Possible Eye Disorders in Children Prenatally Exposed to Either Methadone or Buprenorphine in Comparison with Other Medications: An Examination of the Food and Drug Administration (FDA) Pharmacovigilance Database
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Methadone and Buprenorphine-Related Eye Disorders Cases Recorded in 0–17-Year-Old Individuals
- a = methadone associated with eye disorders = 17;
- b = methadone without eye disorders = 2297 − 17 = 2280;
- c = buprenorphine with eye disorders = 15;
- d = buprenorphine without eye disorders = 1199 − 15 = 1184.
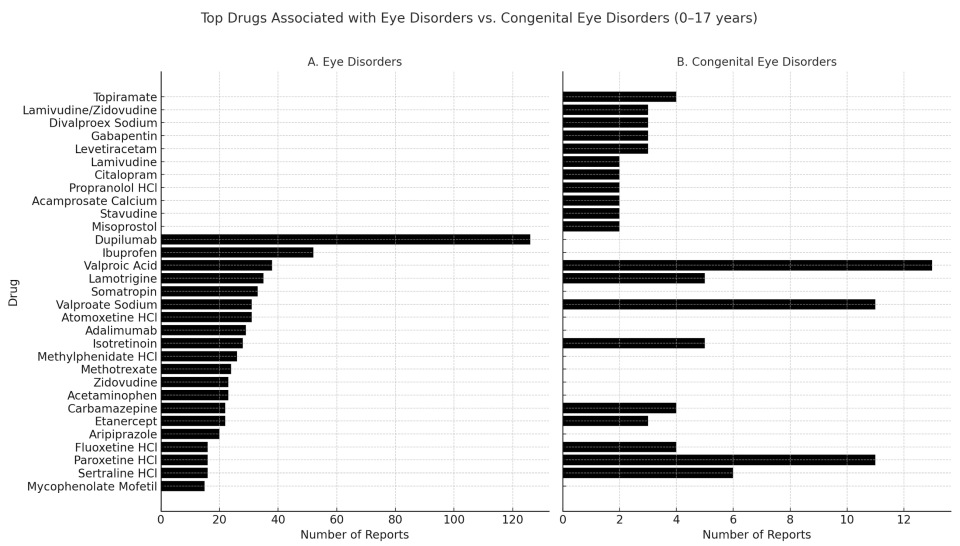
3.2. Top 20 Medications Associated with Eye Disorders and Congenital Eye Disorders Involving Subjects Aged 0–17 Years Old Exposed In Utero According to the FAERS
3.3. Analysis of FAERS Data Regarding Eye Disorders in 0–17-Year-Old Children Exposed During Pregnancy to Psychotropics Different from Opiates/Opioids
4. Discussion
4.1. Dataset Characteristics and General Findings
4.2. Pharmacovigilance Issues
4.3. Comparative Drug Reporting and Absence of Opioid Signals
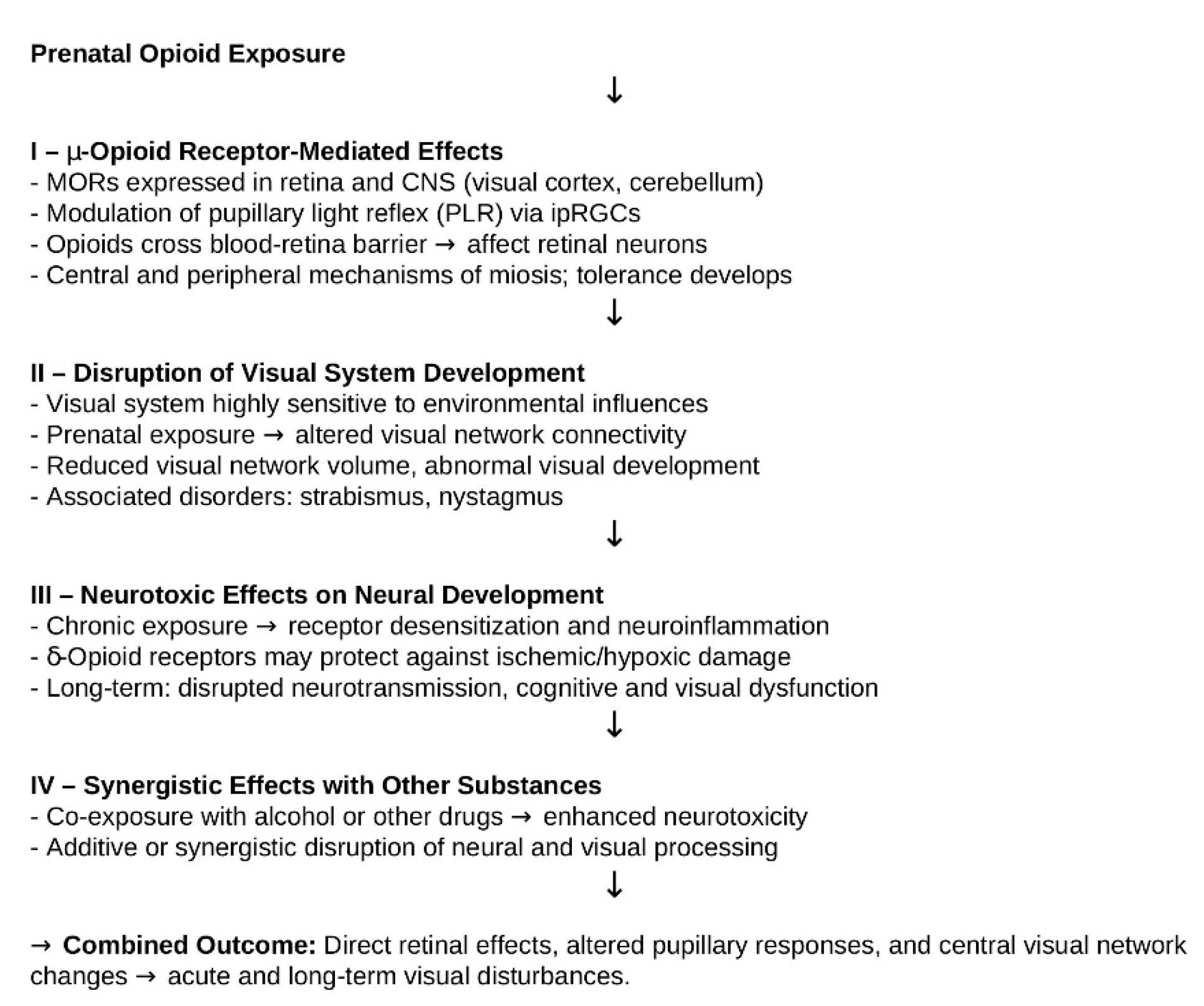
4.4. Potential Mechanisms of Opioid-Related Visual Effects
4.5. Study Limitations
4.6. Research Implications and Clinical Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haas, D.M.; Marsh, D.J.; Dang, D.T.; Parker, C.B.; Wing, D.A.; Simhan, H.N.; Grobman, W.A.; Mercer, B.M.; Silver, R.M.; Hoffman, M.K.; et al. Prescription and Other Medication Use in Pregnancy. Obstet. Gynecol. 2018, 131, 789–798. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Breastfeeding and Maternal Medication. Recommendations for Drugs in the Eleventh WHO Model List of Essential Drugs. 2002. Available online: https://www.who.int/publications/i/item/55732 (accessed on 16 September 2025).
- CDC. Treatment of Opioid Use Disorder Before, During, and After Pregnancy. 2025. Available online: https://www.cdc.gov/opioid-use-during-pregnancy/treatment/index.html (accessed on 25 September 2025).
- American College of Obstetricians and Gynecologists (ACOG). Opioid Use and Opioid Use Disorder in Pregnancy Committee Opinion Number 711 (August 2017). Available online: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/08/opioid-use-and-opioid-use-disorder-in-pregnancy (accessed on 25 September 2025).
- CRAT—Reference Center for Teratogenic Agents. Buprenorphine—Pregnancy. 2022. Available online: https://www.lecrat.fr/12748 (accessed on 25 September 2025).
- CRAT—Reference Center for Teratogenic Agents. Methadone—Pregnancy. 2022. Available online: https://www.lecrat.fr/12738/ (accessed on 25 September 2025).
- UK Teratology Information Service (UKTIS). Use of Buprenorphine in Pregnancy. 2017. Available online: https://uktis.org/monographs/use-of-buprenorphine-in-pregnancy/ (accessed on 25 September 2025).
- UK Teratology Information Service (UKTIS). USE OF HEROIN IN PREGNANCY. 2010. Available online: https://uktis.org/monographs/use-of-heroin-in-pregnancy/ (accessed on 25 September 2025).
- The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update. J. Addict. Med. 2020, 14 (Suppl. S1), 1–91. [CrossRef]
- Mactier, H.; Hamilton, R. Prenatal opioid exposure—Increasing evidence of harm. Early Hum. Dev. 2020, 150, 105188. [Google Scholar] [CrossRef]
- Aslaksen, A.K.; Vikesdal, G.H.; Voie, M.T.; Rowlands, M.; Skranes, J.; Haugen, O.H. Visual function in Norwegian children aged 5–13 years with prenatal exposure to opioid maintenance therapy: A case-control study. Acta Ophthalmol. 2024, 102, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.; Mulvihill, A.; Butler, L.; Chow, A.; Irving, E.; McCulloch, D.L.; McNeil, A.; Michael, K.; Spowart, K.M.; Waterson-Wilson, J.; et al. Impaired vision in children prenatally exposed to methadone: An observational cohort study. Eye 2024, 38, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, Z.; Conti, A.A.; Baldacchino, A. Ophthalmic outcomes in children exposed to opioid maintenance treatment in utero: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 136, 104601. [Google Scholar] [CrossRef]
- Tinelli, F.; Guzzetta, A.; Bertini, C.; Ricci, D.; Mercuri, E.; Ladavas, E.; Cioni, G. Greater sparing of visual search abilities in children after congenital rather than acquired focal brain damage. Neurorehabil. Neural Repair 2011, 25, 721–728. [Google Scholar] [CrossRef]
- Bush, E.; Nidey, N.; Merhar, S.; Wexelblatt, S.; Schwartz, T.; Weber, S.; McAllister, J. Instrument-based vision screening and outcomes in young children following prenatal exposure to buprenorphine or methadone: A retrospective cohort study. J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2025, 29, 104259. [Google Scholar] [CrossRef]
- Hamilton, R.; McGlone, L.; MacKinnon, J.R.; Russell, H.C.; Bradnam, M.S.; Mactier, H. Ophthalmic, clinical and visual electrophysiological findings in children born to mothers prescribed substitute methadone in pregnancy. Br. J. Ophthalmol. 2010, 94, 696–700. [Google Scholar] [CrossRef]
- Konijnenberg, C.; Melinder, A. Neurodevelopmental investigation of the mirror neurone system in children of women receiving opioid maintenance therapy during pregnancy. Addiction 2013, 108, 154–160. [Google Scholar] [CrossRef] [PubMed]
- McGlone, L.; Hamilton, R.; McCulloch, D.L.; MacKinnon, J.R.; Bradnam, M.; Mactier, H. Visual outcome in infants born to drug-misusing mothers prescribed methadone in pregnancy. Br. J. Ophthalmol. 2014, 98, 238–245. [Google Scholar] [CrossRef]
- European Medicines Agency. PRAC Strategy on Measuring the Impact of Pharmacovigilance Activities (Revision 2). 2022. Available online: https://www.ema.europa.eu/en/documents/other/prac-strategy-measuring-impact-pharmacovigilance-activities_en.pdf (accessed on 16 September 2025).
- Bate, A.; Reynolds, R.F.; Caubel, P. The hope, hype and reality of Big Data for pharmacovigilance. Ther. Adv. Drug Saf. 2018, 9, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Schifano, F.; Chiappini, S. Is there such a thing as a ‘lope’ dope? Analysis of loperamide-related European Medicines Agency (EMA) pharmacovigilance database reports. PLoS ONE 2018, 13, e0204443. [Google Scholar] [CrossRef]
- Chiappini, S.; Schifano, F. A Decade of Gabapentinoid Misuse: An Analysis of the European Medicines Agency’s ‘Suspected Adverse Drug Reactions’ Database. CNS Drugs 2016, 30, 647–654. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA In Brief: FDA Approves New Packaging for Brand-Name Over-the-Counter Loperamide to Help Curb Abuse and Misuse. 2022. Available online: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-approves-new-packaging-brand-name-over-counter-loperamide-help-curb-abuse-and-misuse (accessed on 16 September 2025).
- GOV.UK. Pregabalin (Lyrica), Gabapentin (Neurontin) and Risk of Abuse and Dependence: New Scheduling Requirements from 1 April; 2019. Available online: https://www.gov.uk/drug-safety-update/pregabalin-lyrica-gabapentin-neurontin-and-risk-of-abuse-and-dependence-new-scheduling-requirements-from-1-april (accessed on 16 September 2025).
- Baldini, S.; Khattak, A.; Capogrosso, P.; Antonini, G.; Dehò, F.; Schifano, F.; Schifano, N. The Possible Role of Prescribing Medications, Including Central Nervous System Drugs, in Contributing to Male-Factor Infertility (MFI): Assessment of the Food and Drug Administration (FDA) Pharmacovigilance Database. Brain Sci. 2023, 13, 1652. [Google Scholar] [CrossRef] [PubMed]
- Spowart, K.M.; Reilly, K.; Mactier, H.; Hamilton, R. Executive functioning, behavioural, emotional, and cognitive difficulties in school-aged children prenatally exposed to methadone. Front. Pediatr. 2023, 11, 1118634. [Google Scholar] [CrossRef] [PubMed]
- Daw, J.R.; Hanley, G.E.; Greyson, D.L.; Morgan, S.G. Prescription drug use during pregnancy in developed countries: A systematic review. Pharmacoepidemiol. Drug Saf. 2011, 20, 895–902. [Google Scholar] [CrossRef]
- Trønnes, J.N.; Lupattelli, A.; Nordeng, H. Safety profile of medication used during pregnancy: Results of a multinational European study. Pharmacoepidemiol. Drug Saf. 2017, 26, 802–811. [Google Scholar] [CrossRef]
- Peragallo, J.; Biousse, V.; Newman, N.J. Ocular manifestations of drug and alcohol abuse. Curr. Opin. Ophthalmol. 2013, 24, 566–573. [Google Scholar] [CrossRef]
- Sundelin Wahlsten, V.; Sarman, I. Neurobehavioural development of preschool-age children born to addicted mothers given opiate maintenance treatment with buprenorphine during pregnancy. Acta Paediatr. 2013, 102, 544–549. [Google Scholar] [CrossRef]
- Kaltenbach, K.; O’Grady, K.E.; Heil, S.H.; Salisbury, A.L.; Coyle, M.G.; Fischer, G.; Martin, P.R.; Stine, S.; Jones, H.E. Prenatal exposure to methadone or buprenorphine: Early childhood developmental outcomes. Drug Alcohol Depend. 2018, 185, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, N.; Wong, S.; Gupta, A.; Tran, J.; Bhambra, N.; Min, K.K.; Dragioti, E.; Barbui, C.; Fiedorowicz, J.G.; Gosling, C.J.; et al. Safety of psychotropic medications in pregnancy: An umbrella review. Mol. Psychiatry 2025, 30, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Ohlsson, H.; Svikis, D.S.; Sundquist, K.; Sundquist, J. The Protective Effect of Pregnancy on Risk for Drug Abuse: A Population, Co-Relative, Co-Spouse, and Within-Individual Analysis. Am. J. Psychiatry 2017, 174, 954–962. [Google Scholar] [CrossRef]
- Peltier, M.R.; Roberts, W.; Verplaetse, T.L.; Burke, C.; Zakiniaeiz, Y.; Moore, K.; McKee, S.A. Licit and illicit drug use across trimesters in pregnant women endorsing past-year substance use: Results from National Survey on Drug Use and Health (2009–2019). Arch. Women’s Ment. Health 2022, 25, 819–827. [Google Scholar] [CrossRef]
- Dubucs, C.; Plaisancié, J.; Courtade-Saidi, M.; Damase-Michel, C. The first review on prenatal drug exposure and ocular malformation occurrence. Front. Pediatr. 2024, 12, 1379875. [Google Scholar] [CrossRef] [PubMed]
- Whelan, P.J.; Remski, K. Buprenorphine vs methadone treatment: A review of evidence in both developed and developing worlds. J. Neurosci. Rural Pract. 2012, 3, 45–50. [Google Scholar] [CrossRef]
- Suarez, E.A.; Huybrechts, K.F.; Straub, L.; Hernández-Díaz, S.; Jones, H.E.; Connery, H.S.; Davis, J.M.; Gray, K.J.; Lester, B.; Terplan, M.; et al. Buprenorphine versus Methadone for Opioid Use Disorder in Pregnancy. N. Engl. J. Med. 2022, 387, 2033–2044. [Google Scholar] [CrossRef]
- Tomson, T.; Battino, D.; Perucca, E. Teratogenicity of antiepileptic drugs. Curr. Opin. Neurol. 2019, 32, 246–252. [Google Scholar] [CrossRef]
- Mulvihill, A.O.; Cackett, P.D.; George, N.D.; Fleck, B.W. Nystagmus secondary to drug exposure in utero. Br. J. Ophthalmol. 2007, 91, 613–615. [Google Scholar] [CrossRef]
- Asdjodi, S.; Rubarth, R.B.; Hardy, J.; Lee, H. The Effects of Opioids During Pregnancy: A Literature Review. Georget. Med. Rev. 2020, 4, 1–12. [Google Scholar] [CrossRef]
- Cleymaet, A.; Berezin, C.; Vigh, J. Endogenous Opioid Signaling in the Mouse Retina Modulates Pupillary Light Reflex. Int. J. Mol. Sci. 2015, 22, 554. [Google Scholar] [CrossRef]
- Murray, R.; Adler, M.; Korczyn, A. The pupillary effects of opioids. Life Sci. 1983, 33, 495–509. [Google Scholar] [CrossRef]
- Larson, M. Mechanism of opioid-induced pupillary effects. Clin. Neurophysiol. 2008, 119, 1358–1364. [Google Scholar] [CrossRef]
- Rollins, M.; Feiner, J.; Lee, J.; Shah, S.; Larson, M. Pupillary Effects of High-dose Opioid Quantified with Infrared Pupillometry. Anesthesiology 2014, 121, 1037–1044. [Google Scholar] [CrossRef]
- Merhar, S.; Jiang, W.; Parikh, N.; Yin, W.; Zhou, Z.; Tkach, J.; Wang, L.; Kline-Fath, B.; He, L.; Braimah, A.; et al. Effects of prenatal opioid exposure on functional networks in infancy. Dev. Cogn. Neurosci. 2021, 51, 100996. [Google Scholar] [CrossRef]
- Corder, G.; Castro, D.; Bruchas, M.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Swingler, M.; Donadoni, M.; Unterwald, E.; Maggirwar, S.; Sariyer, I. Molecular and cellular basis of mu-opioid receptor signaling: Mechanisms underlying tolerance and dependence development. Front. Neurosci. 2025, 19, 1597922. [Google Scholar] [CrossRef] [PubMed]
- Reeves, K.; Shah, N.; Muñoz, B.; Atwood, B. Opioid Receptor-Mediated Regulation of Neurotransmission in the Brain. Front. Mol. Neurosci. 2022, 15, 919773. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Abdul, Y.; Potter, D.E. Non-analgesic effects of opioids: Neuroprotection in the retina. Curr. Pharm. Des. 2012, 18, 6101–6108. [Google Scholar] [CrossRef]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef]
- Hasford, J.; Goettler, M.; Munter, K.H.; Müller-Oerlinghausen, B. Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J. Clin. Epidemiol. 2002, 55, 945–950. [Google Scholar] [CrossRef]
- Imbrici, P.; De Bellis, M.; Liantonio, A.; De Luca, A. Investigating the Benefit-Risk Profile of Drugs: From Spontaneous Reporting Systems to Real-World Data for Pharmacovigilance. Methods Mol. Biol. 2025, 2834, 333–349. [Google Scholar] [CrossRef]
- European Medicines Agency. Guide on the Interpretation of Spontaneous Case Reports of Suspected Adverse Reactions to Medicines. 2011. Available online: https://www.ema.europa.eu/en/documents/report/guide-interpretation-spontaneous-case-reports-suspected-adverse-reactions-medicines_en.pdf (accessed on 16 September 2025).
- Galli, J.; Loi, E.; Franzoni, A.; Accorsi, P.; Micheletti, S.; Pansera, L.; Fazzi, E. Long-Term Visual and Neurodevelopmental Outcomes in Two Children with Congenital Nystagmus Secondary to Methadone Exposure In utero. Neuropediatrics 2023, 54, 412–416. [Google Scholar] [CrossRef]
- MotherToBaby|Fact Sheets [Internet]; Organization of Teratology Information Specialists (OTIS): Brentwood, TN, USA, 2025; Buprenorphine (Buprenex®). Available online: https://www.ncbi.nlm.nih.gov/books/NBK582609/ (accessed on 25 September 2025).
- MotherToBaby|Fact Sheets [Internet]; Organization of Teratology Information Specialists (OTIS): Brentwood, TN, USA, 2023; Methadone. Available online: https://www.ncbi.nlm.nih.gov/books/NBK582830/ (accessed on 25 September 2025).
- Conradt, E.; Flannery, T.; Aschner, J.L.; Annett, R.D.; Croen, L.A.; Duarte, C.S.; Friedman, A.M.; Guille, C.; Hedderson, M.M.; Hofheimer, J.A.; et al. Prenatal Opioid Exposure: Neurodevelopmental Consequences and Future Research Priorities. Pediatrics 2019, 144, e20190128. [Google Scholar] [CrossRef]
- Anbalagan, S.; Falkowitz, D.M.; Mendez, M.D. Neonatal Abstinence Syndrome. [Updated 1 April 2024]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551498/ (accessed on 27 September 2025).
- CDC. Treat and Manage Infants Affected by Prenatal Opioid Exposure. 2025. Available online: https://www.cdc.gov/opioid-use-during-pregnancy/treatment/infants-opioid.html?utm_source=chatgpt.com%20%22Treat%20and%20Manage%20Infants%20Affected%20by%20Prenatal%20Opioid%20...%22 (accessed on 27 September 2025).
- Yen, E.; Davis, J.M. The immediate and long-term effects of prenatal opioid exposure. Front. Pediatr. 2022, 10, 1039055. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Exposure to Medicinal Products During Pregnancy: Need for Post-Authorisation Data. 2005. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-exposure-medicinal-products-during-pregnancy-need-post-authorisation-data_en.pdf (accessed on 16 September 2025).

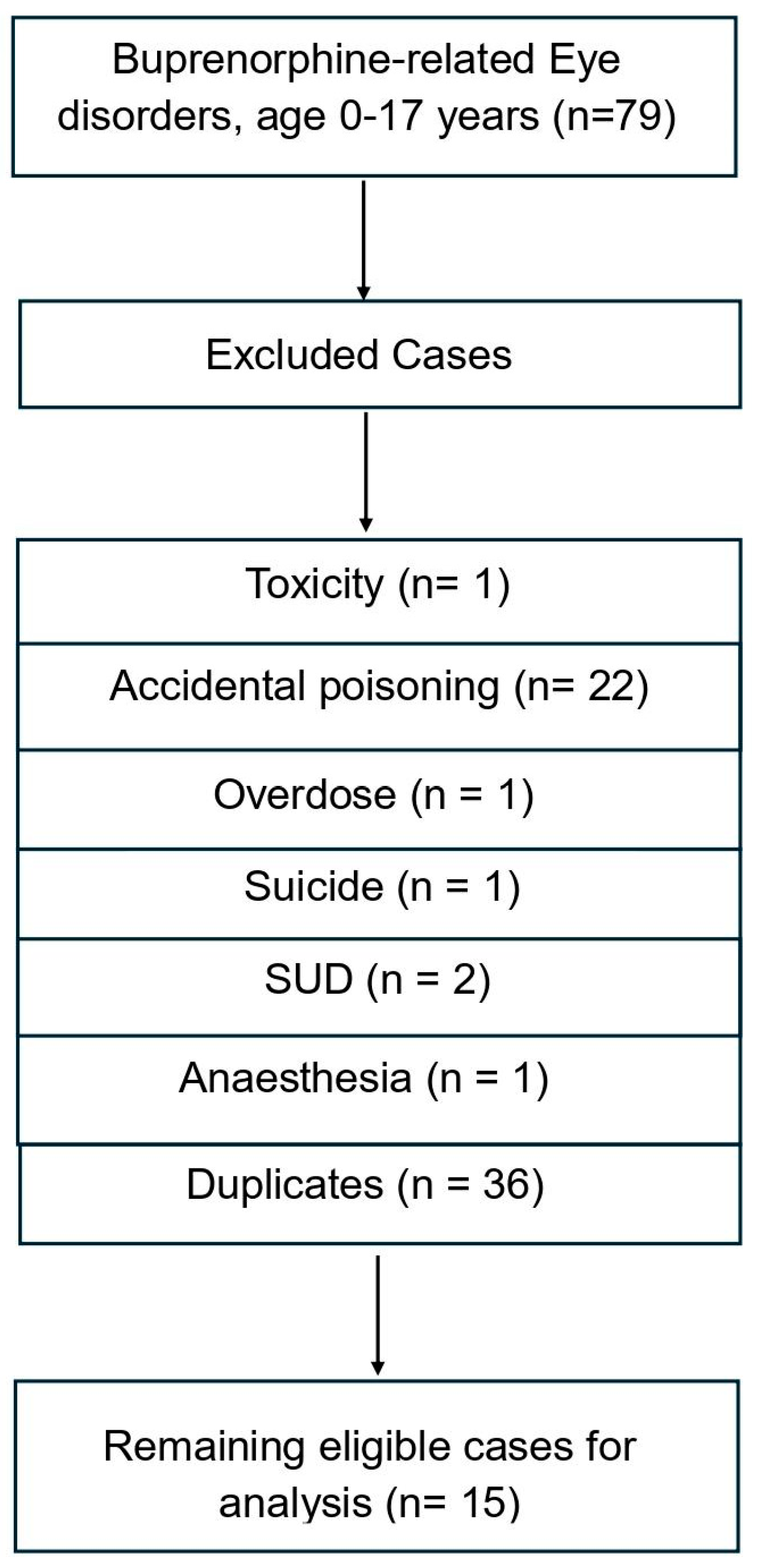
| General Reactions | Eye-Related Reactions | Outcome | Sex | Age | |
|---|---|---|---|---|---|
| Methadone: 17 cases | Foetal Exposure During Pregnancy 6 Maternal Exposure During Pregnancy 5 Small For Dates Baby 3 Developmental Delay 6 Drug Withdrawal Syndrome Neonatal 9 | Strabismus 7 Amblyopia 3 Miosis 3 Visus acuity reduced 3 Nystagmus 6 Optic Nerve Hypoplasia 3 Visual Impairment 4 Refraction disorder 3 | Other 11 Congenital anomaly 9 Hospitalised 1 Disabled 3 Died 2 Life-threatening 2 | F 6 M 4 Not Specified sex 7 | 1–12 months 9 13–24 months 2–5 years 2 NA 4 |
| Buprenorphine: 15 cases | Toxicity To Various Agents 4 Lethargy 3 Somnolence 3 Drug Withdrawal Syndrome 3 Premature Baby 3 Drug Withdrawal Syndrome Neonatal 6 Tremor 4 Agitation 3 | Miosis 6 | Other 8 Congenital anomaly 5 Hospitalised 14 Disabled 1 Died Life-threatening 4 NA 1 | M 7 F 2 Not Specified sex 1 | 0–12 months 11 13–24 months 1 2–5 years 1 |
| Substance | Total Eye Disorders | Total Eye Disorders 0–17 Years | Final Validated Cases |
|---|---|---|---|
| Benzodiazepines | |||
| Diazepam | 2791 | 240 | 7 |
| Alprazolam | 2999 | 97 | 5 |
| Clonazepam | 2862 | 242 | 7 |
| Lorazepam | 2246 | 117 | 5 |
| Bromazepam | 580 | 27 | 1 |
| Anticholinergic drugs | |||
| Biperiden | 35 | 6 | 0 |
| Benztropine | 91 | 15 | 0 |
| Trihexyphenidyl | 166 | 31 | 1 |
| Gabapentinoids | |||
| Pregabalin | 12,170 | 200 | 0 |
| Gabapentin | 3501 | 89 | 5 |
| Antipsychotics | |||
| Quetiapine | 2054 | 156 | 3 |
| Olanzapine | 3973 | 326 | 7 |
| Clozapine | 1354 | 45 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappini, S.; Orsolini, L.; Corkery, J.M.; Guirguis, A.; Mosca, A.; Arillotta, D.; Martinotti, G.; Schifano, F. Possible Eye Disorders in Children Prenatally Exposed to Either Methadone or Buprenorphine in Comparison with Other Medications: An Examination of the Food and Drug Administration (FDA) Pharmacovigilance Database. Brain Sci. 2025, 15, 1177. https://doi.org/10.3390/brainsci15111177
Chiappini S, Orsolini L, Corkery JM, Guirguis A, Mosca A, Arillotta D, Martinotti G, Schifano F. Possible Eye Disorders in Children Prenatally Exposed to Either Methadone or Buprenorphine in Comparison with Other Medications: An Examination of the Food and Drug Administration (FDA) Pharmacovigilance Database. Brain Sciences. 2025; 15(11):1177. https://doi.org/10.3390/brainsci15111177
Chicago/Turabian StyleChiappini, Stefania, Laura Orsolini, John Martin Corkery, Amira Guirguis, Alessio Mosca, Davide Arillotta, Giovanni Martinotti, and Fabrizio Schifano. 2025. "Possible Eye Disorders in Children Prenatally Exposed to Either Methadone or Buprenorphine in Comparison with Other Medications: An Examination of the Food and Drug Administration (FDA) Pharmacovigilance Database" Brain Sciences 15, no. 11: 1177. https://doi.org/10.3390/brainsci15111177
APA StyleChiappini, S., Orsolini, L., Corkery, J. M., Guirguis, A., Mosca, A., Arillotta, D., Martinotti, G., & Schifano, F. (2025). Possible Eye Disorders in Children Prenatally Exposed to Either Methadone or Buprenorphine in Comparison with Other Medications: An Examination of the Food and Drug Administration (FDA) Pharmacovigilance Database. Brain Sciences, 15(11), 1177. https://doi.org/10.3390/brainsci15111177











