Menstrual Cycle Modulation of Verbal Performance and Hemispheric Asymmetry
Abstract
1. Introduction
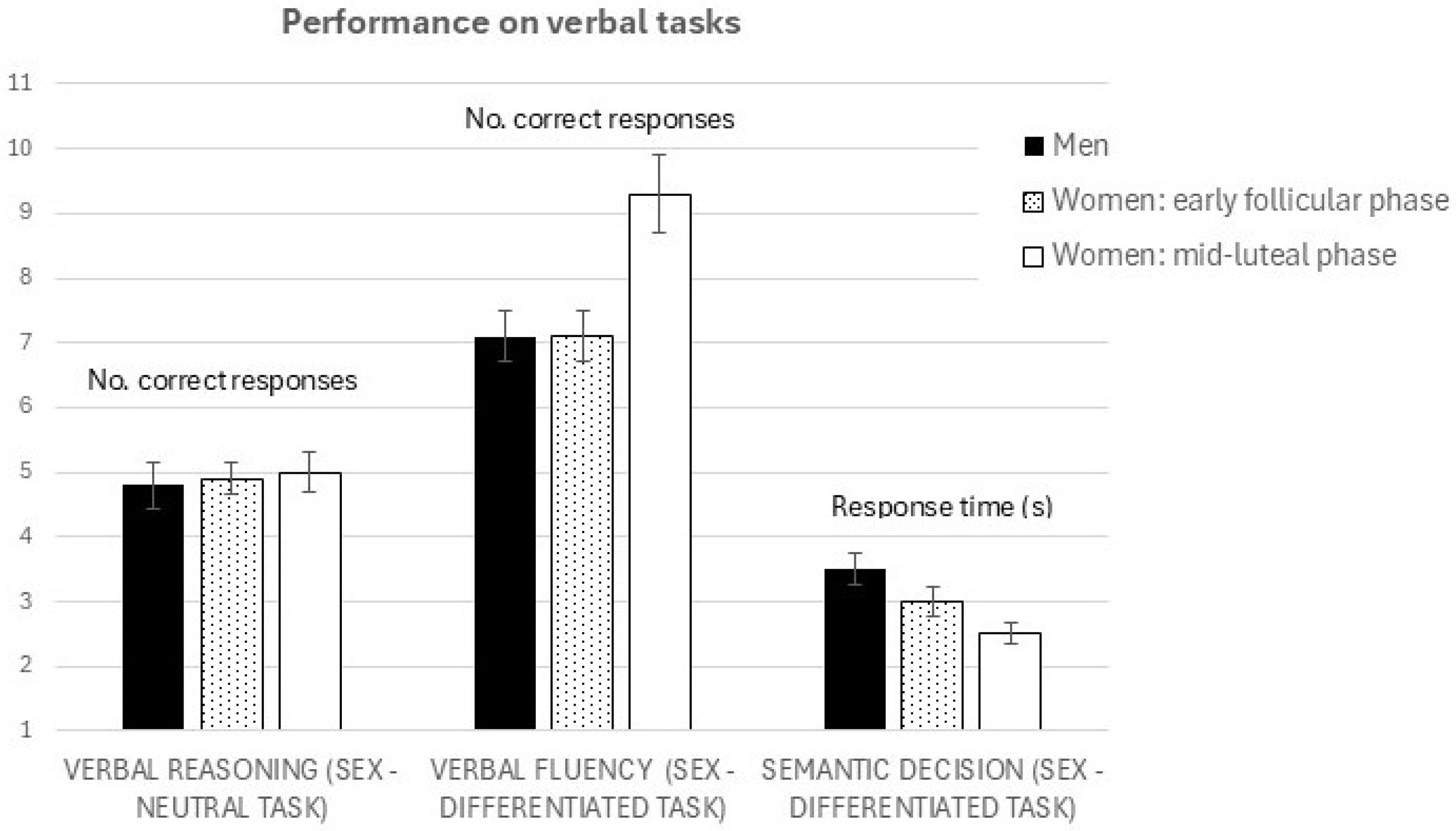
- Behavioral performance: We expected task performance to vary as a function of menstrual cycle phase only for the sex-differentiated tasks (verbal fluency and semantic decision), but not for the sex-neutral task (verbal reasoning). Specifically, we hypothesized that women would perform better during the mid-luteal phase (characterized by high estrogen and progesterone levels) compared to the early follicular phase (low hormone levels).
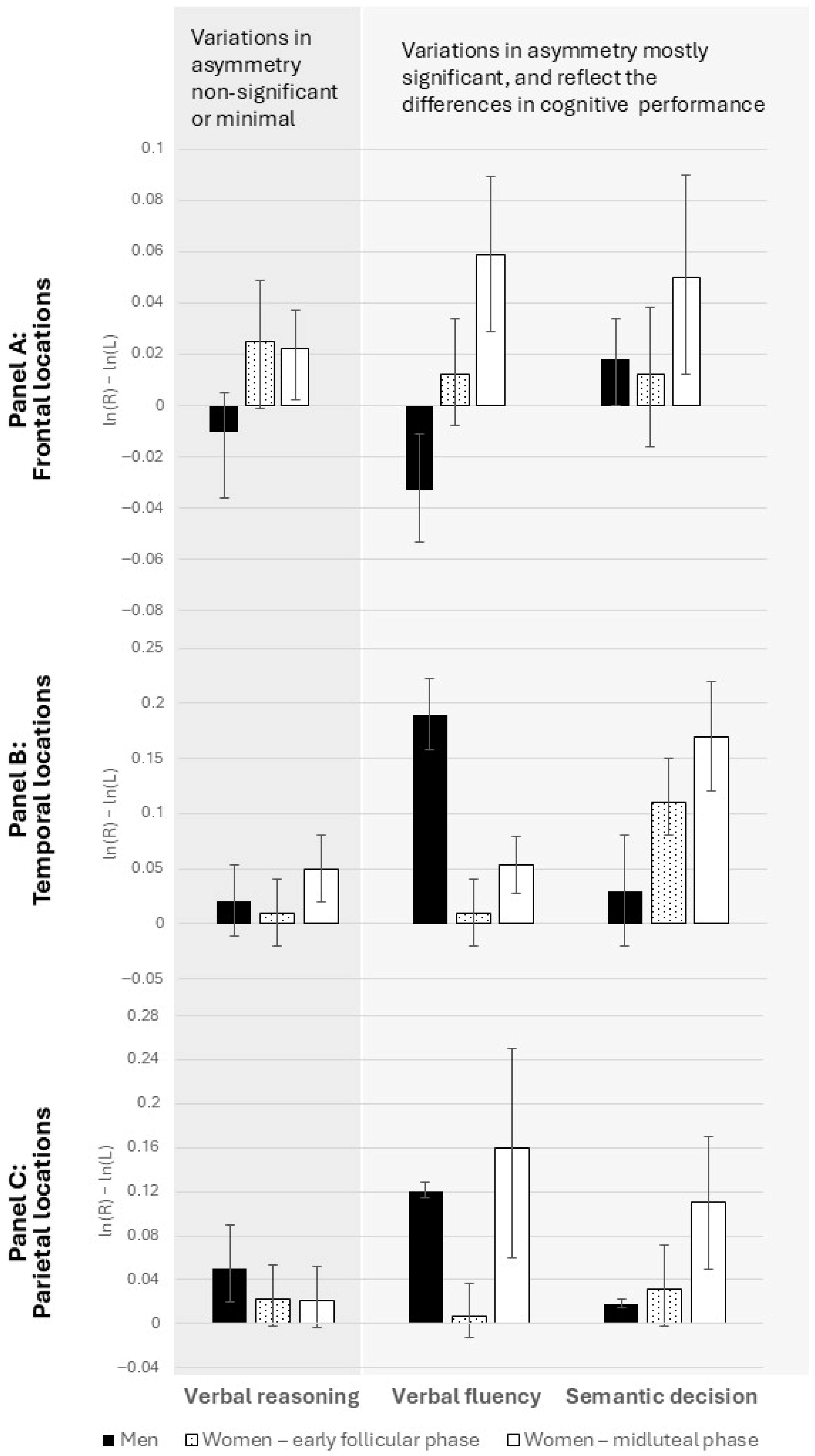
- Neural asymmetries: We further hypothesized that changes in hemispheric activation patterns, as reflected in EEG asymmetry indices, would occur across menstrual cycle phases only for the sex-differentiated tasks (verbal fluency and semantic decision), but not for the sex-neutral task (verbal reasoning).
2. Materials and Methods
2.1. Participants and Procedure
2.2. Tasks and Questionnaires
2.2.1. Verbal Reasoning Task
2.2.2. Semantic Task
2.2.3. Verbal Fluency
2.2.4. The Mood (a Control Variable)
3. Results
3.1. Cognitive Performance
3.2. Hemispheric Asymmetries
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maccoby, E.E.; Jacklin, C.N. The Psychology of Sex Differences; Stanford University Press: Stanford, CA, USA, 1974. [Google Scholar]
- McGuiness, D. Sex differences in the organisation of perception and cognition. In Exploring Sex Differences; Lloyd, B., Archer, J., Eds.; Academic Press: London, UK, 1976; pp. 123–156. [Google Scholar]
- Halpern, D.F. Sex Differences in Cognitive Abilities, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1992. [Google Scholar]
- Martin, D.J.; Hoover, H.D. Sex differences in educational achievement: A longitudinal study. J. Early Adolesc. 1987, 7, 65–83. [Google Scholar] [CrossRef]
- Hyde, J.S.; Linn, M.C. Gender differences in verbal ability: A meta-analysis. Psychol. Bull. 1988, 104, 53–69. [Google Scholar] [CrossRef]
- Hampson, E. Sexual differentiation of spatial functions in humans. In Sexual Differentiation of the Brain; Matsumoto, A., Ed.; CRC Press: London, UK, 2000; pp. 279–300. [Google Scholar]
- Geary, D.C. Male, Female: The Evolution of Human Sex Differences; American Psychological Association: Washington, DC, USA, 2005. [Google Scholar]
- Hirnstein, M.; Hugdahl, K.; Hausmann, M. Cognitive sex differences and hemispheric asymmetry: A critical review of 40 years of research. Laterality 2019, 24, 204–252. [Google Scholar] [CrossRef]
- Mellet, E.; Zago, L.; Jobard, G.; Crivello, F.; Petit, L.; Joliot, M.; Mazoyer, B.; Tzourio-Mazoyer, N. Weak language lateralization affects both verbal and spatial skills: An fMRI study in 297 subjects. Neuropsychologia 2014, 65, 56–62. [Google Scholar] [CrossRef]
- Kljajevic, V. Verbal learning and hemispheric asymmetry. Front. Psychol. 2022, 12, 809192. [Google Scholar] [CrossRef] [PubMed]
- Hampson, E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn. 1990, 14, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Hampson, E. Estrogen-related variations in human spatial and articulatory motor skills. Psychoneuroendocrinology 1990, 15, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Kimura, D. Estrogen replacement therapy may protect against intellectual decline in postmenopausal women. Horm. Behav. 1995, 29, 312–363. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; McCleary, C.A.; Murdock, G.A.; Wilshire, T.W.; Buckwalter, D.K.; Bretsky, P.; Marmol, L.; Gorsuch, R.L.; Buckwalter, J.G. Lifelong estrogen exposure and cognitive performance in elderly women. Brain Cogn. 1999, 39, 203–218. [Google Scholar] [CrossRef]
- Slabbekorn, D.; van Goozen, S.H.M.; Megens, J.; Gooren, J.L.G.; Cohen-Kettenis, P.T. Activating effects of cross-sex hormones on cognitive functioning: A study of short-term and long-term hormone effects in transsexuals. Psychoneuroendocrinology 1999, 24, 423–447. [Google Scholar] [CrossRef]
- Springer, S.P.; Deutsch, G. Left Brain Right Brain, 6th ed.; W.H. Freeman: San Francisco, CA, USA, 2001. [Google Scholar]
- Weiss, S.; Mueller, H.M. The contribution of EEG coherence to the investigation of language. Brain Lang. 2003, 85, 325–343. [Google Scholar] [CrossRef]
- Khateb, A.; Michel, C.M.; Pegna, A.J.; Thut, G.; Landis, T.; Annoni, J.M. The time course of semantic category processing in the cerebral hemispheres: An electrophysiological study. Cogn. Brain Res. 2001, 10, 251–264. [Google Scholar] [CrossRef]
- Davidson, R.J.; Chapman, J.P.; Chapman, L.J.; Henriques, J.B. Asymmetrical brain electrical activity discriminates between psychometrically-matched verbal and spatial cognitive tasks. Psychophysiology 1990, 27, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Hoptman, M.J.; Davidson, R.J. Baseline EEG asymmetries and performance on neuropsychological tasks. Neuropsychologia 1998, 36, 1343–1353. [Google Scholar] [CrossRef]
- Sheppard, W.D.; Boyer, R.W. Pretrial EEG coherence as a predictor of semantic priming effects. Brain Lang. 1990, 39, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J. EEG measures of cerebral asymmetry: Conceptual and methodological issues. Int. J. Neurosci. 1988, 39, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Sutton, S.K.; Davidson, R.J. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychol. Sci. 1997, 8, 204–210. [Google Scholar] [CrossRef]
- Sutton, S.K.; Davidson, R.J. Prefrontal brain electrical asymmetry predicts the evaluation of affective stimuli. Neuropsychologia 2000, 38, 1723–1733. [Google Scholar] [CrossRef]
- Davidson, R.J.; Hughdahl, K. (Eds.) Brain Asymmetry; MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Nettle, D. Hand laterality and cognitive ability: A multiple regression approach. Brain Cogn. 2003, 52, 390–398. [Google Scholar] [CrossRef]
- Gizewski, E.R.; Krause, E.; Wanke, I.; Forsting, M.; Senf, W. Gender-specific cerebral activation during cognitive tasks using functional MRI: Comparison of women in mid-luteal phase and men. Neuroradiology 2005, 48, 14–20. [Google Scholar] [CrossRef]
- Hausmann, M.; Güntürkün, O. Steroid fluctuations modify functional cerebral asymmetries: The hypothesis of progesterone-mediated interhemispheric decoupling. Neuropsychologia 2000, 38, 1362–1374. [Google Scholar] [CrossRef]
- Hausmann, M.; Becker, C.; Gather, U.; Güntürkün, O. Functional cerebral asymmetries during the menstrual cycle: A cross-sectional and longitudinal analysis. Neuropsychologia 2002, 40, 808–816. [Google Scholar] [CrossRef]
- Compton, R.J.; Costello, C.; Diepold, J. Interhemispheric integration during the menstrual cycle: Failure to confirm progesterone-mediated interhemispheric decoupling. Neuropsychologia 2004, 42, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Fernández, G.; Weis, S.; Stoffel-Wagner, B.; Tendolkar, I.; Reuber, M.; Beyenburg, S.; Klaver, P.; Fell, J.; de Greiff, A.; Ruhlmann, J.; et al. Menstrual cycle-dependent neural plasticity in the adult human brain is hormone, task, and region specific. J. Neurosci. 2003, 23, 3790–3795. [Google Scholar] [CrossRef]
- Weis, S.; Hausmann, M.; Stoffers, B.; Vohn, R.; Kellermann, T.; Sturm, W. Estradiol modulates functional brain organization during the menstrual cycle: An analysis of interhemispheric inhibition. J. Neurosci. 2008, 28, 13401–13410. [Google Scholar] [CrossRef]
- Gevins, A.; Smith, M.E.; McEvoy, L.; Yu, D. High resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cereb. Cortex 1997, 7, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Rappelsberger, P. EEG coherence within the 13–18 Hz band as a correlate of a distinct lexical organization of concrete and abstract nouns in humans. Neurosci. Lett. 1996, 209, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Gevins, A.; Smith, M.E. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb. Cortex 2000, 10, 829–839. [Google Scholar] [CrossRef]
- Volf, N.V.; Razumnikova, O.M. Sex differences in EEG coherence during a verbal memory task in young adults. Brain Topogr. 2010, 23, 117–126. [Google Scholar] [CrossRef]
- Corsi-Cabrera, M.; Solís-Ortiz, S.; Guevara, M. Stability of EEG inter- and intrahemispheric correlation in women. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 248–255. [Google Scholar] [CrossRef]
- Blake, K.R.; Dixson, B.J.; O’Dean, S.M.; Denson, T.F. Standardized protocols for characterizing women’s fertility: A data-driven approach. Horm. Behav. 2016, 81, 74–83. [Google Scholar] [CrossRef]
- Gangestad, S.W.; Haselton, M.G.; Welling, L.L.; Gildersleeve, K.; Pillsworth, E.G.; Burriss, R.P.; Larson, C.M.; Puts, D.A. How valid are assessments of conception probability in ovulatory cycle research? Evaluations, recommendations, and theoretical implications. Evol. Hum. Behav. 2016, 37, 85–96. [Google Scholar] [CrossRef]
- Schneider, W.; Eschman, A.; Zuccolotto, A. E-Prime User’s Guide; Psychology Software Tools, Inc.: Pittsburgh, PA, USA, 2002. [Google Scholar]
- Taub, J.M.; Berger, R.J. Acute shifts in the sleep-wakefulness cycle: Effects on performance and mood. Psychosom. Med. 1974, 36, 164–173. [Google Scholar] [CrossRef]
- Prizmic, Z. Correlation Among the CTQ Results and the Parameters of Circadian Variations of Oral Temperature, Pulse, and Mood Dimensions. Master’s Thesis, University of Zagreb, Zagreb, Croatia, 1988. [Google Scholar]
- Baehr, E.; Rosenfeld, P.; Miller, L.; Baehr, R. Premenstrual dysphoric disorder and changes in frontal alpha asymmetry. Int. J. Psychophysiol. 2004, 52, 159–167. [Google Scholar] [CrossRef] [PubMed]
| Early Follicular Phase | Mid-Luteal Phase | ||||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | F | p | ηp2 | |
| Anxiety | 4.97 | 4.23 | 4.35 | 4.00 | 0.06 | 0.82 | 0.002 |
| Depression | 5.48 | 4.67 | 4.15 | 3.80 | 0.62 | 0.44 | 0.021 |
| Friendliness | 14.37 | 4.58 | 14.30 | 4.03 | 0.02 | 0.89 | 0.001 |
| Joy | 11.07 | 4.35 | 11.03 | 4.04 | 0.01 | 0.97 | 0.001 |
| Fatigue | 11.67 | 7.49 | 8.43 | 5.92 | 4.61 | 0.04 | 0.137 |
| Hostility | 5.58 | 2.77 | 4.73 | 3.30 | 0.37 | 0.55 | 0.013 |
| Focus | 20.30 | 5.72 | 20.83 | 5.27 | 0.33 | 0.57 | 0.011 |
| Energy | 10.17 | 4.67 | 11.17 | 3.66 | 1.69 | 0.20 | 0.055 |
| Task: Verbal Reasoning | |||||
|---|---|---|---|---|---|
| Frontal | Source | df | F | p | ηp2 |
| Menstrual cycle phase | 1 | 0.011 | 0.918 | 0.001 | |
| Mid-luteal baseline | 1 | 2.016 | 0.173 | 0.101 | |
| Early follicular baseline | 1 | 3.555 | 0.076 | 0.165 | |
| Error (cycle) | 29 | ||||
| Temporal | Source | df | F | p | ηp2 |
| Menstrual cycle phase | 1 | 2.732 | 0.117 | 0.138 | |
| Mid-luteal baseline | 1 | 0.230 | 0.638 | 0.013 | |
| Early follicular baseline | 1 | 1.825 | 0.194 | 0.097 | |
| Error (cycle) | 29 | ||||
| Parietal | Source | df | F | p | ηp2 |
| Menstrual cycle phase | 1 | 0.002 | 0.969 | 0.000 | |
| Mid-luteal baseline | 1 | 0.007 | 0.935 | 0.000 | |
| Early follicular baseline | 1 | 0.111 | 0.742 | 0.006 | |
| Error (cycle) | 29 | ||||
| Task: Semantic Decision | |||||
|---|---|---|---|---|---|
| Frontal | Source | df | F | p | ηp2 |
| Menstrual cycle phase | 1 | 3.194 | 0.088 | 0.127 | |
| Mid-luteal baseline | 1 | 3.418 | 0.078 | 0.134 | |
| Early follicular baseline | 1 | 2.730 | 0.113 | 0.110 | |
| Error (cycle) | 27 | ||||
| Temporal | Source | df | F | p | ηp2 |
| Menstrual cycle phase | 1 | 4.712 | 0.04 | 0.159 | |
| Mid-luteal baseline | 1 | 0.100 | 0.754 | 0.004 | |
| Early follicular baseline | 1 | 0.877 | 0.358 | 0.034 | |
| Error (cycle) | 29 | ||||
| Parietal | Source | df | F | p | ηp2 |
| Menstrual cycle phase | 1 | 4.859 | 0.042 | 0.222 | |
| Mid-luteal baseline | 1 | 0.128 | 0.725 | 0.007 | |
| Early follicular baseline | 1 | 1.718 | 0.207 | 0.092 | |
| Error (cycle) | 29 | ||||
| Task: Verbal Fluency | |||||
|---|---|---|---|---|---|
| Frontal | Source | df | F | p | ηp2 |
| Menstrual cycle phase | 1 | 5.753 | 0.023 | 0.166 | |
| Mid-luteal baseline | 1 | 1.614 | 0.214 | 0.053 | |
| Early follicular baseline | 1 | 0.964 | 0.334 | 0.032 | |
| Error (cycle) | 29 | ||||
| Temporal | Source | df | F | p | ηp2 |
| Menstrual cycle phase | 1 | 4.275 | 0.049 | 0.141 | |
| Mid-luteal baseline | 1 | 0.809 | 0.377 | 0.030 | |
| Early follicular baseline | 1 | 0.390 | 0.538 | 0.015 | |
| Error (cycle) | 29 | ||||
| Parietal | Source | df | F | p | ηp2 |
| Menstrual cycle phase | 1 | 10.467 | 0.003 | 0.279 | |
| Mid-luteal baseline | 1 | 0.288 | 0.596 | 0.011 | |
| Early follicular baseline | 1 | 1.894 | 0.180 | 0.066 | |
| Error (cycle) | 29 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hromatko, I.; Tadinac, M. Menstrual Cycle Modulation of Verbal Performance and Hemispheric Asymmetry. Brain Sci. 2025, 15, 1141. https://doi.org/10.3390/brainsci15111141
Hromatko I, Tadinac M. Menstrual Cycle Modulation of Verbal Performance and Hemispheric Asymmetry. Brain Sciences. 2025; 15(11):1141. https://doi.org/10.3390/brainsci15111141
Chicago/Turabian StyleHromatko, Ivana, and Meri Tadinac. 2025. "Menstrual Cycle Modulation of Verbal Performance and Hemispheric Asymmetry" Brain Sciences 15, no. 11: 1141. https://doi.org/10.3390/brainsci15111141
APA StyleHromatko, I., & Tadinac, M. (2025). Menstrual Cycle Modulation of Verbal Performance and Hemispheric Asymmetry. Brain Sciences, 15(11), 1141. https://doi.org/10.3390/brainsci15111141







