Targeting Executive Function and Language Impairments with tACS Combined with Behavioral Intervention in Primary Progressive Aphasia: A Case-Series, Pilot Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
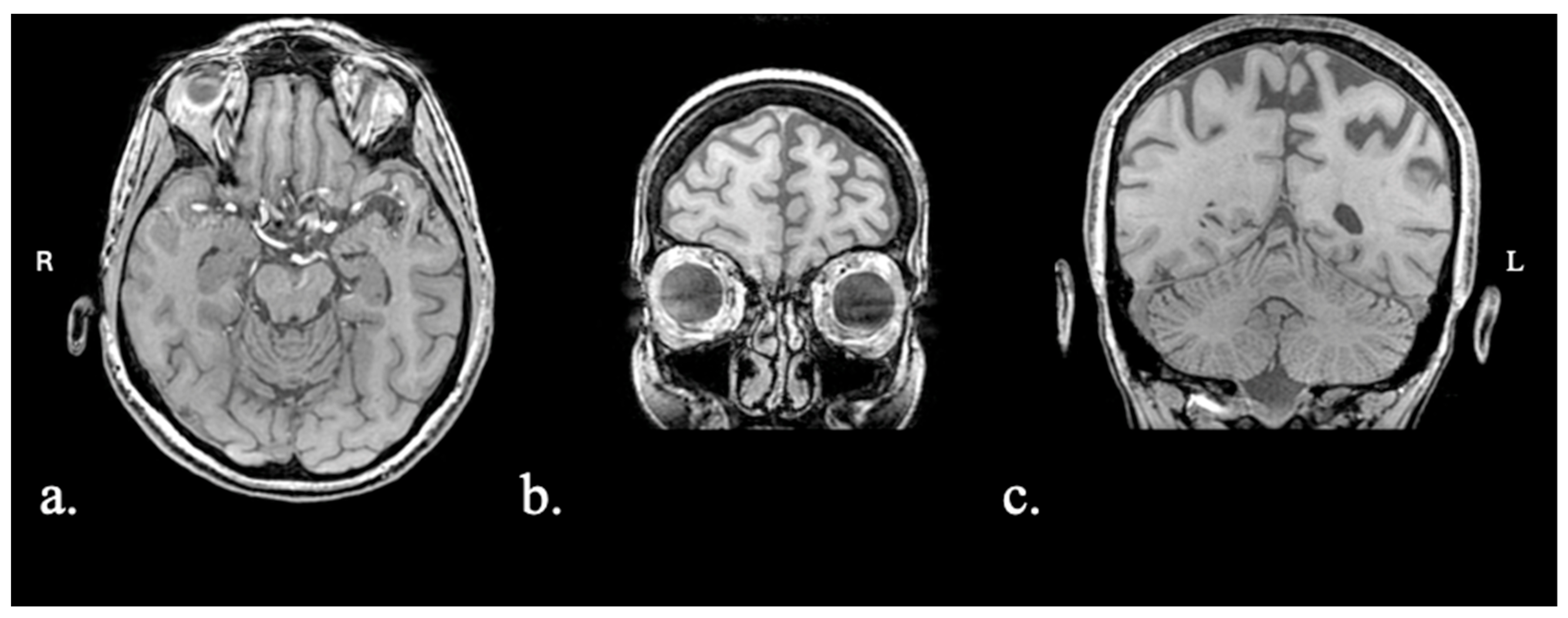
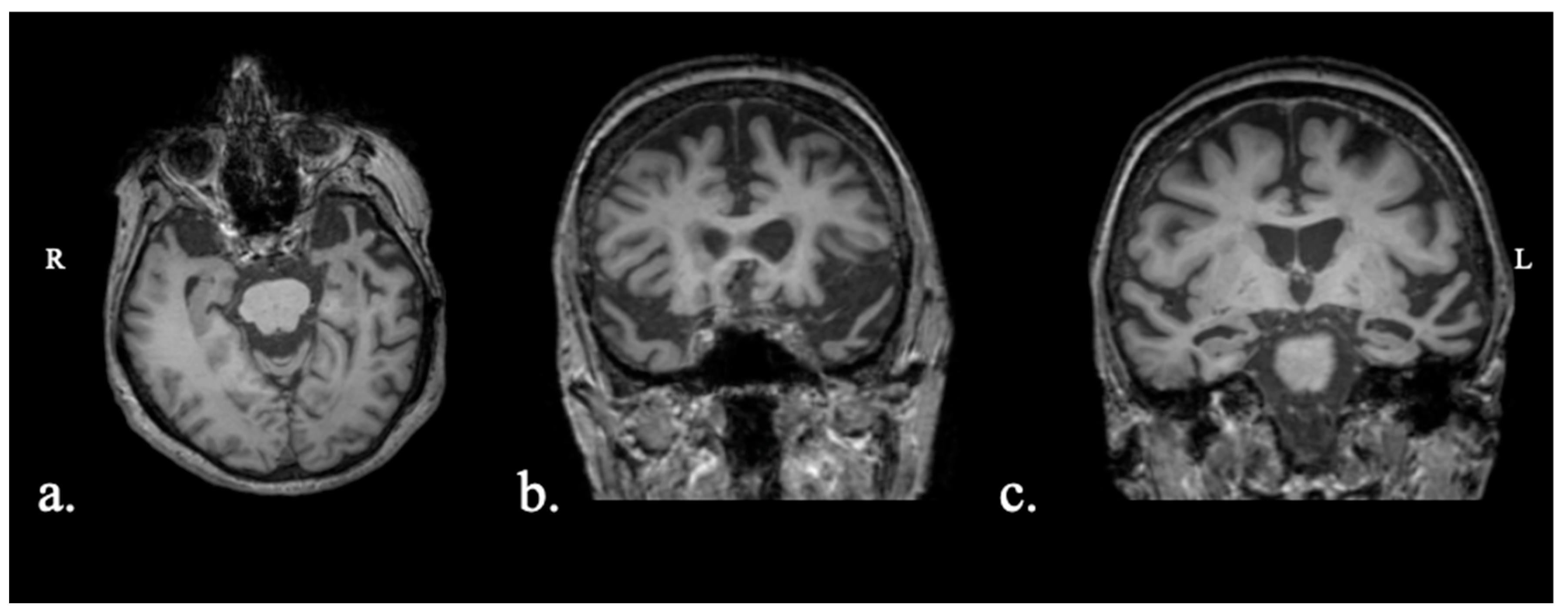
2.1.1. Patient FAY
2.1.2. Patient XTY
2.1.3. Patient ADY
2.1.4. Patient IZS
2.2. Treatment Protocol
2.2.1. Behavioral
2.2.2. Neuromodulation
2.3. Executive Function and Language Assessment
2.3.1. Executive Function Tasks
- Trail Making Test (TMT): Draw a line between 24 consecutive circles that are randomly arranged on a page to connect numbers in increasing order (TMT-A) or to connect numbers and letters in increasing order (TMT-B). Time (in seconds) to task completion is measured. Administration and scoring are as in [63].
- N-back: Determine if the current stimulus matches the one shown ‘n’ trials ago (n = 1). D-prime—a measurement of signal sensitivity that takes into account hits and false alarms—is calculated.
2.3.2. Language Tasks
- Spoken Naming: Name objects shown on black-and-white pictures (n = 30). Total word accuracy is measured. (Note: While performance was evaluated on 30 items, the available normative data differed with respect to two items. Therefore, normative data (z-scores and percentiles) are in reference to the 28 items that the two lists had in common.).
- Picture Description: Produce a narrative based on the Cookie Theft Picture [68]. No time restrictions were applied (i.e., participants were free to speak for as long as they wanted). Proportion of nouns and verbs relative to the total number of words produced is calculated.
- Semantic and Phonemic Fluency: Name as many items as possible that belong to a given semantic category (animals, fruits, and objects) or that start with a specific letter (Χ, Σ, A) [69]. The total number of items produced across the three prompts (per fluency type) is calculated.
2.4. Statistical Analysis
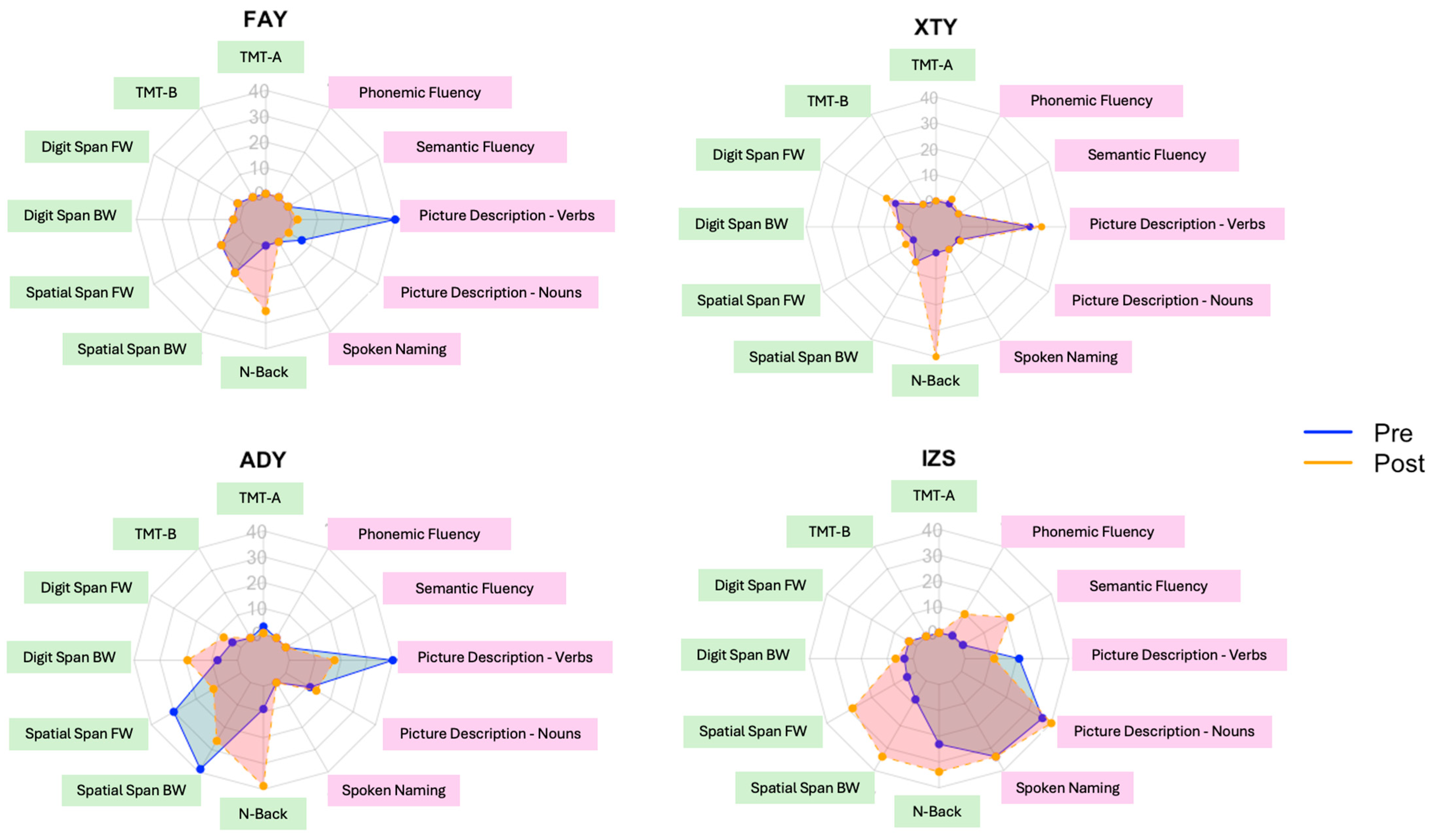
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Clément, F.; Gauthier, S.; Belleville, S. Executive Functions in Mild Cognitive Impairment: Emergence and Breakdown of Neural Plasticity. Cortex 2013, 49, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Favieri, F.; Boncompagni, I.; Agostini, F.; Cantone, M.; Casagrande, M. Executive Functions in Alzheimer Disease: A Systematic Review. Front. Aging Neurosci. 2019, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Basaglia-Pappas, S.; Laurent, B.; Getenet, J.-C.; Boulangé, A.; Rendón de laCruz, A.; Simoes Loureiro, I.; Lefebvre, L. Executive Profile of the Logopenic Variant of Primary Progressive Aphasia: Comparison with the Semantic and Non-Fluent Variants and Alzheimer’s Disease. Brain Sci. 2023, 13, 406. [Google Scholar] [CrossRef]
- Coemans, S.; Keulen, S.; Savieri, P.; Tsapkini, K.; Engelborghs, S.; Chrispeels, N.; Vandenborre, D.; Paquier, P.; Wilssens, I.; Declerck, M.; et al. Executive Functions in Primary Progressive Aphasia: A Meta-Analysis. Cortex 2022, 157, 304–322. [Google Scholar] [CrossRef]
- Eikelboom, W.S.; Janssen, N.; Jiskoot, L.C.; van den Berg, E.; Roelofs, A.; Kessels, R.P.C. Episodic and Working Memory Function in Primary Progressive Aphasia: A Meta-Analysis. Neurosci. Biobehav. Rev. 2018, 92, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Robbins, T.W. The Role of Prefrontal Cortex in Cognitive Control and Executive Function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Vanderhasselt, M.-A. Working Memory Improvement with Non-Invasive Brain Stimulation of the Dorsolateral Prefrontal Cortex: A Systematic Review and Meta-Analysis. Brain Cogn. 2014, 86, 1–9. [Google Scholar] [CrossRef]
- Imburgio, M.J.; Orr, J.M. Effects of Prefrontal tDCS on Executive Function: Methodological Considerations Revealed by Meta-Analysis. Neuropsychologia 2018, 117, 156–166. [Google Scholar] [CrossRef]
- Tippett, D.C.; Neophytou, K.; Tao, Y.; Gallegos, J.; Morrow, C.; Onyike, C.U.; Tsapkini, K. Long-Term, Home-Based Transcranial Direct Current Stimulation Coupled with Computerized Cognitive Training in Frontotemporal Dementia: A Case Report. J. Cent. Nerv. Syst. Dis. 2024, 16, 11795735241258435. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Petesi, M.; Brambilla, M.; Cosseddu, M.; Zanetti, O.; Miniussi, C.; Padovani, A.; Borroni, B. Treatment of Primary Progressive Aphasias by Transcranial Direct Current Stimulation Combined with Language Training. J. Alzheimers Dis. JAD 2014, 39, 799–808. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Paternicò, D.; Cosseddu, M.; Brambilla, M.; Petesi, M.; Premi, E.; Gasparotti, R.; Zanetti, O.; Padovani, A.; et al. Grey Matter Density Predicts the Improvement of Naming Abilities After tDCS Intervention in Agrammatic Variant of Primary Progressive Aphasia. Brain Topogr. 2016, 29, 738–751. [Google Scholar] [CrossRef]
- Roncero, C.; Service, E.; De Caro, M.; Thiel, A.; Probst, S.; Chertkow, H. Maximizing the Treatment Benefits of tDCS in Neurodegenerative Anomia. Front. Neurosci. 2019, 13, 1231. [Google Scholar] [CrossRef]
- Foxe, J.J.; Snyder, A.C. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG Alpha Oscillations: The Inhibition–Timing Hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Doppelmayr, M.; Pecherstorfer, T.; Freunberger, R.; Hanslmayr, S. EEG Alpha Synchronization and Functional Coupling during Top-down Processing in a Working Memory Task. Hum. Brain Mapp. 2005, 26, 148–155. [Google Scholar] [CrossRef]
- Nishida, K.; Yoshimura, M.; Isotani, T.; Yoshida, T.; Kitaura, Y.; Saito, A.; Mii, H.; Kato, M.; Takekita, Y.; Suwa, A.; et al. Differences in Quantitative EEG between Frontotemporal Dementia and Alzheimer’s Disease as Revealed by LORETA. Clin. Neurophysiol. 2011, 122, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Lejko, N.; Larabi, D.I.; Herrmann, C.S.; Aleman, A.; Ćurčić-Blake, B. Alpha Power and Functional Connectivity in Cognitive Decline: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2020, 78, 1047–1088. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, G.; Wendling, F.; Merlet, I.; Molaee-Ardekani, B.; Mekonnen, A.; Salvador, R.; Soria-Frisch, A.; Grau, C.; Dunne, S.; Miranda, P.C. Transcranial Current Brain Stimulation (tCS): Models and Technologies. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Paulus, W. Transcranial Alternating Current Stimulation (tACS). Front. Hum. Neurosci. 2013, 7, 317. [Google Scholar] [CrossRef]
- Kraft, J.D.; Hampstead, B.M. A Systematic Review of tACS Effects on Cognitive Functioning in Older Adults Across the Healthy to Dementia Spectrum. Neuropsychol. Rev. 2024, 34, 1165–1190. [Google Scholar] [CrossRef]
- Jones, K.T.; Ostrand, A.E.; Gazzaley, A.; Zanto, T.P. Enhancing Cognitive Control in Amnestic Mild Cognitive Impairment via At-Home Non-Invasive Neuromodulation in a Randomized Trial. Sci. Rep. 2023, 13, 7435. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.G.; Vellage, A.-K.; Heinze, H.-J.; Zaehle, T. Entrainment of Human Alpha Oscillations Selectively Enhances Visual Conjunction Search. PLoS ONE 2015, 10, e0143533. [Google Scholar] [CrossRef] [PubMed]
- Yaple, Z.; Martinez-Saito, M.; Awasthi, B.; Feurra, M.; Shestakova, A.; Klucharev, V. Transcranial Alternating Current Stimulation Modulates Risky Decision Making in a Frequency-Controlled Experiment. eNeuro 2017, 4, ENEURO.0136-17.2017. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Jeong, H.; Roh, D.; Kim, D.H. tACS as a Promising Therapeutic Option for Improving Cognitive Function in Mild Cognitive Impairment: A Direct Comparison between tACS and tDCS. J. Psychiatr. Res. 2021, 141, 248–256. [Google Scholar] [CrossRef]
- Lang, S.; Gan, L.S.; Alrazi, T.; Monchi, O. Theta Band High Definition Transcranial Alternating Current Stimulation, but Not Transcranial Direct Current Stimulation, Improves Associative Memory Performance. Sci. Rep. 2019, 9, 8562. [Google Scholar] [CrossRef]
- Senkowski, D.; Sobirey, R.; Haslacher, D.; Soekadar, S.R. Boosting Working Memory: Uncovering the Differential Effects of tDCS and tACS. Cereb. Cortex Commun. 2022, 3, tgac018. [Google Scholar] [CrossRef]
- Hartwigsen, G. Flexible Redistribution in Cognitive Networks. Trends Cogn. Sci. 2018, 22, 687–698. [Google Scholar] [CrossRef]
- Mesulam, M.-M. Primary Progressive Aphasia—Differentiation from Alzheimer’s Disease. Ann. Neurol. 1987, 22, 533–534. [Google Scholar] [CrossRef]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of Primary Progressive Aphasia and Its Variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Kamath, V.; Chaney, G.-A.S.; DeRight, J.; Onyike, C.U. A Meta-Analysis of Neuropsychological, Social Cognitive, and Olfactory Functioning in the Behavioral and Language Variants of Frontotemporal Dementia. Psychol. Med. 2019, 49, 2669–2680. [Google Scholar] [CrossRef]
- Karpathiou, N.; Kambanaros, M. Comparing Individuals with PPA to Individuals with AD: Cognitive and Linguistic Profiles. Front. Commun. 2022, 7, 893471. [Google Scholar] [CrossRef]
- Peristeri, E.; Durrleman, S.; Papageorgiou, S.; Potagas, C.; Frantzidis, C.; Kotrotsios, A.; Scarmeas, N.; Tsapkini, K. Theory of Mind Deficits in Non-Fluent Primary Progressive Aphasia. Cortex 2025, 186, 116–127. [Google Scholar] [CrossRef]
- Neophytou, K.; Williamson, K.; Herrmann, O.; Afthinos, A.; Gallegos, J.; Martin, N.; Tippett, D.C.; Tsapkini, K. Home-Based Transcranial Direct Current Stimulation in Primary Progressive Aphasia: A Pilot Study. Brain Sci. 2024, 14, 391. [Google Scholar] [CrossRef] [PubMed]
- Tsapkini, K.; Webster, K.T.; Ficek, B.N.; Desmond, J.E.; Onyike, C.U.; Rapp, B.; Frangakis, C.E.; Hillis, A.E. Electrical Brain Stimulation in Different Variants of Primary Progressive Aphasia: A Randomized Clinical Trial. Alzheimers Dement. Transl. Res. Clin. Interv. 2018, 4, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.M.; Goldberg, E.B.; Sebastian, R.; Walker, A.; Meier, E.L.; Hillis, A.E. Transcranial Direct Current Stimulation Paired With Verb Network Strengthening Treatment Improves Verb Naming in Primary Progressive Aphasia: A Case Series. Am. J. Speech Lang. Pathol. 2022, 31, 1736–1754. [Google Scholar] [CrossRef] [PubMed]
- Shah-Basak, P.; Fernandez, A.; Armstrong, S.E.M.; Hodzic-Santor, B.H.; Lavoie, M.; Jokel, R.; Meltzer, J.A. Behavioural and Neurophysiological Responses to Written Naming Treatment and High Definition tDCS: A Case Study in Advanced Primary Progressive Aphasia. Aphasiology 2022, 36, 1182–1205. [Google Scholar] [CrossRef]
- Nickels, K.; Beeson, P.M.; Kielar, A. Addressing Phonological Deficit in Primary Progressive Aphasia With Behavioral Intervention and Transcranial Direct Current Stimulation. J. Speech Lang. Hear. Res. 2025, 68, 2348–2385. [Google Scholar] [CrossRef]
- George, A.; McConathey, E.; Vogel-Eyny, A.; Galletta, E.; Pilloni, G.; Charvet, L. Feasibility of Home-Based Transcranial Direct Current Stimulation Combined with Personalized Word Retrieval for Improving Naming in Primary Progressive Aphasia. Front. Neurol. 2025, 16, 1543712. [Google Scholar] [CrossRef]
- Salis, C.; Hwang, F.; Howard, D.; Lallini, N. Short-Term and Working Memory Treatments for Improving Sentence Comprehension in Aphasia: A Review and a Replication Study. Semin. Speech Lang. 2017, 38, 29–39. [Google Scholar] [CrossRef]
- Zakarias, L.; Salis, C.; Wartenburger, I. Transfer Effects on Spoken Sentence Comprehension and Functional Communication after Working Memory Training in Stroke Aphasia. J. Neurolinguistics 2018, 48, 47–63. [Google Scholar] [CrossRef]
- Paraskevas, G.P.; Kasselimis, D.; Kourtidou, E.; Constantinides, V.; Bougea, A.; Potagas, C.; Evdokimidis, I.; Kapaki, E. Cerebrospinal Fluid Biomarkers as a Diagnostic Tool of the Underlying Pathology of Primary Progressive Aphasia. J. Alzheimers Dis. 2017, 55, 1453–1461. [Google Scholar] [CrossRef]
- Potagas, C.; Nikitopoulou, Z.; Angelopoulou, G.; Kasselimis, D.; Laskaris, N.; Kourtidou, E.; Constantinides, V.C.; Bougea, A.; Paraskevas, G.P.; Papageorgiou, G. Silent Pauses and Speech Indices as Biomarkers for Primary Progressive Aphasia. Medicina 2022, 58, 1352. [Google Scholar] [CrossRef]
- Patel, N.; Peterson, K.A.; Ingram, R.U.; Storey, I.; Cappa, S.F.; Catricala, E.; Halai, A.; Patterson, K.E.; Lambon Ralph, M.A.; Rowe, J.B.; et al. A ‘Mini Linguistic State Examination’ to Classify Primary Progressive Aphasia. Brain Commun. 2022, 4, fcab299. [Google Scholar] [CrossRef] [PubMed]
- Tsapkini, K.; Vlahou, C.H.; Potagas, C. Adaptation and Validation of Standardized Aphasia Tests in Different Languages: Lessons from the Boston Diagnostic Aphasia Examination—Short Form in Greek. Behav. Neurol. 2010, 22, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Simos, P.G.; Kasselimis, D.; Mouzaki, A. Age, Gender, and Education Effects on Vocabulary Measures in Greek. Aphasiology 2011, 25, 475–491. [Google Scholar] [CrossRef]
- Simos, P.G.; Kasselimis, D.; Potagas, C.; Evdokimidis, I. Verbal Comprehension Ability in Aphasia: Demographic and Lexical Knowledge Effects. Behav. Neurol. 2014, 2014, 258303. [Google Scholar] [CrossRef][Green Version]
- Simos, P.G.; Sideridis, G.D.; Kasselimis, D.; Mouzaki, A. Reading Fluency Estimates of Current Intellectual Function: Demographic Factors and Effects of Type of Stimuli. J. Int. Neuropsychol. Soc. 2013, 19, 355–361. [Google Scholar] [CrossRef]
- Basak, C.; Qin, S.; O’Connell, M.A. Differential Effects of Cognitive Training Modules in Healthy Aging and Mild Cognitive Impairment: A Comprehensive Meta-Analysis of Randomized Controlled Trials. Psychol. Aging 2020, 35, 220. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.T.M.; Mowszowski, L.; Naismith, S.L.; Chadwick, V.L.; Valenzuela, M.; Lampit, A. Computerized Cognitive Training in Older Adults With Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Am. J. Psychiatry 2017, 174, 329–340. [Google Scholar] [CrossRef]
- Lampit, A.; Hallock, H.; Valenzuela, M. Computerized Cognitive Training in Cognitively Healthy Older Adults: A Systematic Review and Meta-Analysis of Effect Modifiers. PLoS Med. 2014, 11, e1001756. [Google Scholar] [CrossRef]
- Carvalho, C.M.; Poltronieri, B.C.; Reuwsaat, K.; Reis, M.E.A.; Panizzutti, R. Digital Cognitive Training for Functionality in Mild Cognitive Impairment: A Randomized Controlled Clinical Trial. GeroScience 2025, 47, 5111–5121. [Google Scholar] [CrossRef]
- Harvey, P.D.; Dowell-Esquivel, C.; Macchiarelli, J.E.; Martinez, A.; Kallestrup, P.; Czaja, S.J. Early Prediction of Mastery of a Computerized Functional Skills Training Program in Participants with Mild Cognitive Impairment. Int. Psychogeriatr. 2024, 36, 1182–1193. [Google Scholar] [CrossRef]
- Valdés, E.G.; Andel, R.; Lister, J.J.; Gamaldo, A.; Edwards, J.D. Can Cognitive Speed of Processing Training Improve Everyday Functioning Among Older Adults With Psychometrically Defined Mild Cognitive Impairment? J. Aging Health 2019, 31, 595–610. [Google Scholar] [CrossRef]
- Duff, K.; Ying, J.; Suhrie, K.R.; Dalley, B.C.A.; Atkinson, T.J.; Porter, S.M.; Dixon, A.M.; Hammers, D.B.; Wolinsky, F.D. Computerized Cognitive Training in Amnestic Mild Cognitive Impairment: A Randomized Clinical Trial. J. Geriatr. Psychiatry Neurol. 2022, 35, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Tomaszczyk, J.C.; Dawson, D.; Turner, G.R.; Colella, B.; Green, R.E.A. Feasibility of Online Self-Administered Cognitive Training in Moderate–Severe Brain Injury. Disabil. Rehabil. 2017, 39, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Mahncke, H.W.; DeGutis, J.; Levin, H.; Newsome, M.R.; Bell, M.D.; Grills, C.; French, L.M.; Sullivan, K.W.; Kim, S.-J.; Rose, A. A Randomized Clinical Trial of Plasticity-Based Cognitive Training in Mild Traumatic Brain Injury. Brain 2021, 144, 1994–2008. [Google Scholar] [CrossRef]
- Wolinsky, F.D.; Vander Weg, M.W.; Howren, M.B.; Jones, M.P.; Dotson, M.M. Effects of Cognitive Speed of Processing Training on a Composite Neuropsychological Outcome: Results at One-Year from the IHAMS Randomized Controlled Trial. Int. Psychogeriatr. 2016, 28, 317–330. [Google Scholar] [CrossRef]
- Gray, N.; Yoon, J.-S.; Charness, N.; Boot, W.R.; Roque, N.A.; Andringa, R.; Harrell, E.R.; Lewis, K.G.; Vitale, T. Relative Effectiveness of General versus Specific Cognitive Training for Aging Adults. Psychol. Aging 2022, 37, 210. [Google Scholar] [CrossRef]
- Meltzer, J.A.; Kates Rose, M.; Le, A.Y.; Spencer, K.A.; Goldstein, L.; Gubanova, A.; Lai, A.C.; Yossofzai, M.; Armstrong, S.E.M.; Bialystok, E. Improvement in Executive Function for Older Adults through Smartphone Apps: A Randomized Clinical Trial Comparing Language Learning and Brain Training. Aging Neuropsychol. Cogn. 2023, 30, 150–171. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.L.; Shaw, J.S.; Hosseini, S.M.H. Toward Personalized Cognitive Training in Older Adults: A Pilot Investigation of the Effects of Baseline Performance and Age on Cognitive Training Outcomes. J. Alzheimers Dis. 2024, 97, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Im, C.-H.; Park, J.-H.; Shim, M.; Chang, W.H.; Kim, Y.-H. Evaluation of Local Electric Fields Generated by Transcranial Direct Current Stimulation with an Extracephalic Reference Electrode Based on Realistic 3D Body Modeling. Phys. Med. Biol. 2012, 57, 2137. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative Data Stratified by Age and Education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Manual for the Wechsler Adult Intelligence Scale-Revised (WAIS-R); Psychological Corp.: San Antonio, TX, USA, 1981. [Google Scholar]
- Corsi, P. Memory and the Medial Temporal Region of the Brain. Ph.D. Dissertation, McGill University, Montreal, QB, Canada, 1972. [Google Scholar]
- Simos, P.G.; Papastefanakis, E.; Panou, T.; Kasselimis, D. The Greek Memory Scale; Laboratory of Applied Psychology, University of Crete: Rethymno, Greece, 2011. [Google Scholar]
- Kessels, R.P.C.; van Zandvoort, M.J.E.; Postma, A.; Kappelle, L.J.; de Haan, E.H.F. The Corsi Block-Tapping Task: Standardization and Normative Data. Appl. Neuropsychol. 2000, 7, 252–258. [Google Scholar] [CrossRef]
- Goodglass, H.; Kaplan, E.; Barresi, B. BDAE-3: Boston Diagnostic Aphasia Examination, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Kosmidis, M.H.; Vlahou, C.H.; Panagiotaki, P.; Kiosseoglou, G. The Verbal Fluency Task in the Greek Population: Normative Data, and Clustering and Switching Strategies. J. Int. Neuropsychol. Soc. 2004, 10, 164–172. [Google Scholar] [CrossRef]
- Crawford, J.R.; Garthwaite, P.H. Comparison of a Single Case to a Control or Normative Sample in Neuropsychology: Development of a Bayesian Approach. Cogn. Neuropsychol. 2007, 24, 343–372. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Makowski, D. The Psycho Package: An Efficient and Publishing-Oriented Workflow for Psychological Science. J. Open Source Softw. 2018, 3, 470. [Google Scholar] [CrossRef]
- Huber, S.; Klein, E.; Moeller, K.; Willmes, K. Comparing a Single Case to a Control Group–Applying Linear Mixed Effects Models to Repeated Measures Data. Cortex 2015, 71, 148–159. [Google Scholar] [CrossRef]
- Van Den Berg, E.; Dijkzeul, J.C.M.; Poos, J.M.; Eikelboom, W.S.; Van Hemmen, J.; Franzen, S.; De Jong, F.J.; Dopper, E.G.P.; Vonk, J.M.J.; Papma, J.M.; et al. Differential Linguistic Features of Verbal Fluency in Behavioral Variant Frontotemporal Dementia and Primary Progressive Aphasia. Appl. Neuropsychol. Adult 2024, 31, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Scheffel, L.; Duffy, J.R.; Strand, E.A.; Josephs, K.A. Word Fluency Test Performance in Primary Progressive Aphasia and Primary Progressive Apraxia of Speech. Am. J. Speech Lang. Pathol. 2021, 30, 2635–2642. [Google Scholar] [CrossRef]
- Kane, M.J.; Conway, A.R.A.; Miura, T.K.; Colflesh, G.J.H. Working Memory, Attention Control, and the n-Back Task: A Question of Construct Validity. J. Exp. Psychol. Learn. Mem. Cogn. 2007, 33, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghen, P.; Basak, C. Ageing and Switching of the Focus of Attention in Working Memory: Results from a Modified N-Back Task. Q. J. Exp. Psychol. Sect. A 2005, 58, 134–154. [Google Scholar] [CrossRef] [PubMed]
- Fromm, A.E.; Trujillo-Llano, C.; Grittner, U.; Meinzer, M.; Flöel, A.; Antonenko, D. Increased Variability in Response to Transcranial Direct Current Stimulation in Healthy Older Compared to Young Adults: A Systematic Review and Meta-Analysis. Brain Stimulat. 2025, 18, 1257–1265. [Google Scholar] [CrossRef] [PubMed]


| Participant | Age | Sex | Education | Years Since Onset | Diagnosis | MoCA |
|---|---|---|---|---|---|---|
| FAY | 63 | F | 16 | 1 | lvPPA | 20 |
| XTY | 76 | F | 15 | 2 | lvPPA | 11 |
| ADY | 76 | F | 16 | 3 | svPPA | 15 |
| IZS | 60 | M | 18 | 1 | lvPPA | 21 |
| MLSE Total | MLSE Articulation | MLSE Phonology | MLSE Semantics | MLSE Syntax | MLSE Working Memory | |
| FAY | 88 | 30 | 29 | 17 | 7 | 5 |
| XTY | 73 | 30 | 24 | 8 | 7 | 4 |
| ADY | 75 | 30 | 27 | 8 | 6 | 4 |
| IZS | 96 | 30 | 29 | 19 | 8 | 10 |
| FAY | XTY | ADY | IZS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain | Task | Metric | Pre | Post | Diff | Pre | Post | Diff | Pre | Post | Diff | Pre | Post | Diff |
| Executive Function | TMT-A | Seconds | 93 | 88 | −5 | 264 | 209 | −55 | 69 | 80 | 11 | 61 | 61 | 0 |
| TMT-B | Seconds | 300 | 300 | 0 | 300 | 300 | 0 | 300 | 300 | 0 | 216 | 195 | −21 | |
| Digit Span FW | Score | 7 | 7 | 0 | 13 | 15 | 2 | 9 | 12 | 3 | 7 | 9 | 2 | |
| Digit Span BW | Score | 4 | 4 | 0 | 7 | 6 | −1 | 8 | 12 | 4 | 7 | 9 | 2 | |
| Spatial Span FW | Score | 6 | 6 | 0 | 3 | 5 | 2 | 7 | 6 | −1 | 5 | 7 | 2 | |
| Spatial Span BW | Score | 6 | 6 | 0 | 4 | 4 | 0 | 7 | 6 | −1 | 5 | 8 | 3 | |
| N-back | d-prime | 1.25 | 2.21 | 0.96 | 0.12 | 1.84 | 1.72 | 2.71 | 4.38 | 1.67 | 3.37 | 3.43 | 0.06 | |
| Language | Spoken Naming | # of correct items | 9 | 10 | 1 | 6 | 5 | −1 | 1 | 1 | 0 | 26 | 28 | 2 |
| Picture Description | % nouns | 0.17 | 0.10 | −0.07 | 0.07 | 0.11 | 0.04 | 0.17 | 0.18 | 0.01 | 0.23 | 0.24 | 0.01 | |
| Picture Description | % verbs | 0.25 | 0.10 | −0.15 | 0.17 | 0.18 | 0.01 | 0.20 | 0.15 | −0.05 | 0.17 | 0.14 | −0.03 | |
| Semantic Fluency | # of items | 5 | 8 | 3 | 5 | 7 | 2 | 11 | 6 | −5 | 31 | 50 | 19 | |
| Phonemic Fluency | # of items | 4 | 4 | 0 | 15 | 22 | 7 | 9 | 5 | −4 | 16 | 29 | 13 | |
| Domain | Task | Patient | Pre | Post | ||
|---|---|---|---|---|---|---|
| Cohen’s d | p-Value | Cohen’s d | p-Value | |||
| Executive Function | TMT-A | FAY | 8.86 | <0.001 * | 8.14 | <0.001 * |
| XTY | 14.51 | <0.001 * | 10.92 | <0.001 * | ||
| ADY | 1.78 | 0.047 * | 2.50 | 0.011 * | ||
| IZS | 4.26 | <0.001 * | 4.26 | <0.001 * | ||
| TMT-B | FAY | 12.66 | <0.001 * | 12.66 | <0.001 * | |
| XTY | 4.51 | <0.001 * | 4.51 | <0.001 * | ||
| ADY | 4.51 | <0.001 * | 4.51 | <0.001 * | ||
| IZS | 8.15 | <0.001 * | 7.02 | <0.001 * | ||
| N-back | FAY | −2.95 | 0.005 * | −1.80 | 0.047 * | |
| XTY | −4.31 | <0.001 * | −2.25 | 0.020 * | ||
| ADY | −1.20 | NS | 0.81 | NS | ||
| IZS | −0.41 | NS | −0.33 | NS | ||
| Language | Spoken Naming | FAY | −6.86 | <0.001 * | −6.46 | <0.001 * |
| XTY | −8.08 | <0.001 * | −8.48 | <0.001 * | ||
| ADY | −10.09 | <0.001 * | −10.09 | <0.001 * | ||
| IZS | 0.00 | NS | 0.81 | NS | ||
| Picture Description—Nouns | FAY | −1.18 | NS | −2.56 | 0.009 * | |
| XTY | −3.08 | 0.003 * | −2.30 | 0.016 * | ||
| ADY | −1.08 | NS | −0.93 | NS | ||
| IZS | 0.08 | NS | 0.23 | NS | ||
| Picture Description—Verbs | FAY | 0.80 | NS | −1.70 | 0.054 ~ | |
| XTY | −0.45 | NS | −0.30 | NS | ||
| ADY | 0.04 | NS | −0.75 | NS | ||
| IZS | −0.50 | NS | −0.97 | NS | ||
| Semantic Fluency | FAY | −4.92 | <0.001 * | −4.62 | <0.001 * | |
| XTY | −4.92 | <0.001 * | −4.72 | <0.001 * | ||
| ADY | −4.33 | <0.001 * | −4.82 | <0.001 * | ||
| IZS | −2.35 | 0.017 * | −0.47 | NS | ||
| Phonemic Fluency | FAY | −4.07 | <0.001 * | −4.07 | <0.001 * | |
| XTY | −2.75 | 0.008 * | −1.90 | 0.040 * | ||
| ADY | −3.47 | 0.002 * | −3.95 | 0.001 * | ||
| IZS | −2.63 | 0.010 * | −1.06 | NS | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neophytou, K.; Kasselimis, D.S.; Angelopoulou, G.; Deligiannaki, A.; Bourtsoukli, R.; Peristeri, E.; Spanou, V.; Papageorgiou, S.G.; Constantinides, V.C.; Potagas, C.; et al. Targeting Executive Function and Language Impairments with tACS Combined with Behavioral Intervention in Primary Progressive Aphasia: A Case-Series, Pilot Investigation. Brain Sci. 2025, 15, 1199. https://doi.org/10.3390/brainsci15111199
Neophytou K, Kasselimis DS, Angelopoulou G, Deligiannaki A, Bourtsoukli R, Peristeri E, Spanou V, Papageorgiou SG, Constantinides VC, Potagas C, et al. Targeting Executive Function and Language Impairments with tACS Combined with Behavioral Intervention in Primary Progressive Aphasia: A Case-Series, Pilot Investigation. Brain Sciences. 2025; 15(11):1199. https://doi.org/10.3390/brainsci15111199
Chicago/Turabian StyleNeophytou, Kyriaki, Dimitrios S. Kasselimis, Georgia Angelopoulou, Areti Deligiannaki, Rafailia Bourtsoukli, Eleni Peristeri, Vasilina Spanou, Sokratis G. Papageorgiou, Vasilios C. Constantinides, Constantin Potagas, and et al. 2025. "Targeting Executive Function and Language Impairments with tACS Combined with Behavioral Intervention in Primary Progressive Aphasia: A Case-Series, Pilot Investigation" Brain Sciences 15, no. 11: 1199. https://doi.org/10.3390/brainsci15111199
APA StyleNeophytou, K., Kasselimis, D. S., Angelopoulou, G., Deligiannaki, A., Bourtsoukli, R., Peristeri, E., Spanou, V., Papageorgiou, S. G., Constantinides, V. C., Potagas, C., & Tsapkini, K. (2025). Targeting Executive Function and Language Impairments with tACS Combined with Behavioral Intervention in Primary Progressive Aphasia: A Case-Series, Pilot Investigation. Brain Sciences, 15(11), 1199. https://doi.org/10.3390/brainsci15111199







