Medication vs. Movement in ADHD: Interaction Between Medication and Physical Activity on Neurocognitive Functioning
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Demographic Questionnaire
2.2.2. Diagnostic Screening Using the Vanderbilt ADHD Diagnostic Parent Rating Scale (VADPRS)
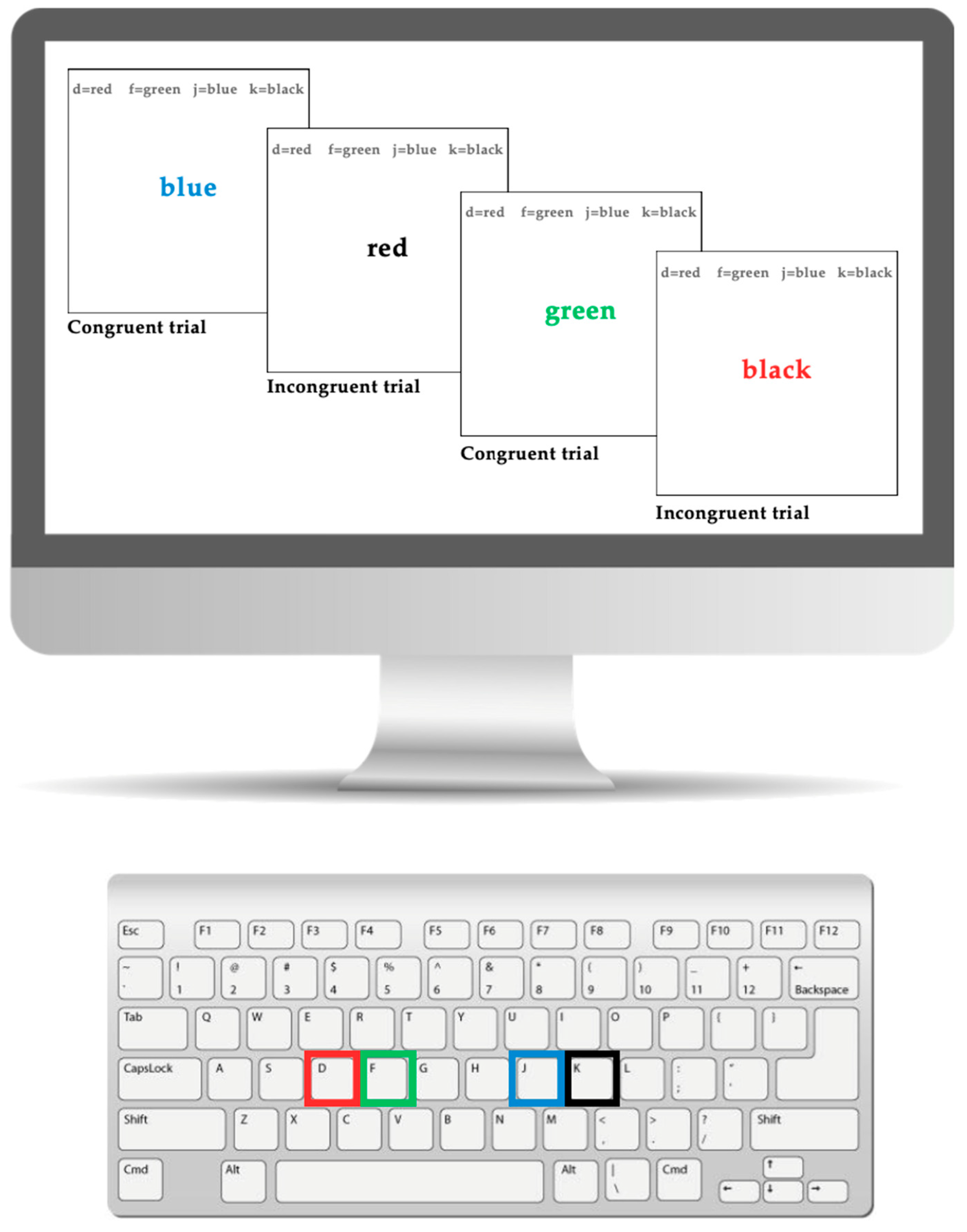
2.2.3. Executive Functioning: Stroop Task
2.2.4. Heart Rate
2.2.5. Functional Near-Infrared Spectroscopy (fNIRS): Left DLPFC
2.3. Design
2.3.1. Movement Condition
2.3.2. Stationary Condition
2.4. Procedure
2.5. Statistical Analysis
2.5.1. fNIRS and Left DLPFC Activity
2.5.2. Executive Functioning
3. Results
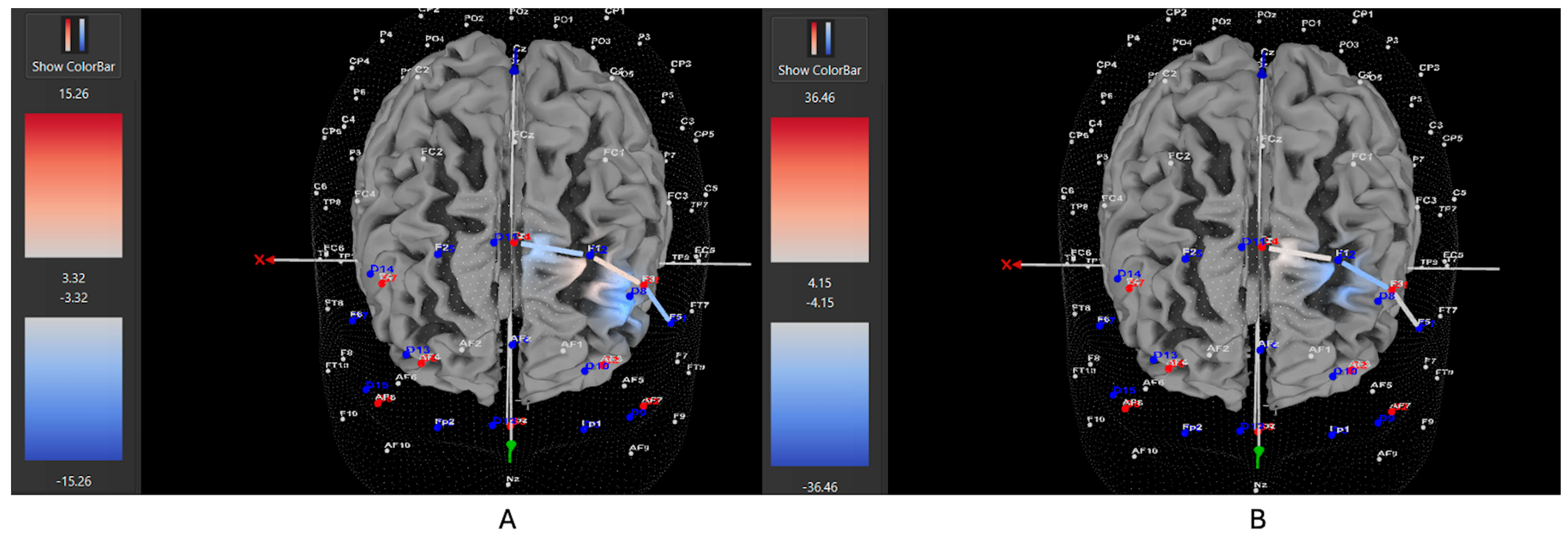
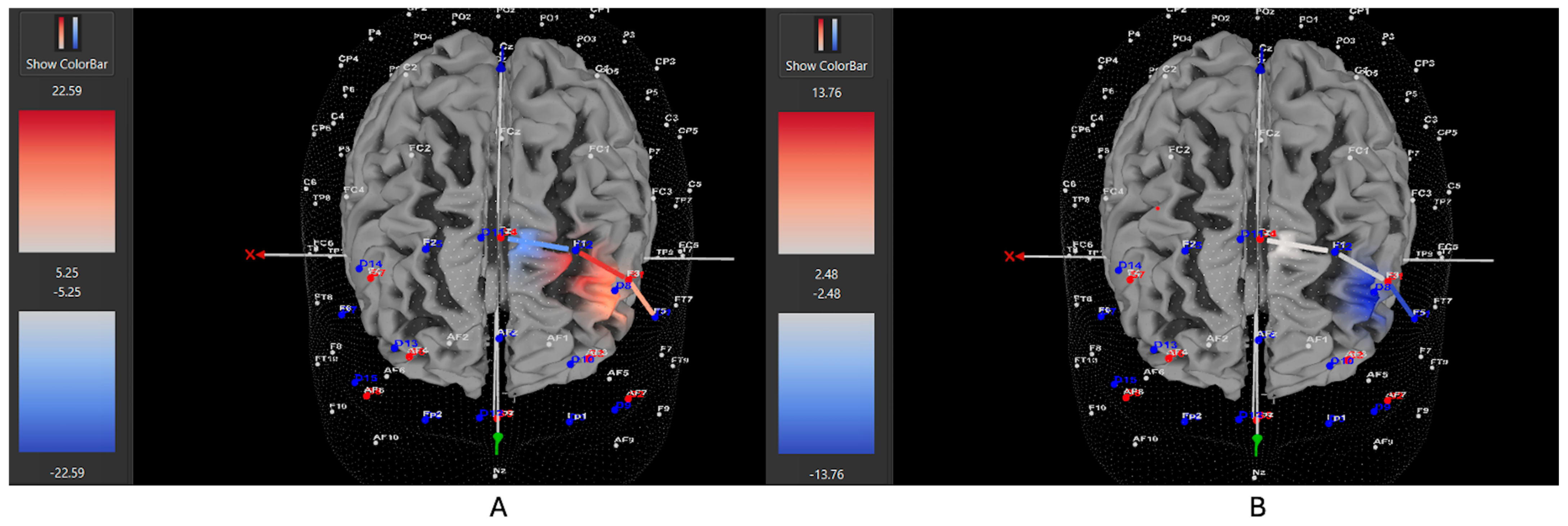
3.1. fNIRS and Left DLPFC Activity
3.2. Executive Functioning: Stroop Task
4. Discussion
4.1. Practical Implications
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazarova, V.A.; Sokolov, A.V.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Treatment of ADHD: Drugs, psychological therapies, devices, complementary and alternative methods as well as the trends in clinical trials. Front. Pharmacol. 2022, 13, 1066988. [Google Scholar] [CrossRef]
- Nanda, A.; Janga, L.S.N.; Sambe, H.G.; Yasir, M.; Man, R.K.; Gogikar, A.; Mohammed, L. Adverse effects of stimulant interventions for attention deficit hyperactivity disorder (ADHD): A comprehensive systematic review. Cureus 2023, 15, e43803. [Google Scholar] [CrossRef]
- Van Riper, S.M.; Tempest, G.D.; Piccirilli, A.; Ma, Q.; Reiss, A.L. Aerobic Exercise, Cognitive Performance, and Brain Activity in Adolescents with Attention-Deficit/Hyperactivity Disorder. Med. Sci. Sports Exerc. 2023, 55, 1445–1455. [Google Scholar] [CrossRef]
- Diamond, A. Attention-deficit disorder (attention-deficit/hyperactivity disorder without hyperactivity): A neurobiologically and behaviorally distinct disorder from attention-deficit/hyperactivity disorder (with hyperactivity). Dev. Psychopathol. 2005, 17, 807–825. [Google Scholar] [CrossRef]
- Antshel, K.M.; Hier, B.O.; Barkley, R.A. Executive Functioning Theory and ADHD. In Handbook of Executive Functioning; Springer: New York, NY, USA, 2014; pp. 107–120. [Google Scholar]
- Ruiter, M.; Görlich, E.; Loyens, S.; Wong, J.; Paas, F. Effects of Desk-Bike Cycling on Phonological Working Memory Performance in Adolescents with Attention Deficit Hyperactivity Disorder. Front. Educ. 2022, 7, 841576. [Google Scholar] [CrossRef]
- Zang, Y.-F.; Jin, Z.; Weng, X.-C.; Zhang, L.; Zeng, Y.-W.; Yang, L.; Wang, Y.-F.; Seidman, L.J.; Faraone, S.V. Functional MRI in attention-deficit hyperactivity disorder: Evidence for hypofrontality. Brain Dev. 2005, 27, 544–550. [Google Scholar] [CrossRef]
- Ueda, S.; Ota, T.; Iida, J.; Yamamuro, K.; Yoshino, H.; Kishimoto, N.; Kishimoto, T. Reduced prefrontal hemodynamic response in adult attention-deficit hyperactivity disorder as measured by near-infrared spectroscopy. Psychiatry Clin. Neurosci. 2018, 72, 380–390. [Google Scholar] [CrossRef]
- Rapport, M.D.; Bolden, J.; Kofler, M.J.; Sarver, D.E.; Raiker, J.S.; Alderson, R.M. Hyperactivity in Boys with Attention-Deficit/Hyperactivity Disorder (ADHD): A Ubiquitous Core Symptom or Manifestation of Working Memory Deficits? J. Abnorm. Child Psychol. 2009, 37, 521–534. [Google Scholar] [CrossRef]
- Berridge, C.W.; Devilbiss, D.M. Psychostimulants as cognitive enhancers: The prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol. Psychiatry 2011, 69, e101–e111. [Google Scholar] [CrossRef]
- Harris, S.S.; Green, S.M.; Kumar, M.; Urs, N.M. A role for cortical dopamine in the paradoxical calming effects of psychostimulants. Sci. Rep. 2022, 12, 3129. [Google Scholar] [CrossRef]
- Cho, S.C.; Hwang, J.W.; Kim, B.N.; Lee, H.Y.; Kim, H.W.; Lee, J.S.; Lee, D.S. The relationship between regional cerebral blood flow and response to methylphenidate in children with attention-deficit hyperactivity disorder: Comparison between non-responders to methylphenidate and responders. J. Psychiatr. Res. 2007, 41, 459–465. [Google Scholar] [CrossRef]
- Hoy, B.-A.; Bi, M.; Lam, M.; Krishnasamy, G.; Abdalmalak, A.; Fenesi, B. Hyperactivity in ADHD: Friend or foe? Brain Sci. 2024, 14, 719. [Google Scholar] [CrossRef]
- Hoy, B.-A.; Feehely, M.; Bi, M.; Lam, M.; Abdalmalak, A.; Fenesi, B. Individual differences in the neurocognitive effect of movement during executive functioning in children with ADHD: Impact of subtype, severity, and gender. Brain Sci. 2025, 15, 623. [Google Scholar] [CrossRef]
- Bigelow, H.; Gottlieb, M.D.; Ogrodnik, M.; Graham, J.D.; Fenesi, B. The Differential Impact of Acute Exercise and Mindfulness Meditation on Executive Functioning and Psycho-Emotional Well-Being in Children and Youth with ADHD. Front. Psychol. 2021, 12, 660845. [Google Scholar] [CrossRef]
- Welsch, L.; Alliott, O.; Kelly, P.; Fawkner, S.; Booth, J.; Niven, A. The effect of physical activity interventions on executive functions in children with ADHD: A systematic review and meta-analysis. Ment. Health Phys. Act. 2021, 20, 100379. [Google Scholar] [CrossRef]
- Benzing, V.; Chang, Y.-K.; Schmidt, M. Acute Physical Activity Enhances Executive Functions in Children with ADHD. Sci. Rep. 2018, 8, 12382. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Li, B.-M. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol. Psychiatry 2005, 57, 1377–1384. [Google Scholar] [CrossRef]
- Sarver, D.E.; Rapport, M.D.; Kofler, M.J.; Raiker, J.S.; Friedman, L.M. Hyperactivity in Attention-Deficit/Hyperactivity Disorder (ADHD): Impairing Deficit or Compensatory Behavior? J. Abnorm. Child Psychol. 2015, 43, 1219–1232. [Google Scholar] [CrossRef]
- Danaher, P.A.; Henderson, R. Moving beyond sedentarism: Conceptual and empirical developments. In Beyond Binaries in Education Research; Routledge: London, UK, 2012; pp. 60–78. [Google Scholar]
- Guirado, T.; Chambonnière, C.; Chaput, J.P.; Metz, L.; Thivel, D.; Duclos, M. Effects of classroom active desks on children and adolescents’ physical activity, sedentary behavior, academic achievements and overall health: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 2828. [Google Scholar] [CrossRef]
- Pontifex, M.B.; Saliba, B.J.; Raine, L.B.; Picchietti, D.L.; Hillman, C.H. Exercise Improves Behavioral, Neurocognitive, and Scholastic Performance in Children with Attention-Deficit/Hyperactivity Disorder. J. Pediatr. 2013, 162, 543–551. [Google Scholar] [CrossRef]
- Cerrillo-Urbina, A.J.; García-Hermoso, A.; Sánchez-Lopez, M.; Pardo-Guijarro, M.J.; Santos Gómez, J.L.; Martínez-Vizcaíno, V. The effects of physical exercise in children with attention deficit hyperactivity disorder: A systematic review and meta-analysis of randomized control trials. Child Care Health Dev. 2015, 41, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Neudecker, C.; Mewes, N.; Reimers, A.K.; Woll, A. Exercise interventions in children and adolescents with ADHD: A systematic review. J. Atten. Disord. 2019, 23, 4. [Google Scholar] [CrossRef]
- Peluso, M.A.M.; Andrade, L.H.S.G. Physical activity and mental health: The association between exercise and mood. Clinics 2005, 60, 61–70. [Google Scholar] [CrossRef]
- Poliakova, E.; Conrad, A.L.; Schieltz, K.M.; O’Brien, M.J. Using fNIRS to evaluate ADHD medication effects on neuronal activity: A systematic literature review. Front. Neuroimaging 2023, 2, 1083036. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Dan, I.; Tsuzuki, D.; Kato, M.; Okamoto, M.; Kyutoku, Y.; Soya, H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage 2010, 50, 1702–1710. [Google Scholar] [CrossRef]
- Almajidy, R.K.; Mankodiya, K.; Abtahi, M.; Hofmann, U.G. A Newcomer’s Guide to Functional Near Infrared Spectroscopy Experiments. IEEE Rev. Biomed. Eng. 2020, 13, 292–308. [Google Scholar] [CrossRef]
- Hyodo, K.; Dan, I.; Suwabe, K.; Kyutoku, Y.; Yamada, Y.; Akahori, M.; Byun, K.; Kato, M.; Soya, H. Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol. Aging 2012, 33, 2621–2632. [Google Scholar] [CrossRef]
- Thomas, A.G.; Dennis, A.; Bandettini, P.A.; Johansen-Berg, H. The Effects of Aerobic Activity on Brain Structure. Front. Psychol. 2012, 3, 86. [Google Scholar] [CrossRef]
- Huang, T.; Gu, Q.; Deng, Z.; Tsai, C.; Xue, Y.; Zhang, J.; Zou, L.; Chen, Z.; Wang, K. Executive Function Performance in Young Adults When Cycling at an Active Workstation: An fNIRS Study. Int. J. Environ. Res. Public Health 2019, 16, 1119. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, N.; Usami, M.; Naya, N.; Tsuji, T.; Mishima, H.; Horie, J.; Iida, J. Efficacy and safety of medication for attention-deficit hyperactivity disorder in children and adolescents with common comorbidities: A systematic review. Neurol. Ther. 2021, 10, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Liu, B.; Kuang, D.; Zhu, X.; Bi, X.; Song, Y.; Ren, Y. The association between physical activity and sleep in adult ADHD patients with stimulant medication use. Front. Psychiatry 2023, 14, 1236636. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Fusetto Veronesi, G.; Gabellone, A.; Margari, A.; Marzulli, L.; Matera, E.; Margari, L. The management of sleep disturbances in children with attention-deficit/hyperactivity disorder (ADHD): An update of the literature. Expert Rev. Neurother. 2024, 24, 585–596. [Google Scholar] [CrossRef]
- Koch, E.D.; Freitag, C.M.; Mayer, J.S.; Medda, J.; Reif, A.; Grimm, O.; Ramos-Quiroga, J.A.; Sanchez, J.P.; Asherson, P.; Kuntsi, J.; et al. The dynamical association between physical activity and affect in the daily life of individuals with ADHD. Eur. Neuropsychopharmacol. 2022, 57, 69–74. [Google Scholar] [CrossRef]
- Zang, Y. Impact of physical exercise on children with attention deficit hyperactivity disorders: Evidence through a meta-analysis. Medicine 2019, 98, e17980. [Google Scholar] [CrossRef]
- Ogundele, M.O.; Ayyash, H.F. ADHD in children and adolescents: Review of current practice of non-pharmacological and behavioural management. AIMS Public Health 2023, 10, 35–51. [Google Scholar] [CrossRef]
- Shrestha, M.; Lautenschleger, J.; Soares, N. Non-pharmacologic management of attention-deficit/hyperactivity disorder in children and adolescents: A review. Transl. Pediatr. 2020, 9, S114–S124. [Google Scholar] [CrossRef]
- Catala-Lopez, F.; Hutton, B.; Nunez-Beltran, A.; Page, M.J.; Ridao, M.; Macias Saint-Gerons, D.; A Catalá, M.; Tabarés-Seisdedos, R.; Moher, D. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS ONE 2017, 12, e0180355. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, F.; Gugnani, J.S.; Kaur, H.; Damera, A.R.; Mane, R.; Sekhri, A.; Singh, G.; Kaur, G. Dietary interventions and supplements for managing attention-deficit/hyperactivity disorder (ADHD): A systematic review of efficacy and recommendations. Cureus 2024, 16, e69804. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Li, G.; Ye, J.; Wu, M.; Xu, R.; Hu, M. Effects of combined physical and cognitive training on executive function of adolescent shooting athletes: A functional near-infrared spectroscopy study. Sports Med. Health Sci. 2023, 5, 220–228. [Google Scholar] [CrossRef]
- Stein, M.A.; Weiss, M.; Hlavaty, L. ADHD treatments, sleep, and sleep problems: Complex associations. Neurotherapeutics 2012, 9, 509–517. [Google Scholar] [CrossRef]
- Smith, Y. Stimulants and Sleep. News-Medical. Available online: https://www.news-medical.net/health/Stimulants-and-Sleep.aspx (accessed on 1 September 2025).
- Lagun, N. The ADHD effort paradox: Lagun’s law models structural effort regulation. Preprints 2025. [Google Scholar] [CrossRef]
- Sergeant, J. The cognitive-energetic model: An empirical approach to attention-deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 2000, 24, 7–12. [Google Scholar] [CrossRef]
- Kofler, M.J.; Rapport, M.D.; Alderson, R.M. Quantifying ADHD classroom inattentiveness, its moderators, and variability: A meta-analytic review. J. Child Psychol. Psychiatry 2008, 49, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Lauth, G.W.; Heubeck, B.G.; Mackowiak, K. Observation of children with attention-deficit hyperactivity (ADHD) problems in three natural classroom contexts. Br. J. Educ. Psychol. 2006, 76, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Adólfsdóttir, S.; Sørensen, L.; Lundervold, A.J. The Attention Network Test: A Characteristic Pattern of Deficits in Children with ADHD. Behav. Brain Funct. 2008, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Konrad, K.; Neufang, S.; Hanisch, C.; Fink, G.R.; Herpertz-Dahlmann, B. Dysfunctional Attentional Networks in Children with Attention Deficit/Hyperactivity Disorder: Evidence from an Event-Related Functional Magnetic Resonance Imaging Study. Biol. Psychiatry 2006, 59, 643–651. [Google Scholar] [CrossRef]



| Characteristic | ADHD Medicated (n = 15) | ADHD Unmedicated (n = 11) | Control (n = 24) |
|---|---|---|---|
| Child’s Age (years), mean (SD) | 9.73 (1.53) | 10.09 (1.76) | 10.21 (1.06) |
| Guardian’s Age (years), mean (SD) | 44.2 (7.17) | 38.82 (8.20) | 45.17 (4.62) |
| Participant’s Sex | |||
| Male | 7 | 9 | 14 |
| Female | 8 | 2 | 8 |
| Guardian’s Sex | |||
| Male | 2 | 3 | 4 |
| Female | 13 | 8 | 20 |
| Guardian’s Education Level (n) | |||
| Some high school, no diploma | 0 | 0 | 0 |
| High school graduate, diploma or the equivalent | 0 | 1 | 0 |
| Some college credit, no degree | 1 | 0 | 2 |
| Trade/technical/vocational training | 3 | 0 | 1 |
| Associate degree | 1 | 2 | 1 |
| Bachelor’s degree | 6 | 3 | 8 |
| Master’s degree | 2 | 3 | 7 |
| Professional degree | 3 | 1 | 5 |
| No response | 0 | 1 | 0 |
| Guardian’s Employment (n) | |||
| Employed for wages | 10 | 7 | 13 |
| Self-employed | 3 | 2 | 6 |
| Unemployed | 1 | 1 | 3 |
| Student | 0 | 1 | 0 |
| Household Income | |||
| Prefer not to say | 1 | 1 | 4 |
| <$30,000 | 0 | 1 | 1 |
| $30,000–$40,000 | 0 | 0 | 1 |
| $40,000–$50,000 | 0 | 1 | 1 |
| $50,000–$60,000 | 1 | 2 | 3 |
| $60,000–$70,000 | 2 | 0 | 1 |
| $70,000–$80,000 | 0 | 0 | 1 |
| $80,000–$90,000 | 2 | 0 | 2 |
| $90,000–$100,000 | 3 | 1 | 0 |
| >$100,000 | 6 | 5 | 8 |
| Age of ADHD diagnosis (n) | |||
| Unsure | 0 | 3 | - |
| 3–5 | 2 | 2 | - |
| 6–8 | 9 | 4 | - |
| 9–12 | 3 | 2 | - |
| ADHD Subtype (n) | |||
| Predominantly inattentive | 3 | 2 | - |
| Predominantly hyperactive | 2 | 4 | - |
| Combined subtype | 11 | 5 | - |
| Unsure/no diagnosis given | - | ||
| ADHD Severity (n) | |||
| Low | 3 | 5 | - |
| Moderate | 6 | 3 | - |
| High | 6 | 3 | - |
| Number of ADHD Medications | |||
| 1 | 13 | 3 | - |
| 2 | 2 | 0 | - |
| Type of ADHD Medication | |||
| Methylphenidate Stimulants (i.e., Concerta, Biphentin, Methylphenidate HCI) | 11 | 2 | - |
| Amphetamine Stimulants (i.e., Vyvanse) | 3 | 1 | - |
| Non-Stimulants (i.e., Strattera, Intuniv) | 3 | 0 | - |
| None | 0 | 8 | - |
| Length of ADHD Medication | |||
| <6 months | 1 | 2 | - |
| 6–11 months | 5 | 1 | - |
| 1–3 years | 6 | 1 | - |
| 3+ years | 2 | 0 | - |
| Comorbid Diagnosis | |||
| Yes | 8 | 0 | 0 |
| No | 7 | 11 | 24 |
| Medicated for Comorbid Diagnosis | |||
| Yes | 4 | 0 | 0 |
| No | 11 | 11 | 24 |
| Unmedicated ADHD (n = 11) | Medicated ADHD (n = 14) | Controls (n = 24) | ||||
|---|---|---|---|---|---|---|
| Stationary M (SD) | Movement M (SD) | Stationary M (SD) | Movement M (SD) | Stationary M (SD) | Movement M (SD) | |
| Total RT | 1144 (809) | 1003 (247) | 1252 (502) | 1083 (404) | 1103 (420) | 968 (200) |
| Congruent RT | 1265 (1264) | 935 (234) | 1241 (618) | 1040 (394) | 1054 (447) | 932 (248) |
| Incongruent RT | 1068 (524) | 1071 (371) | 1272 (469) | 1134 (466) | 1131 (475) | 1007 (233) |
| Total PC | 0.87 (0.13) | 0.95 (0.07) | 0.95 (0.06) | 0.91 (0.11) | 0.90 (0.13) | 0.92 (0.11) |
| Congruent PC | 0.95 (0.15) | 0.97 (0.09) | 0.96 (0.09) | 0.90 (0.16) | 0.96 (0.12) | 0.93 (0.12) |
| Incongruent PC | 0.80 (0.18) | 0.93 (0.11) | 0.93 (0.11) | 0.92 (0.13) | 0.84 (0.23) | 0.89 (0.17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoy, B.-A.; Bi, M.; Lam, M.; Abdalmalak, A.; Fenesi, B. Medication vs. Movement in ADHD: Interaction Between Medication and Physical Activity on Neurocognitive Functioning. Brain Sci. 2025, 15, 1107. https://doi.org/10.3390/brainsci15101107
Hoy B-A, Bi M, Lam M, Abdalmalak A, Fenesi B. Medication vs. Movement in ADHD: Interaction Between Medication and Physical Activity on Neurocognitive Functioning. Brain Sciences. 2025; 15(10):1107. https://doi.org/10.3390/brainsci15101107
Chicago/Turabian StyleHoy, Beverly-Ann, Michelle Bi, Matthew Lam, Androu Abdalmalak, and Barbara Fenesi. 2025. "Medication vs. Movement in ADHD: Interaction Between Medication and Physical Activity on Neurocognitive Functioning" Brain Sciences 15, no. 10: 1107. https://doi.org/10.3390/brainsci15101107
APA StyleHoy, B.-A., Bi, M., Lam, M., Abdalmalak, A., & Fenesi, B. (2025). Medication vs. Movement in ADHD: Interaction Between Medication and Physical Activity on Neurocognitive Functioning. Brain Sciences, 15(10), 1107. https://doi.org/10.3390/brainsci15101107







