rTg4510 Tauopathy Mice Exhibit Non-Spatial Memory Deficits Prevented by Doxycycline Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavioral Tests
2.2.1. Spontaneous Physical Activities
2.2.2. Trace Eyeblink Conditioning
2.2.3. Contextual Fear Conditioning
2.3. Statistical Analysis
2.4. Statement on the Use of Generative AI
3. Results
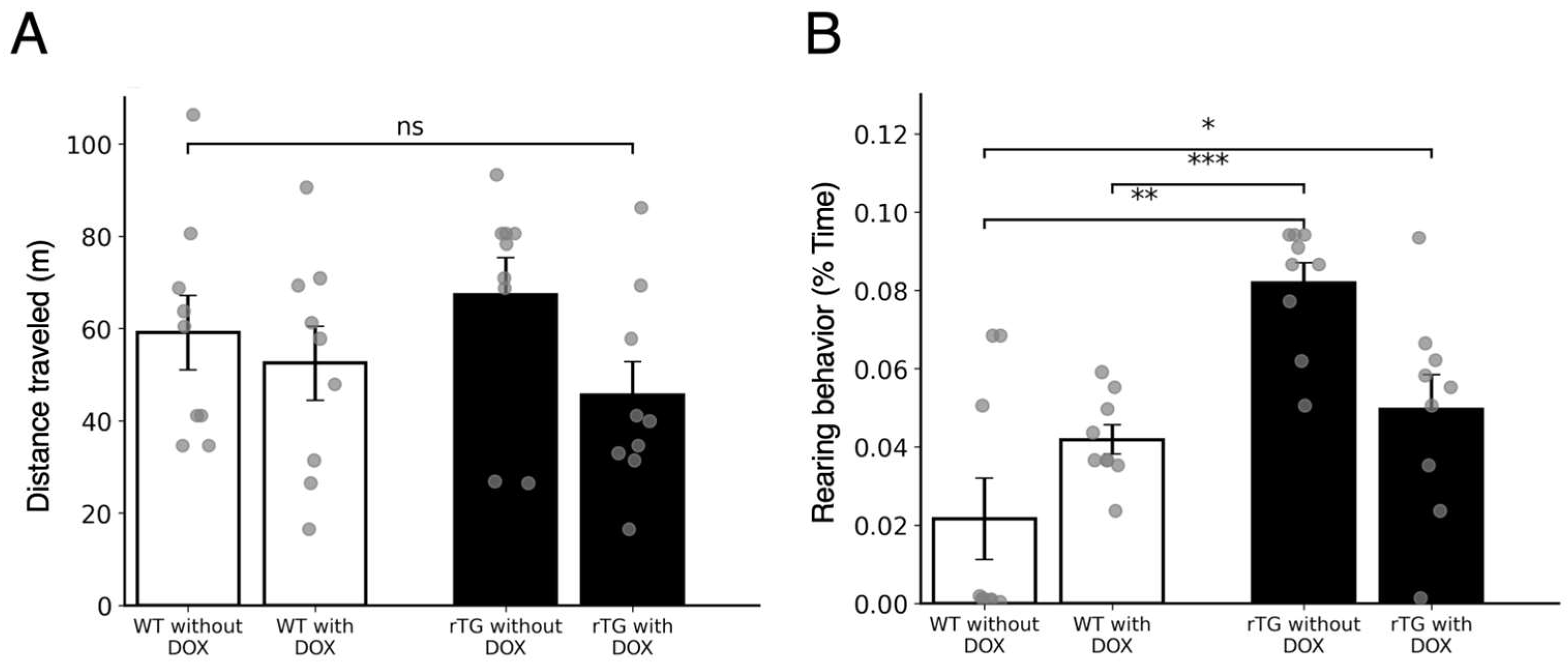
3.1. Spontaneous Physical Activity Assessment Reveals Preserved Motor Function with Increased Rearing Behavior in rTg4510 Mice Without DOX
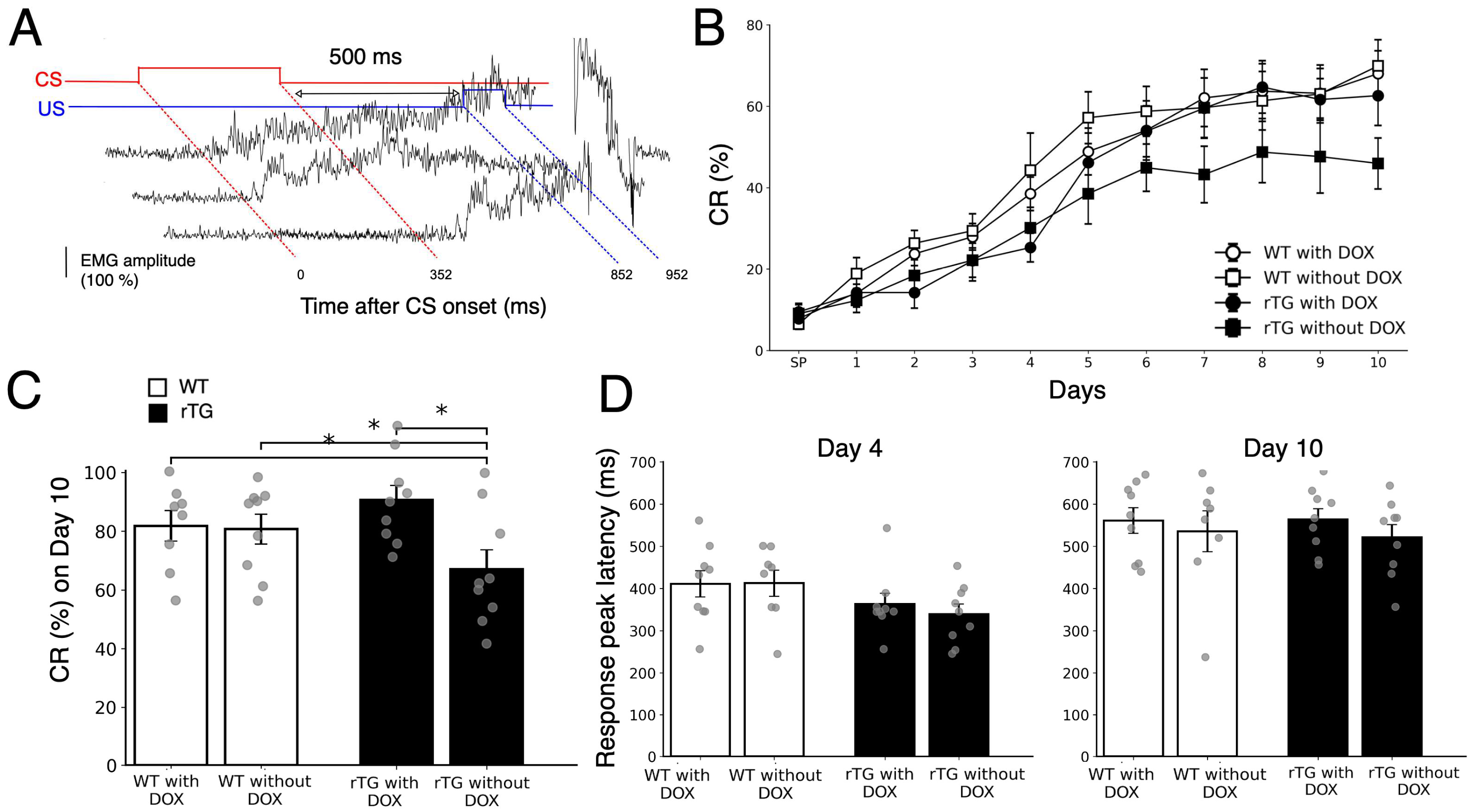
3.2. rTg4510 Mice Show DOX-Dependent Deficits in Trace Eyeblink Conditioning
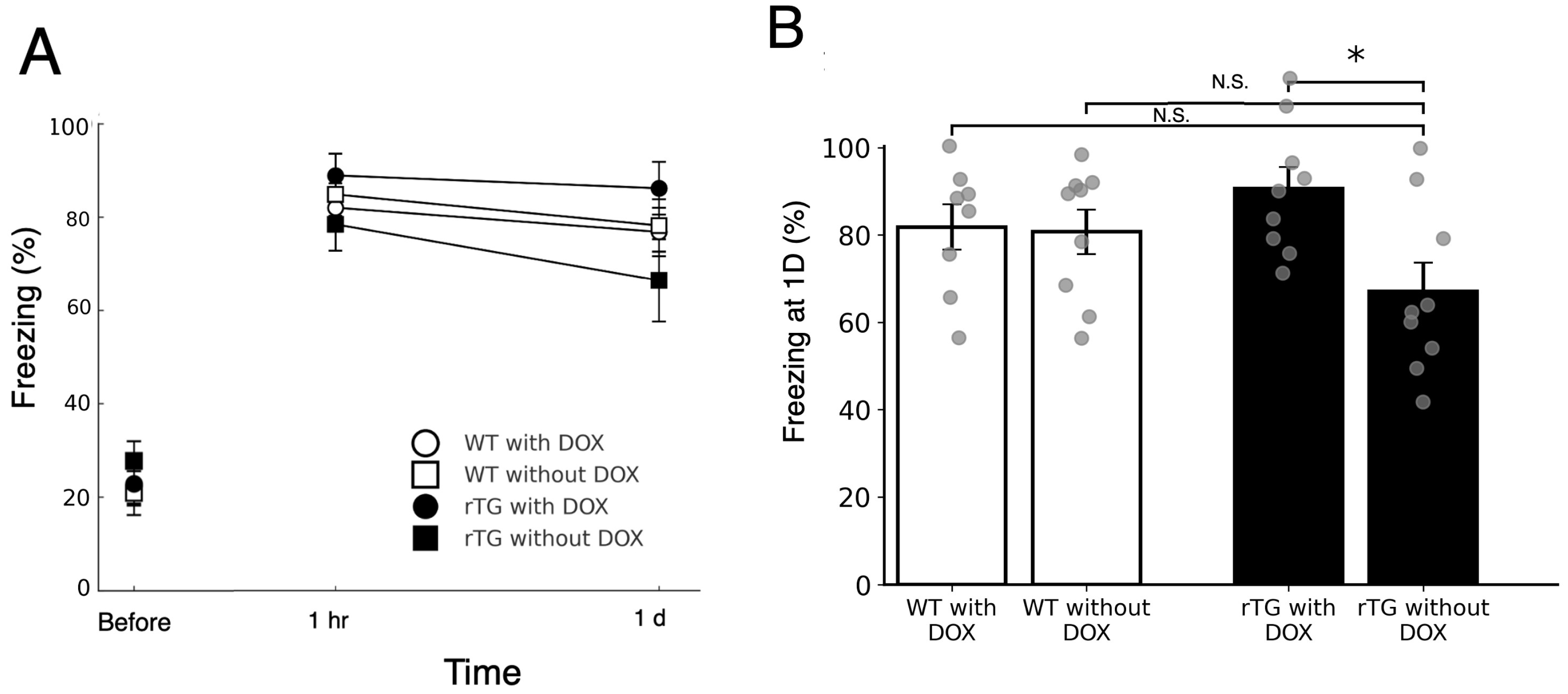
3.3. rTg4510 Mice Exhibit Mild DOX-Dependent Impairment in Contextual Fear Memory Retention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | β-amyloid |
| AD | Alzheimer’s disease |
| APP | amyloid-β precursor protein; |
| CR | conditioned response; |
| TI | trace interval |
References
- Lee, V.M.; Goedert, M.; Trojanowski, Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Yin, X.; Chen, K.; Hu, Z.; Zhou, Q.; Cao, X.; Chen, Z.; Liu, D. The role of pathological tau in synaptic dysfunction in Alzheimer’s disease. Transl. Neurodegener. 2021, 10, 45. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Qiao, Y.; Chi, Y.; Zhang, Q.; Ma, Y. Safety and efficacy of lecanemab for Alzheimer’s disease: A systematic review and meta-analysis of randomized clinical trials. Front. Aging Neurosci. 2023, 15, 1169499. [Google Scholar] [CrossRef]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Del Tredici, K.; et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef]
- Herrup, K. How Not to Study a Disease: The Story of Alzheimer’s; MIT Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Kopeikina, K.J.; Polydoro, M.; Tai, H.C.; Yaeger, E.; Carlson, G.A.; Pitstick, R.; Hyman, B.T.; Spires-Jones, T.L. Synaptic alterations in the rTg4510 mouse model of tauopathy. J. Comp. Neurol. 2013, 521, 1334–1353. [Google Scholar] [CrossRef]
- Lewis, J.; McGowan, E.; Rockwood, J.; Melrose, H.; Nacharaju, P.; Van Slegtenhorst, M.; Gwinn-Hardy, K.; Paul Murphy, M.; Baker, M.; Yu, X.; et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000, 25, 402–405. [Google Scholar] [CrossRef]
- SantaCruz, K.; Lewis, J.; Spires, T.; Paulson, J.; Kotilinek, L.; Ingelsson, M.; Guimaraes, A.; DeTure, M.; Ramsden, M.; McGowan, E.; et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef]
- Ramsden, M.; Kotilinek, L.; Forster, C.; Paulson, J.; McGowan, E.; SantaCruz, K.; Guimaraes, A.; Yue, M.; Lewis, J.; Carlson, G.; et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J. Neurosci. 2005, 25, 10637–10647. [Google Scholar] [CrossRef]
- Kubota, T.; Kirino, Y. Age-dependent impairment of memory and neurofibrillary tangle formation. Brain Res. 2021, 1765, 147496. [Google Scholar] [CrossRef]
- Squire, L.R. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992, 99, 195–231. [Google Scholar] [CrossRef]
- Eichenbaum, H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron 2004, 44, 109–120. [Google Scholar] [CrossRef]
- Gilbert, P.E.; Kesner, R.P.; Lee, I. Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus 2001, 11, 626–636. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Knierim, J.J. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front. Behav. Neurosci. 2011, 5, 69. [Google Scholar] [CrossRef]
- Beer, Z.; Vavra, P.; Atucha, E.; Rentzing, K.; Heinze, H.J.; Sauvage, M.M. The memory for time and space differentially engages the proximal and distal parts of the hippocampal subfields CA1 and CA3. PLoS Biol. 2018, 16, e2006100. [Google Scholar] [CrossRef]
- MacDonald, C.J.; Lepage, K.Q.; Eden, U.T.; Eichenbaum, H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 2011, 71, 737–749. [Google Scholar] [CrossRef]
- Huerta, P.T.; Sun, L.D.; Wilson, M.A.; Tonegawa, S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron 2000, 25, 473–480. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Nakazawa, K.; Tonegawa, S.; Kirino, Y.; Kano, M. Hippocampal CA3 NMDA receptors are crucial for adaptive timing of trace eyeblink conditioned response. J. Neurosci. 2006, 26, 1562–1570. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kawahara, S.; Mori, H.; Mishina, M.; Kirino, Y. Long-trace interval eyeblink conditioning is impaired in mutant mice lacking the NMDA receptor subunit epsilon 1. Eur. J. Neurosci. 2001, 13, 1221–1227. [Google Scholar] [CrossRef]
- de Calignon, A.; Polydoro, M.; Suárez-Calvet, M.; William, C.; Adamowicz, D.H.; Kopeikina, K.J.; Pitstick, R.; Sahara, N.; Ashe, K.H.; Carlson, G.A.; et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 2012, 73, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011, 121, 171–181. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Yanamandra, K.; Kfoury, N.; Jiang, H.; Mahan, T.E.; Ma, S.; Maloney, S.E.; Wozniak, D.F.; Diamond, M.I.; Holtzman, D.M. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 2013, 80, 402–414. [Google Scholar] [CrossRef]
- Monteiro, C.; Toth, B.; Brunstein, F.; Bobbala, A.; Datta, S.; Ceniceros, R.; Sanabria Bohorquez, S.M.; Anania, V.G.; Wildsmith, K.R.; Schauer, S.P.; et al. Randomized phase II study of the safety and efficacy of Semorinemab in participants with mild-to-moderate Alzheimer disease: Lauriet. Neurology 2023, 101, e1391–e1401. [Google Scholar] [CrossRef]
- Roughan, J.V.; Wright-Williams, S.L.; Flecknell, P.A. Automated analysis of postoperative behaviour: Assessment of HomeCageScan as a novel method to rapidly identify pain and analgesic effects in mice. Lab. Anim. 2009, 43, 17–26. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Cagniard, B.; Yamazaki, M.; Nakayama, J.; Sakimura, K.; Kirino, Y.; Kano, M. Task-specific enhancement of hippocampus-dependent learning in mice deficient in monoacylglycerol lipase, the major hydrolyzing enzyme of the endocannabinoid 2-Arachidonoylglycerol. Front. Behav. Neurosci. 2015, 9, 134. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Oku, I.; Nishigawa, A.; Nishimoto, A.; Kirino, Y. Impaired long-trace1 eyeblink conditioning in a Tg2576 mouse model of Alzheimer’s disease. Neurosci. Lett. 2012, 506, 155–159. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kirino, Y. Presenilin 2 mutation accelerates the onset of impairment in trace eyeblink conditioning in a mouse model of Alzheimer’s disease overexpressing human mutant amyloid precursor protein. Neurosci. Lett. 2013, 538, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Fukumoto, K.; Nagai, M.; Mizuguchi, A.; Kobashi, Y. Early contextual fear memory deficits in a double-transgenic amyloid-β precursor protein/presenilin 2 mouse model of Alzheimer’s disease. Int. J. Alz. Dis. 2017, 2017, 8584205. [Google Scholar] [CrossRef]
- Jul, P.; Volbracht, C.; de Jong, I.E.M.; Helboe, L.; Elvang, A.B.; Pedersen, J.T. Hyperactivity with agitative-like behavior in a mouse tauopathy model. J. Alz.Dis. 2016, 49, 783–795. [Google Scholar] [CrossRef]
- Papanikolaou, A.; Rodrigues, F.R.; Holeniewska, J.; Phillips, K.G.; Saleem, A.B.; Solomon, S.G. Plasticity in visual cortex is disrupted in a mouse model of tauopathy. Commun. Biol. 2022, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.F.; Steinmetz, J.E. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience 2009, 162, 732–755. [Google Scholar] [CrossRef] [PubMed]
- Woodruff-Pak, D.S.; Disterhoft, J.F. Where is the trace in trace conditioning? Trends Neurosci. 2008, 31, 105–112. [Google Scholar] [CrossRef]
- Solomon, P.R.; Vander Schaaf, E.R.; Thompson, R.F.; Weisz, D.J. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 1986, 100, 729–744. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Barulli, D.; Stern, Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef]
- Park, D.C.; Reuter-Lorenz, P.A. The adaptive brain: Aging and neurocognitive scaffolding. Annu. Rev. Psychol. 2009, 60, 173–196. [Google Scholar] [CrossRef]
- Rajah, M.N.; D’Esposito, M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain 2005, 128, 1964–1983. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Sperling, R.A. Large-scale functional brain network abnormalities in Alzheimer’s disease: Insights from functional neuroimaging. Behav. Neurol. 2009, 21, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016, 17, 777–792. [Google Scholar] [CrossRef]
- Santos, S.F.; Pierrot, N.; Octave, J.N. Network excitability dysfunction in Alzheimer’s disease: Insights from in vitro and in vivo models. Rev. Neurosci. 2010, 21, 153–171. [Google Scholar] [CrossRef]
- Gibbons, G.S.; Lee, V.M.Y.; Trojanowski, J.Q. Mechanisms of cell-to-cell transmission of pathological tau: A Review. JAMA Neurol. 2019, 76, 101–108. [Google Scholar] [CrossRef]
- Duff, K.; Knight, H.; Refolo, L.M.; Sanders, S.; Yu, X.; Picciano, M.; Malester, B.; Hutton, M.; Adamson, J.; Goedert, M.; et al. Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol. Dis. 2000, 7, 87–98. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 22–35. [Google Scholar] [CrossRef]
- Kovacs, G.G. Tauopathies. Handb. Clin. Neurol. 2018, 145, 355–368. [Google Scholar] [CrossRef]
- Siegel, J.J.; Taylor, W.; Gray, R.; Kalmbach, B.; Zemelman, B.V.; Desai, N.S.; Johnston, D.; Chitwood, R.A. Trace eyeblink conditioning in mice is dependent upon the dorsal medial prefrontal cortex, cerebellum, and amygdala: Behavioral characterization and functional circuitry. eNeuro 2015, 2, e0051-14. [Google Scholar] [CrossRef]
- Hattori, S.; Yoon, T.; Disterhoft, J.F.; Weiss, C. Functional reorganization of a prefrontal cortical network mediating consolidation of trace eyeblink conditioning. J. Neurosci. 2014, 34, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Crimins, J.L.; Rocher, A.B.; Luebke, J.I. Electrophysiological changes precede morphological changes to frontal cortical pyramidal neurons in the rTg4510 mouse model of progressive tauopathy. Acta Neuropathol. 2012, 124, 777–795. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.B.; Lashley, R.; Zhao, F.; Wang, X.; Kung, N.; Askwith, C.C.; Lin, L.; Shultis, M.W.; Hodgetts, K.J.; Lin, C.G. Enhancement of tripartite synapses as a potential therapeutic strategy for Alzheimer’s disease: A preclinical study in rTg4510 mice. Alz. Res. Ther. 2019, 23, 75. [Google Scholar] [CrossRef]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.L.; et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Colom-Cadena, M.; Davies, C.; Sirisi, S.; Lee, J.E.; Simzer, E.M.; Tzioras, M.; Querol-Vilaseca, M.; Sánchez-Aced, É.; Chang, Y.Y.; Holt, K.; et al. Synaptic oligomeric tau in Alzheimer’s disease—A potential culprit in the spread of tau pathology through the brain. Neuron 2023, 111, 2170–2183.e6. [Google Scholar] [CrossRef]
- Bejanin, A.; Schonhaut, D.R.; La Joie, R.; Kramer, J.H.; Baker, S.L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 2017, 140, 3286–3300. [Google Scholar] [CrossRef]
- Liu, L.; Drouet, V.; Wu, J.W.; Witter, M.P.; Small, S.A.; Clelland, C.; Duff, K. Trans-synaptic spread of tau pathology in vivo. PLoS ONE 2012, 7, e31302. [Google Scholar] [CrossRef]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef]
- Kovacs, G.G. Invited review: Neuropathology of tauopathies: Principles and practice. Neuropathol. Appl. Neurobiol. 2015, 41, 3–23. [Google Scholar] [CrossRef]
- Dam, T.; Boxer, A.L.; Golbe, L.; Höglinger, G.U.; Morris, H.R.; Litvan, I.; Lang, A.E.; Corvol, J.C.; Aiba, I.; Grundman, M.; et al. Safety and efficacy of anti-tau monoclonal antibody gosuranemab in progressive supranuclear palsy: A phase 2, randomized, placebo-controlled trial. Nat. Med. 2021, 27, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Boxer, A.L.; Qureshi, I.A.; Ahlijanian, M.; Grundman, M.; Golbe, L.I.; Litvan, I.; Honig, L.S.; Tuite, P.; McFarland, N.R.; O’Suilleabhain, P.; et al. Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: A randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol. 2019, 18, 549–558. [Google Scholar] [CrossRef]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Imbimbo, B.P.; Balducci, C.; Ippati, S.; Watling, M. Initial failures of anti-tau antibodies in Alzheimer’s disease are reminiscent of the amyloid-β story. Neural Regen. Res. 2023, 18, 117–118. [Google Scholar] [CrossRef]
- Cheng, D.T.; Disterhoft, J.F.; Power, J.M.; Ellis, D.A.; Desmond, J.E. Neural substrates underlying human delay and trace eyeblink conditioning. Proc. Natl. Acad. Sci. USA 2008, 105, 8108–8113. [Google Scholar] [CrossRef] [PubMed]
- Reeb-Sutherland, B.C.; Fox, N.A. Eyeblink conditioning: A non-invasive biomarker for neurodevelopmental disorders. J. Autism Dev. Disord. 2015, 45, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Binyamin, D.; Werbner, N.; Nuriel-Ohayon, M.; Uzan, A.; Mor, H.; Abbas, A.; Ziv, O.; Teperino, R.; Gutman, R.; Koren, O. The aging mouse microbiome has obesogenic characteristics. Genome Med. 2020, 12, 87. [Google Scholar] [CrossRef]
- Paris, J.J.; Singh, H.D.; Ganno, M.L.; Jackson, P.; McLaughlin, J.P. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology 2014, 231, 2349–2360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishimoto, Y.; Kubota, T.; Nakashima, K.; Kirino, Y. rTg4510 Tauopathy Mice Exhibit Non-Spatial Memory Deficits Prevented by Doxycycline Treatment. Brain Sci. 2025, 15, 1183. https://doi.org/10.3390/brainsci15111183
Kishimoto Y, Kubota T, Nakashima K, Kirino Y. rTg4510 Tauopathy Mice Exhibit Non-Spatial Memory Deficits Prevented by Doxycycline Treatment. Brain Sciences. 2025; 15(11):1183. https://doi.org/10.3390/brainsci15111183
Chicago/Turabian StyleKishimoto, Yasushi, Takashi Kubota, Kentaro Nakashima, and Yutaka Kirino. 2025. "rTg4510 Tauopathy Mice Exhibit Non-Spatial Memory Deficits Prevented by Doxycycline Treatment" Brain Sciences 15, no. 11: 1183. https://doi.org/10.3390/brainsci15111183
APA StyleKishimoto, Y., Kubota, T., Nakashima, K., & Kirino, Y. (2025). rTg4510 Tauopathy Mice Exhibit Non-Spatial Memory Deficits Prevented by Doxycycline Treatment. Brain Sciences, 15(11), 1183. https://doi.org/10.3390/brainsci15111183






