Synthetic Cannabinoids Versus Marijuana. Pediatrics 2017, 139, e20162675. [Google Scholar] [CrossRef] [PubMed]
- Köck, P.; Walter, M. Personality disorder and substance use disorder—An update. Ment. Health Prev. 2018, 12, 82–89. [Google Scholar] [CrossRef]
- Moeller, F.G.; Dougherty, D.M. Antisocial personality disorder, alcohol, and aggression. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2001, 25, 5–11. [Google Scholar]
- Blackburn, R. “What works” with mentally disordered offenders. Psychol. Crime Law 2004, 10, 297–308. [Google Scholar] [CrossRef]
- Herpertz, S.; Saß, H. Persönlichkeitsstörungen; Georg Thieme Verlag: Stuttgart, Germany, 2003. [Google Scholar] [CrossRef]
- Giammarco, E.A.; Atkinson, B.; Baughman, H.M.; Veselka, L.; Vernon, P.A. The relation between antisocial personality and the perceived ability to deceive. Personal. Individ. Differ. 2013, 54, 246–250. [Google Scholar] [CrossRef]
- Winstock, A.R.; Barratt, M.J. Synthetic cannabis: A comparison of patterns of use and effect profile with natural cannabis in a large global sample. Drug Alcohol Depend. 2013, 131, 106–111. [Google Scholar] [CrossRef]
- Higgins, K.; O’Neill, N.; O’Hara, L.; Jordan, J.A.; McCann, M.; O’Neill, T.; Clarke, M.; O’Neill, T.; Campbell, A. Chapter 7. Synthetic cannabinoids. In Evidence for Public Health on Novel Psychoactive Substance Use: A Mixed-Methods Study; Public Health Research, No. 7.14; NIHR Journals Library: Southampton, UK, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544991/ (accessed on 3 October 2025).
| Guenzburg (n = 110) M (SD)/n (%) | Kaufbeuren (n = 417) M (SD)/n (%) | Statistics | |
|---|---|---|---|
| Sex | FET = 11.438, p = 0.009, Cramer-V = 0.147 | ||
| male | 107 (97%) | 417 (100%) | |
| female | 3 (3%) | 0 | |
| Age at the beginning of leave (in years) | 36.01 (8.46) | 36.43 (9.05) | t(522) = −0.442, p = 0.659, Cohen’s d = −0.047 |
| Primary diagnosis (according to ICD-10) | Chi2(8) = 23.661, p = 0.002, Cramer-V = 0.212 | ||
| Mental and behavioral disorders due to the use of | |||
| ... alcohol (F10) | 22 (20.0%) | 70 (16.8%) | |
| ... opioids (F11) | 9 (8.2%) | 25 (6.0%) | |
| ... cannabinoids (F12) | 25 (22.7%) ° | 51 (12.2%) | |
| ... sedatives or hypnotics (F13) | 0 | 1 (0.2%) | |
| ... cocaine (F14) | 9 (8.2%) | 57 (13.7%) | |
| ... other stimulants (F15) | 10 (9.1%) | 17 (4.1%) | |
| ... multiple drug use (F19) | 34 (30.9%) ^ | 194 (46.5%) | |
| Schizophrenia (F20) | 0 | 2 (0.5) | |
| Reaction to severe stress, and adjustment disorders (F43) | 1 (0.9%) | 0 | |
| Comorbid personality disorder | 37 (34%) | 67 (16%) | Chi2(1) = 16.962, p < 0.001, Cramer-V = 0.179 |
| On substitution treatment | 9 (8%) | 11 (3%) | Chi2(1) = 7.327, p = 0.012, Cramer-V = 0.118 |
| Number of prior convictions (according to criminal record extract) | 6.06 (4.85) | 7.05 (5.74) | Z = −1.313, p = 0.189, Cohen’s d = 0.115 |
| Age at first conviction (in years) | 23.60 (9.53) | 23.04 (9.26) | t (523) = 0.562, p = 0.574, Cohen’s d = 0.061 |
| Violent index offense or history of violent offenses | 58 (53%) | 245 (59%) | Chi2(1) = 1.293, p = 0.279, Cramer-V = 0.050 |
| Duration of hospitalization in forensic psychiatric care (in months) | 22.26 (6.41) | 17.64 (5.52) | t (524) = 7.536, p < 0.001, Cohen’s d = 0.396 |
| Time since leave (in days) | 146.85 (86.56) | 133.08 (111.45) | t (159.702) = 0.993, p = 0.369, Cohen’s d = 0.132 |
| Guenzburg n (%) | Kaufbeuren n (%) | Total | |
|---|---|---|---|
| Tested negative | 53 (77.9%) | 155 (96.3%) | 208 |
| Tested positive | 15 (22.1%) | 6 (3.7%) | 21 |
| Total | 68 | 161 |
| Predictors | Regression Coefficient | p | Odds-Ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Hospital | 1.967 | <0.001 | 7.147 | 2.628 | 19.437 |
| Time since leave (in days) | 0 | 0.962 | 1.000 | 0.995 | 1.005 |
| Tested Positive n (%) | |
|---|---|
| JWH | 2 (5%) |
| A | 5 (14%) |
| B | 0 |
| C | 2 (5%) |
| E | 1 (3%) |
| F | 4 (11%) |
| H | 11 (30%) |
| M | 12 (32%) |
| N | 0 |
| P | 0 |
| S | 0 |
| T | 0 |
| U | 0 |
| XLR | 0 |
| Tested Negative n (%) | Tested Positive n (%) | Total | |
|---|---|---|---|
| 2015 | 0 | 1 | 1 |
| 2016 | 7 | 0 | 7 |
| 2017 | 5 | 1 | 6 |
| 2018 | 13 | 2 | 15 |
| 2019 | 32 | 7 | 39 |
| 2020 | 19 | 2 | 21 |
| 2021 | 41 | 2 | 43 |
| 2022 | 32 | 0 | 32 |
| 2023 | 37 | 3 | 40 |
| 2024 | 20 | 3 | 23 |
| 2025 | 2 | 0 | 2 |
| Total | 208 | 21 | 229 |
| Tested Negative (n = 208) M (SD)/n (%) | Tested Positive (n = 21) M (SD)/n (%) | Statistics | |
|---|---|---|---|
| Sex | Chi2(1) = 0.101, p = 1.000, Cramer-V = 0.021 | ||
| male | 207 (99.5%) | 21 (100%) | |
| female | 1 (0.5%) | 0 | |
| Age at the beginning of leave (in years) | 35.17 (8.41) | 34.19 (5.78) | t (225) = 0.518, p = 0.605, Cohen’s d = 0.119 |
| Primary diagnosis (according to ICD-10) | Chi2(7) = 7.136, p = 0.357, Cramer-V = 0.177 | ||
| Mental and behavioral disorders due to the use of | |||
| ... alcohol (F10) | 26 (12.5%) | 4 (19.0%) | |
| ... opioids (F11) | 11 (5.3%) | 0 | |
| ... cannabinoids (F12) | 35 (16.8%) | 3 (14.3%) | |
| ... cocaine (F14) | 26 (12.5%) | 0 | |
| ... other stimulants (F15) | 10 (4.8%) | 0 | |
| ... multiple drug use (F19) | 98 (47.1%) | 14 (66.7%) | |
| Schizophrenia (F20) | 1 (0.5%) | 0 | |
| Reaction to severe stress, and adjustment disorders (F43) | 1 (0.5%) | 0 | |
| Comorbid personality disorder | 53 (26%) | 4 (19%) | Chi2(1) = 0.422, p = 0.363, Cramer-V = 0.043 |
| On substitution treatment | 10 (5%) | 4 (19%) | Chi2(1) = 6.739, p = 0.029, Cramer-V = 0.172 |
| Number of prior convictions (according to criminal record extract) | 6.38 (5.17)) | 8.43 (5.03) | Z = −1.862, p = 0.063, Cohen’s d = 0.248 |
| Age at first conviction (in years) | 22.39 (9.06) | 19.33 (4.87) | t(36.136) = 2.471, p =0.018, Cohen’s d = 0.349 |
| Violent index offense or history of violent offenses | 111 (53%) | 18 (86%) | Chi2(1) = 8.114, p = 0.003, Cramer-V = 0.188 |
| Duration of hospitalization in forensic psychiatric care (in months) | 19.06 (6.16) | 19.07 (6.57) | t(227) = −0.010, p = 0.992, Cohen’s d = 0.002 |
| Time since leave (in days) | 135.79 (107.03) | 142.29 (81.04) | t(216) = −0.270, p = 0.787, Cohen’s d = −0.062 |
| Predictors | Regression Coefficient | p | Odds-Ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| On substitution treatment | 1.244 | 0.067 | 3.468 | 0.916 | 13.138 |
| Age at first conviction (in years) | −0.018 | 0.650 | 0.982 | 0.910 | 1.061 |
| Violent index offense or history of violent offenses | 1.397 | 0.038 | 4.043 | 1.083 | 15.086 |
| Time since leave (in days) | 0.001 | 0.007 | 1.001 | 0.997 | 1.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritz, M.; Funk, H.; Montiel, F.; Streb, J.; Dudeck, M. Routine Hair Testing Unmasks Hidden Synthetic Cannabinoid Use in Forensic Psychiatric Patients: A 10-Year Comparative Study in Two Bavarian Clinics. Brain Sci. 2025, 15, 1240. https://doi.org/10.3390/brainsci15111240
Fritz M, Funk H, Montiel F, Streb J, Dudeck M. Routine Hair Testing Unmasks Hidden Synthetic Cannabinoid Use in Forensic Psychiatric Patients: A 10-Year Comparative Study in Two Bavarian Clinics. Brain Sciences. 2025; 15(11):1240. https://doi.org/10.3390/brainsci15111240
Chicago/Turabian StyleFritz, Michael, Hannah Funk, Felipe Montiel, Judith Streb, and Manuela Dudeck. 2025. "Routine Hair Testing Unmasks Hidden Synthetic Cannabinoid Use in Forensic Psychiatric Patients: A 10-Year Comparative Study in Two Bavarian Clinics" Brain Sciences 15, no. 11: 1240. https://doi.org/10.3390/brainsci15111240
APA StyleFritz, M., Funk, H., Montiel, F., Streb, J., & Dudeck, M. (2025). Routine Hair Testing Unmasks Hidden Synthetic Cannabinoid Use in Forensic Psychiatric Patients: A 10-Year Comparative Study in Two Bavarian Clinics. Brain Sciences, 15(11), 1240. https://doi.org/10.3390/brainsci15111240







