Dynamic Brain Activation and Connectivity in Elite Golfers During Distinct Golf Swing Phases: An fMRI Study
Abstract
1. Introduction
2. Methods
2.1. Participants
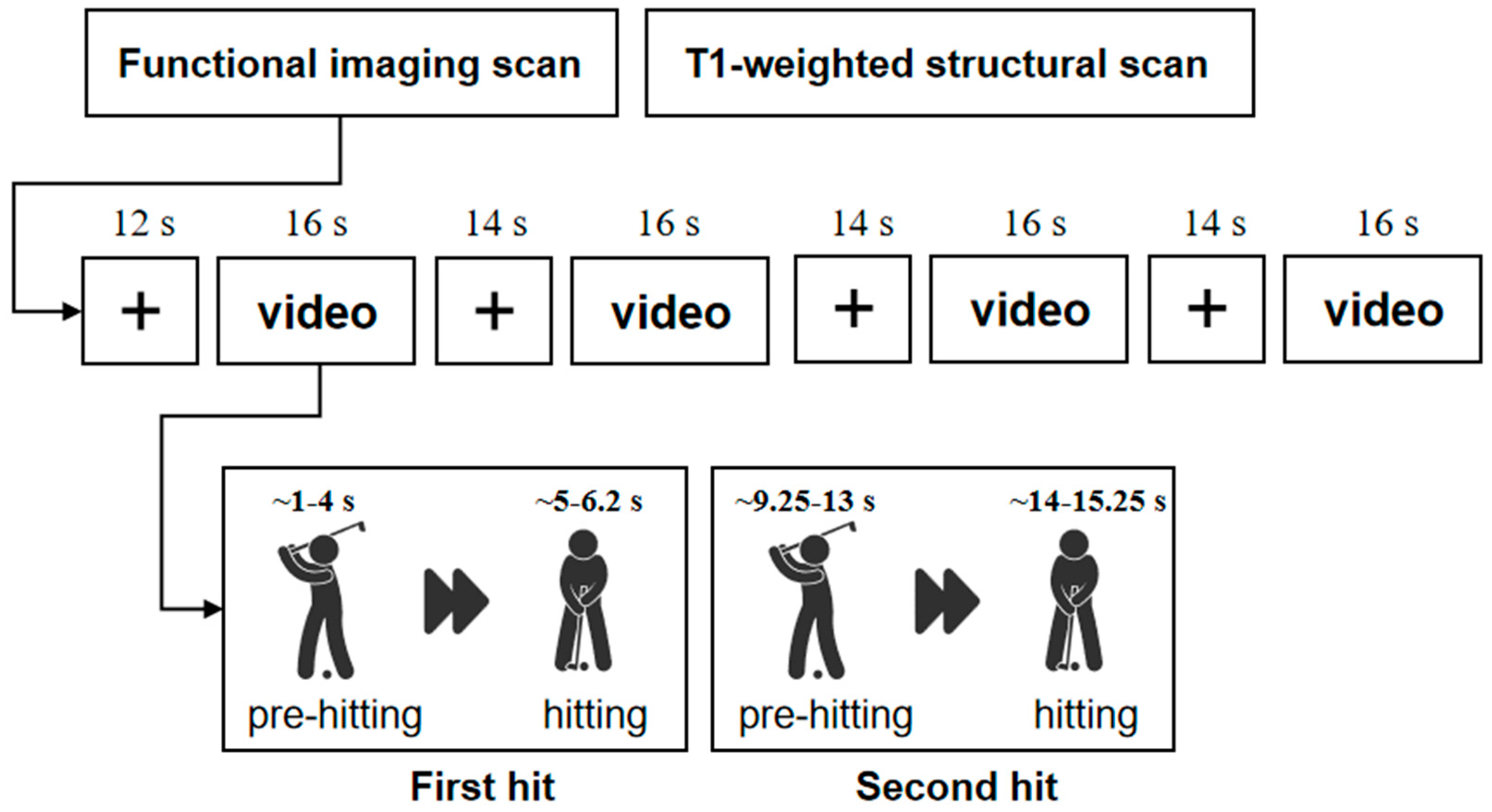
2.2. Experimental Design
2.3. Image Acquisition and Preprocessing
2.4. Generalized Linear Models (GLMs)
2.5. Generalized Psychophysiological Interaction (gPPI)
3. Results
3.1. Regions Activated Between Groups
3.2. The Difference Functional Connectivity in Conditions
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulder, T. Motor imagery and action observation: Cognitive tools for rehabilitation. J. Neural. Transm. 2007, 114, 1265–1278. [Google Scholar] [CrossRef]
- Calvo-Merino, B.; Glaser, D.E.; Grèzes, J.; Passingham, R.E.; Haggard, P. Action observation and acquired motor skills: An FMRI study with expert dancers. Cereb. Cortex 2005, 15, 1243–1249. [Google Scholar] [CrossRef]
- Jeannerod, M. Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage 2001, 14 Pt 2, S103–S109. [Google Scholar] [CrossRef] [PubMed]
- Avenanti, A.; Candidi, M.; Urgesi, C. Vicarious motor activation during action perception: Beyond correlational evidence. Front. Hum. Neurosci. 2013, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Eaves, D.L.; Riach, M.; Holmes, P.S.; Wright, D.J. Motor Imagery during Action Observation: A Brief Review of Evidence, Theory and Future Research Opportunities. Front. Neurosci. 2016, 10, 514. [Google Scholar] [CrossRef]
- Wright, M.J.; Bishop, D.T.; Jackson, R.C.; Abernethy, B. Brain regions concerned with the identification of deceptive soccer moves by higher-skilled and lower-skilled players. Front. Hum. Neurosci. 2013, 7, 851. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.T.; Wright, M.J.; Jackson, R.C.; Abernethy, B. Neural bases for anticipation skill in soccer: An FMRI study. J. Sport Exerc. Psychol. 2013, 35, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Meirhaeghe, N.; Sohn, H.; Jazayeri, M. A precise and adaptive neural mechanism for predictive temporal processing in the frontal cortex. Neuron 2021, 109, 2995–3011.e5. [Google Scholar] [CrossRef]
- Shipp, S.; Adams, R.A.; Friston, K.J. Reflections on agranular architecture: Predictive coding in the motor cortex. Trends Neurosci. 2013, 36, 706–716. [Google Scholar] [CrossRef]
- Churchland, M.M.; Yu, B.M.; Ryu, S.I.; Santhanam, G.; Shenoy, K.V. Neural variability in premotor cortex provides a signature of motor preparation. J. Neurosci. 2006, 26, 3697–3712. [Google Scholar] [CrossRef]
- Caminiti, R.; Borra, E.; Visco-Comandini, F.; Battaglia-Mayer, A.; Averbeck, B.B.; Luppino, G. Computational Architecture of the Parieto-Frontal Network Underlying Cognitive-Motor Control in Monkeys. eNeuro 2017, 4, ENEURO.0306-16.2017. [Google Scholar] [CrossRef]
- Tin, C.; Poon, C.S. Internal models in sensorimotor integration: Perspectives from adaptive control theory. J. Neural. Eng. 2005, 2, S147–S163. [Google Scholar] [CrossRef]
- Tsay, J.S.; Haith, A.M.; Ivry, R.B.; Kim, H.E. Interactions between sensory prediction error and task error during implicit motor learning. PLoS Comput. Biol. 2022, 18, e1010005. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Soh, K.G.; Jaafar, W.M.W.; Soh, K.L.; Deng, N.; Cao, S.; Li, M.; Liu, H. Mental fatigue in golf: A systematic review. PLoS ONE 2025, 20, e0310403. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.T.; Wang, K.P.; Huang, C.J.; Hung, T.M. Nonlinear refinement of functional brain connectivity in golf players of different skill levels. Sci. Rep. 2022, 12, 2365. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Han, J.K.; Kim, B.N.; Han, D.H. Brain networks governing the golf swing in professional golfers. J. Sports Sci. 2015, 33, 1980–1987. [Google Scholar] [CrossRef]
- Shao, X.; Luo, D.; Zhou, Y.; Xiao, Z.; Wu, J.; Tan, L.H.; Qiu, S.; Yuan, D. Myeloarchitectonic plasticity in elite golf players’ brains. Hum. Brain Mapp. 2022, 43, 3461–3468. [Google Scholar] [CrossRef]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [CrossRef]
- McLaren, D.G.; Ries, M.L.; Xu, G.; Johnson, S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage 2012, 61, 1277–1286. [Google Scholar] [CrossRef]
- Gong, D.; He, H.; Liu, D.; Ma, W.; Dong, L.; Luo, C.; Yao, D. Enhanced functional connectivity and increased gray matter volume of insula related to action video game playing. Sci. Rep. 2015, 5, 9763. [Google Scholar] [CrossRef]
- Milton, J.; Solodkin, A.; Hlustík, P.; Small, S.L. The mind of expert motor performance is cool and focused. Neuroimage 2007, 35, 804–813. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, M.; Qi, Y.; Chen, A.; Mou, H.; Jia, X.; Wang, Y. A systematic review and coordinate-based meta-analysis of resting-state fMRI in athletes from open and closed skills sports. Sci. Rep. 2025, 15, 21870. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, F.; Zhu, H.; Xiang, M.; Zhou, L. Neural Efficiency and Acquired Motor Skills: An fMRI Study of Expert Athletes. Front. Psychol. 2019, 10, 2752. [Google Scholar] [CrossRef]
- Wang, D.; Li, D.; Mirifar, A.; Zhou, C.; Luan, M. The neural dynamics of integrating prior and kinematic information during action anticipation in sport. Neuroimage 2025, 315, 121291. [Google Scholar] [CrossRef]
- Qiu, F.; Pi, Y.; Liu, K.; Zhu, H.; Li, X.; Zhang, J.; Wu, Y. Neural efficiency in basketball players is associated with bidirectional reductions in cortical activation and deactivation during multiple-object tracking task performance. Biol. Psychol. 2019, 144, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Mehrkanoon, S.; Boonstra, T.W.; Breakspear, M.; Hinder, M.; Summers, J.J. Upregulation of cortico-cerebellar functional connectivity after motor learning. Neuroimage 2016, 128, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Thulborn, K.R.; Corcos, D.M. Neural basis for the processes that underlie visually guided and internally guided force control in humans. J. Neurophysiol. 2003, 90, 3330–3340. [Google Scholar] [CrossRef]
- Cao, B.; Guo, Y.; Xia, F.; Li, L.; Ren, Z.; Lu, M.; Wang, J.; Huang, R. Dynamic reconfiguration of brain functional networks in world class gymnasts: A resting-state functional MRI study. Brain Commun. 2025, 7, fcaf083. [Google Scholar] [CrossRef] [PubMed]
- Ionta, S.; Heydrich, L.; Lenggenhager, B.; Mouthon, M.; Fornari, E.; Chapuis, D.; Gassert, R.; Blanke, O. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron 2011, 70, 363–374. [Google Scholar] [CrossRef]
- Farrell, M.J.; Robertson, I.H. The automatic updating of egocentric spatial relationships and its impairment due to right posterior cortical lesions. Neuropsychologia 2000, 38, 585–595. [Google Scholar] [CrossRef]
- Seidler, R.D.; Kwak, Y.; Fling, B.W.; Bernard, J.A. Neurocognitive mechanisms of error-based motor learning. Adv. Exp. Med. Biol. 2013, 782, 39–60. [Google Scholar] [PubMed]
- Tan, H.; Jenkinson, N.; Brown, P. Dynamic neural correlates of motor error monitoring and adaptation during trial-to-trial learning. J. Neurosci. 2014, 34, 5678–5688. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.A.; Seidler, R.D.; Hassevoort, K.M.; Benson, B.L.; Welsh, R.C.; Wiggins, J.L.; Jaeggi, S.M.; Buschkuehl, M.; Monk, C.S.; Jonides, J.; et al. Resting state cortico-cerebellar functional connectivity networks: A comparison of anatomical and self-organizing map approaches. Front. Neuroanat. 2012, 6, 31. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129 Pt 3, 564–583. [Google Scholar] [CrossRef]
- Fattori, P.; De Vitis, M.; Filippini, M.; Vaccari, F.E.; Diomedi, S.; Gamberini, M.; Galletti, C. Visual sensitivity at the service of action control in posterior parietal cortex. Front. Physiol. 2024, 15, 1408010. [Google Scholar] [CrossRef] [PubMed]
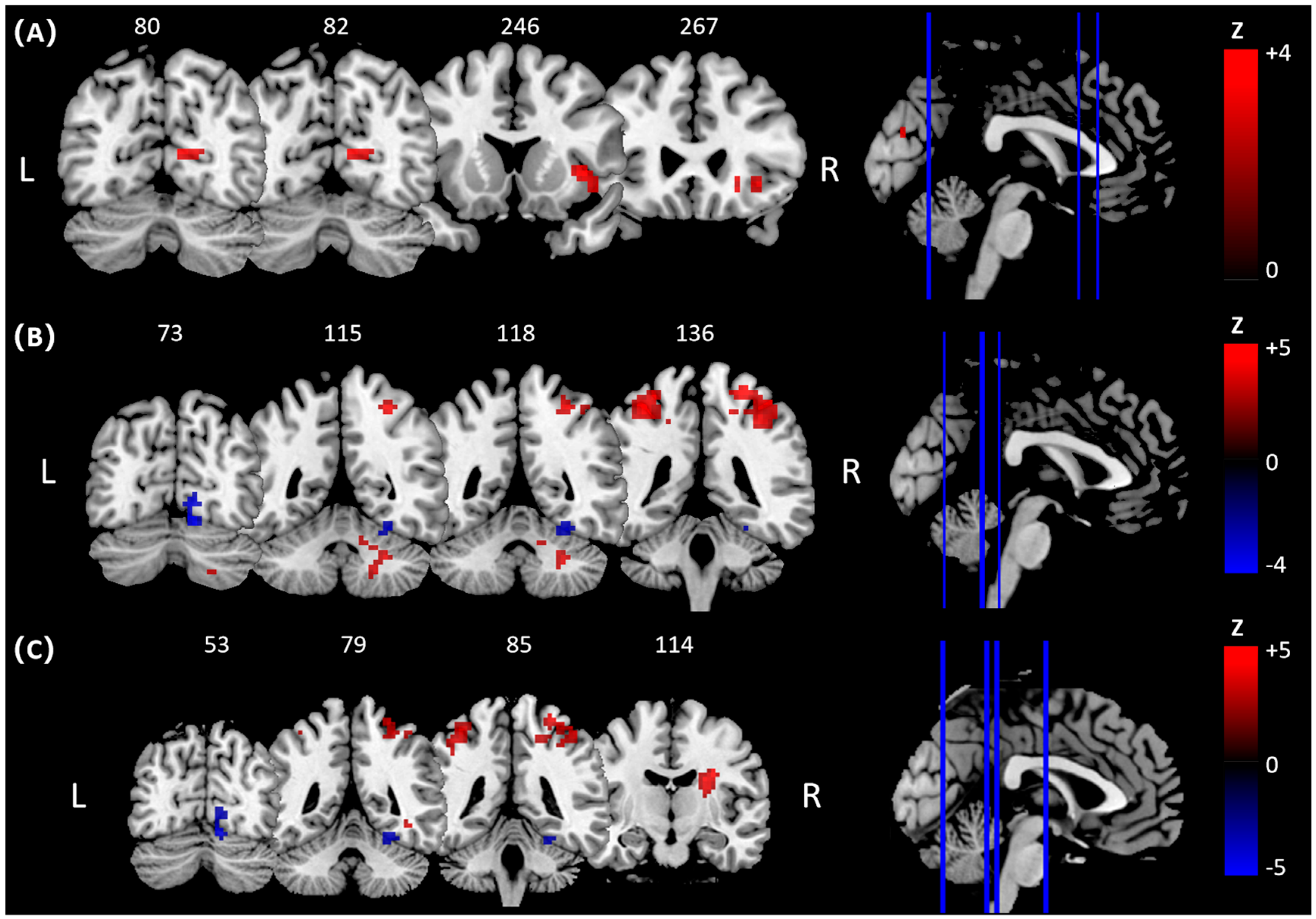

| Comparisons | Brain Regions/BA | Peak MNI Coordinates | Cluster Voxels | Peak Z Values | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Pre-hitting | Golf group > Control group | R Insula/BA 48 | 39 | 15 | 0 | 67 | 3.90 a |
| R Posterior Cingulate Cortex (PCC)/BA 17 | 18 | −60 | 12 | 69 | 3.85 a | ||
| Hitting | Golf group > Control group | R Cerebelum/BA 19 | 18 | −57 | −27 | 99 | 4.36 a |
| L Postcentral Gyrus/BA 2 | −36 | −39 | 57 | 105 | 4.26 a | ||
| R Postcentral Gyrus/BA 2 | 27 | −39 | 66 | 255 | 4.04 a | ||
| Golf group < Control group | R Cerebellum/BA 18 | 12 | −72 | −15 | 114 | 4.05 a | |
| Hitting > Pre-hitting | Golf group < Control group | L Postcentral Gyrus/BA 2 | −36 | −39 | 57 | 175 | 4.54 a |
| R Postcentral Gyrus/BA 4 | 33 | −33 | 66 | 199 | 4.06 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, X.; Tang, D.; Zhou, Y.; Zhou, X.; Zhao, S.; Xu, Q.; Zhu, Z. Dynamic Brain Activation and Connectivity in Elite Golfers During Distinct Golf Swing Phases: An fMRI Study. Brain Sci. 2025, 15, 1215. https://doi.org/10.3390/brainsci15111215
Shao X, Tang D, Zhou Y, Zhou X, Zhao S, Xu Q, Zhu Z. Dynamic Brain Activation and Connectivity in Elite Golfers During Distinct Golf Swing Phases: An fMRI Study. Brain Sciences. 2025; 15(11):1215. https://doi.org/10.3390/brainsci15111215
Chicago/Turabian StyleShao, Xueyun, Dongsheng Tang, Yulong Zhou, Xinyi Zhou, Shirui Zhao, Qiaoling Xu, and Zhiqiang Zhu. 2025. "Dynamic Brain Activation and Connectivity in Elite Golfers During Distinct Golf Swing Phases: An fMRI Study" Brain Sciences 15, no. 11: 1215. https://doi.org/10.3390/brainsci15111215
APA StyleShao, X., Tang, D., Zhou, Y., Zhou, X., Zhao, S., Xu, Q., & Zhu, Z. (2025). Dynamic Brain Activation and Connectivity in Elite Golfers During Distinct Golf Swing Phases: An fMRI Study. Brain Sciences, 15(11), 1215. https://doi.org/10.3390/brainsci15111215





