Anorexia Nervosa Dampens Subjective and Facial Pain Responsiveness
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials and Procedure
2.3. Assessment of Pain Thresholds and Pain Tolerance Thresholds
2.4. Assessment of Subjective and Facial Responses to Painful Heat Stimuli
2.5. Statistical Analysis
3. Results
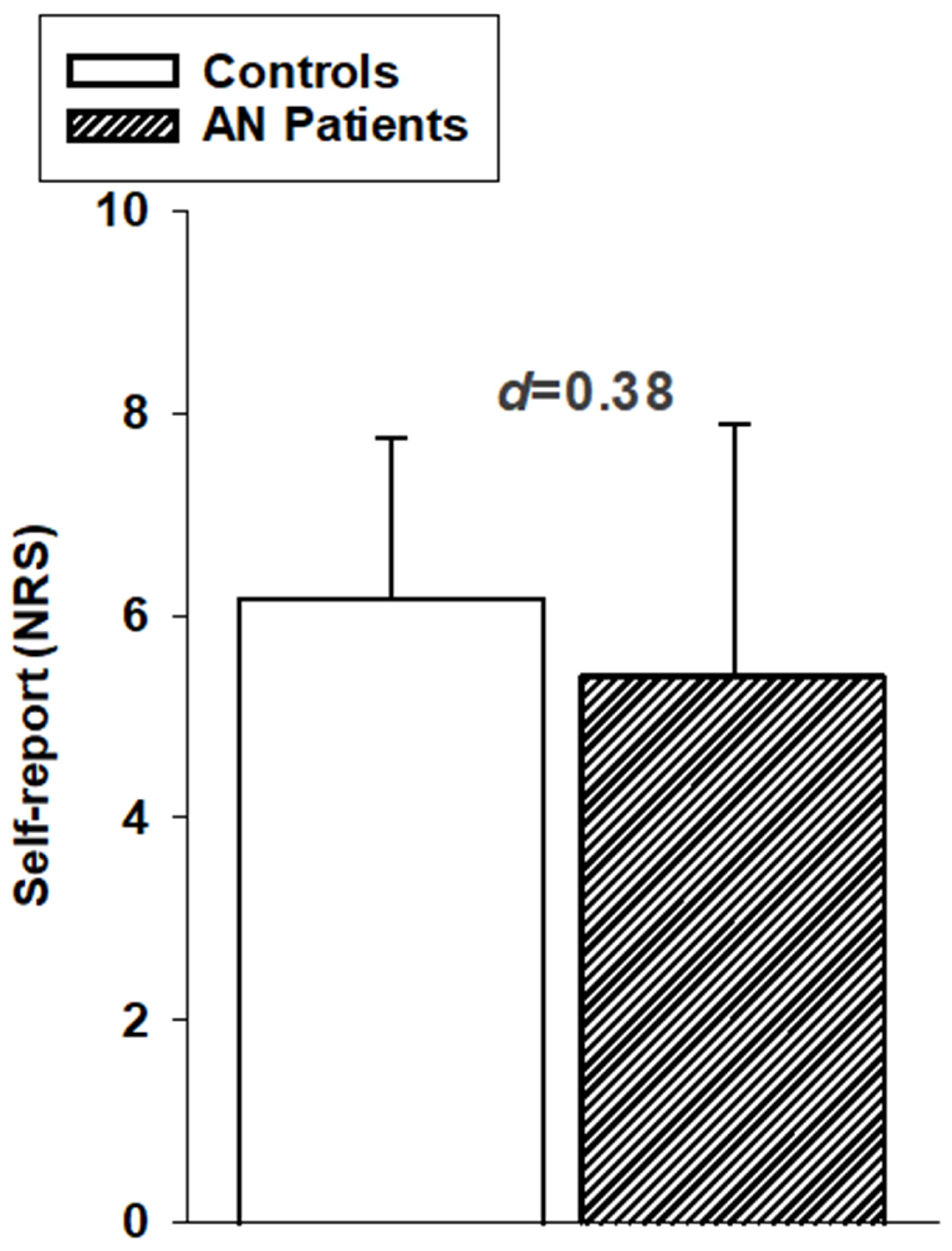
3.1. Psychophysical Data/Subjective Responses to Pain
3.2. Facial Expression of Pain to Painful Heat Stimulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AN | Anorexia Nervosa |
| AUs | Action Units |
| BDI-II | Beck Depression Inventory-II |
| BMI | body mass index |
| EAT-26 | Eating Attitudes Test |
| EDI-2 | Eating Disorder Inventory-2 |
| FACS | Facial Action Coding System |
| MDD | Major Depressive Disorder |
| NRS | Numerical Rating Scale |
| sqrt | Square root |
References
- Beumont, P.J. Clinical presentation of anorexia nervosa and bulimia nervosa. In Eating Disorders and Obesity: A Comprehensive Handbook; Guilford Press: New York, NY, USA, 2002; Volume 2, pp. 162–170. [Google Scholar]
- Calugi, S.; Chignola, E.; El Ghoch, M.; Dalle Grave, R. Starvation symptoms in patients with anorexia nervosa: A longitudinal study. Eat. Disord. 2018, 26, 523–537. [Google Scholar] [CrossRef]
- Krieg, J.C.; Roscher, S.; Strian, F.; Pirke, K.M.; Lautenbacher, S. Pain sensitivity in recovered anorexics, restrained and unrestrained eaters. J. Psychosom. Res. 1993, 37, 595–601. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Pauls, A.M.; Strian, F.; Pirke, K.M.; Krieg, J.C. Pain perception in patients with eating disorders. Psychosom. Med. 1990, 52, 673–682. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Pauls, A.M.; Strian, F.; Pirke, K.M.; Krieg, J.C. Pain sensitivity in anorexia nervosa and bulimia nervosa. Biol. Psychiatry 1991, 29, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Papezová, H.; Yamamotová, A.; Uher, R. Elevated pain threshold in eating disorders: Physiological and psychological factors. J. Psychiatr. Res. 2005, 39, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Pauls, A.M.; Lautenbacher, S.; Strian, F.; Pirke, K.M.; Krieg, J.C. Assessment of somatosensory indicators of polyneuropathy in patients with eating disorders. Eur. Arch. Psychiatry Clin. Neurosci. 1991, 241, 8–12. [Google Scholar] [CrossRef]
- Raymond, N.C.; Faris, P.L.; Thuras, P.D.; Eiken, B.; Howard, L.A.; Hofbauer, R.D.; Eckert, E.D. Elevated pain threshold in anorexia nervosa subjects. Biol. Psychiatry 1999, 45, 1389–1392. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Barth, K.; Friess, E.; Strian, F.; Pirke, K.M.; Krieg, J.C. Dieting and pain sensitivity: A validation of clinical findings. Physiol. Behav. 1991, 50, 629–631. [Google Scholar] [CrossRef]
- de Zwaan, M.; Biener, D.; Bach, M.; Wiesnagrotzki, S.; Stacher, G. Pain sensitivity, alexithymia, and depression in patients with eating disorders: Are they related? J. Psychosom. Res. 1996, 41, 65–70. [Google Scholar] [CrossRef]
- Fazia, G.; Carbone, E.A.; Rania, M.; Quirino, D.; Aloi, M.; de Filippis, R.; De Fazio, P.; Colloca, L.; Segura-García, C. Pain experience in eating disorders: The mediating role of depression, alexithymia and interoceptive awareness. Eur. Eat. Disord. Rev. 2024, 32, 148–160. [Google Scholar] [CrossRef]
- Bär, K.J.; Berger, S.; Schwier, C.; Wutzler, U.; Beissner, F. Insular dysfunction and descending pain inhibition in anorexia nervosa. Acta Psychiatr. Scand. 2013, 127, 269–278. [Google Scholar] [CrossRef]
- Bär, K.J.; de la Cruz, F.; Berger, S.; Schultz, C.C.; Wagner, G. Structural and functional differences in the cingulate cortex relate to disease severity in anorexia nervosa. J. Psychiatry Neurosci. 2015, 40, 269–279. [Google Scholar] [CrossRef]
- Kunz, M.; Mylius, V.; Schepelmann, K.; Lautenbacher, S. On the relationship between self-report and facial expression of pain. J. Pain 2004, 5, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Chen, J.I.; Lautenbacher, S.; Rainville, P. Brain mechanisms associated with facial encoding of affective states. Cogn. Affect. Behav. Neurosci. 2023, 23, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Seminowicz, D.A.; Moayedi, M. The dorsolateral prefrontal cortex in acute and chronic pain. J. Pain 2017, 18, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Dapelo, M.M.; Hart, S.; Hale, C.; Morris, R.; Lynch, T.R.; Tchanturia, K. Facial expression of positive emotions in individuals with eating disorders. Psychiatry Res. 2015, 230, 70–77. [Google Scholar] [CrossRef]
- Davies, H.; Schmidt, U.; Stahl, D.; Tchanturia, K. Evoked facial emotional expression and emotional experience in people with anorexia nervosa. Int. J. Eat. Disord. 2011, 44, 531–539. [Google Scholar] [CrossRef]
- Davies, H.; Wolz, I.; Leppanen, J.; Fernandez-Aranda, F.; Schmidt, U.; Tchanturia, K. Facial expression to emotional stimuli in non-psychotic disorders: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 252–271. [Google Scholar] [CrossRef]
- Lang, K.; Larsson, E.E.C.; Mavromara, L.; Simic, M.; Treasure, J.; Tchanturia, K. Diminished facial emotion expression and associated clinical characteristics in Anorexia Nervosa. Psychiatry Res. 2016, 236, 165–172. [Google Scholar] [CrossRef]
- Leppanen, J.; Dapelo, M.M.; Davies, H.; Lang, K.; Treasure, J.; Tchanturia, K. Computerised analysis of facial emotion expression in eating disorders. PLoS ONE 2017, 12, e0178972. [Google Scholar] [CrossRef]
- Ekman, P.E.; Friesen, W.V. Facial Action Coding System; Consulting Psychologists Press: Palo Alto, CA, USA, 1978. [Google Scholar]
- Lautenbacher, S.; Bär, K.J.; Eisold, P.; Kunz, M. Understanding Facial Expressions of Pain in Patients with Depression. J. Pain 2017, 18, 376–384. [Google Scholar] [CrossRef]
- The DSM series and experience with DSM-IV. Psychopathology 2002, 35, 67–71. [CrossRef] [PubMed]
- Garner, D.M.; Olmsted, M.P.; Bohr, Y.; Garfinkel, P.E. The Eating Attitudes Test: Psychometric features and clinical correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Meermann, R.; Vandereycken, W. Therapie der Magersucht und Bulimia Nervosa: Ein Klinischer Leitfaden für den Praktiker; Walter de Gruyter GmbH & Co KG: Berlin, Germany; New York, NY, USA, 1987. [Google Scholar]
- Thiel, A.; Jacobi, C.; Horstmann, S.; Paul, T.; Nutzinger, D.O.; Schüssler, G. A German Version of the Eating Disorder Inventory EDI-2. Psychotherapie, Psychosomatik. Med. Psychol. 1997, 47, 365–376. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II; Psychological Assessment; American Psychological Association APA: Washington, DC, USA, 1996. [Google Scholar]
- Bär, K.J.; Brehm, S.; Boettger, M.K.; Boettger, S.; Wagner, G.; Sauer, H. Pain perception in major depression depends on pain modality. Pain 2005, 117, 97–103. [Google Scholar] [CrossRef]
- Boettger, M.K.; Grossmann, D.; Bär, K.J. Thresholds and perception of cold pain, heat pain, and the thermal grill illusion in patients with major depressive disorder. Psychosom. Med. 2013, 75, 281–287. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Roscher, S.; Strian, D.; Fassbender, K.; Krumrey, K.; Krieg, J.C. Pain perception in depression: Relationships to symptomatology and naloxone-sensitive mechanisms. Psychosom. Med. 1994, 56, 345–352. [Google Scholar] [CrossRef]
- Craig, K.D.; Prkachin, K.M.; Grunau, R. The facial expression of pain. In Handbook of Pain Assessment; Turk, D., Melzack, R., Eds.; Guilford Press: New York, NY, USA, 2011; pp. 117–133. [Google Scholar]
- Karmann, A.J.; Lautenbacher, S.; Kunz, M. The role of inhibitory mechanisms in the regulation of facial expressiveness during pain. Biol. Psychol. 2015, 104, 82–89. [Google Scholar] [CrossRef]
- Karmann, A.J.; Lautenbacher, S.; Bauer, F.; Kunz, M. The influence of communicative relations on facial responses to pain: Does it matter who is watching? Pain Res. Manag. 2014, 19, 15–22. [Google Scholar] [CrossRef]
- Schneider, P.; Lautenbacher, S.; Kunz, M. Sex differences in facial expressions of pain: Results from a combined sample. Pain 2024, 165, 1784–1792. [Google Scholar] [CrossRef]
- Kunz, M.; Meixner, D.; Lautenbacher, S. Facial muscle movements encoding pain-a systematic review. Pain 2019, 160, 535–549. [Google Scholar] [CrossRef]
- Gilam, G.; Gross, J.J.; Wager, T.D.; Keefe, F.J.; Mackey, S.C. What is the relationship between pain and emotion? Bridging constructs and communities. Neuron 2020, 107, 17–21. [Google Scholar] [CrossRef]
- Kunz, M.; Lautenbacher, S.; LeBlanc, N.; Rainville, P. Are both the sensory and the affective dimensions of pain encoded in the face? Pain 2012, 153, 350–358. [Google Scholar] [CrossRef]
- Corstorphine, E.; Mountford, V.; Tomlinson, S.; Waller, G.; Meyer, C. Distress tolerance in the eating disorders. Eat. Behav. 2007, 8, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wildes, J.E.; Ringham, R.M.; Marcus, M.D. Emotion avoidance in patients with anorexia nervosa: Initial test of a functional model. Int. J. Eat. Disord. 2010, 43, 398–404. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, D.M.; Spargo, E.; Wassif, W.S.; Newham, D.J.; Peters, T.J.; Lantos, P.L.; Russell, G.F. Structural and functional changes in skeletal muscle in anorexia nervosa. Acta Neuropathol. 1998, 95, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Fellows, H.A.; Humphries, L.L. Neurologic complications of anorexia nervosa. Acta Neurol. Scand. 1994, 89, 111–116. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Eddy, K.T.; Rutkove, S.B.; Breithaupt, L. Anorexia nervosa and muscle health: A systematic review of our current understanding and future recommendations for study. Int. J. Eat. Disord. 2023, 56, 483–500. [Google Scholar] [CrossRef]
- Raffone, F.; Atripaldi, D.; Barone, E.; Marone, L.; Carfagno, M.; Mancini, F.; Saliani, A.M.; Martiadis, V. Exploring the Role of Guilt in Eating Disorders: A Pilot Study. Clin. Pract. 2025, 15, 56. [Google Scholar] [CrossRef]



| Parameter | Healthy Controls | AN Patients | p |
|---|---|---|---|
| Number of participants (N) | 18 | 17 | |
| Male/female (N) | 0/18 | 0/17 | |
| Age in years (mean (SD)) | 25.6 (5.3) | 27.3 (8.9) | 0.46 |
| BMI (mean (SD)) | 22.1 (2.5) | 15.3 (1.7) | <0.001 |
| Education | |||
| 10 years at school | 1 | 7 | 0.012 |
| 12 years at school (A-Level) | 17 | 10 | |
| Smoker/non-smoker (N) | 3/15 | 5/12 | 0.369 |
| Coffee consumption yes/no (N) | 9/9 | 13/5 | 0.086 |
| Sport (yes/no) (N) | 10/8 | 10/7 | 0.845 |
| Depression scales | |||
| BDI (mean (SD)) | 3.1 (2.1) | 23.2 (8.5) | <0.001 |
| Eating Disorder scales | |||
| EDI (mean (SD)) | - | 3.6 (6.8) | |
| EAT 26 (mean (SD)) | - | 1.3 (0.6) | |
| Pain outcomes Pain threshold (mean (SD)) | 44.0 (3.3) | 45.6 (2.1) | |
| Tolerance threshold (mean (SD)) | 48.5 (1.3) | 48.7 (0.7) | |
| Self-report, NRS (mean (SD)) | 6.2 (1.6) | 5.5 (2.4) |
| Healthy Controls | AN Patients | ||||
|---|---|---|---|---|---|
| Frequency | d | Frequency | d | ||
| AU 1/2 | raised eyebrows | 9% | 0.33 | - | - |
| * AU 4 | furrowed brows | 18% | 0.97 | 12% | 0.54 |
| * AU 6/7 | narrowed eyes | 86% | 1.51 | 37% | 1.21 |
| * AU 9/10 | wrinkled nose | 43% | 1.35 | 11% | 0.63 |
| AU 14 | dimpler | 14% | 0.13 | 7% | 0.21 |
| AU 17 | chin raiser | 7% | 0.34 | - | - |
| * AU 25/26/27 | opened mouth | 39% | 0.51 | 15% | 1.53 |
| AU 43 | closed eyes | - | - | 6% | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lautenbacher, S.; Kunz, M.; Bär, K.-J. Anorexia Nervosa Dampens Subjective and Facial Pain Responsiveness. Brain Sci. 2025, 15, 1082. https://doi.org/10.3390/brainsci15101082
Lautenbacher S, Kunz M, Bär K-J. Anorexia Nervosa Dampens Subjective and Facial Pain Responsiveness. Brain Sciences. 2025; 15(10):1082. https://doi.org/10.3390/brainsci15101082
Chicago/Turabian StyleLautenbacher, Stefan, Miriam Kunz, and Karl-Jürgen Bär. 2025. "Anorexia Nervosa Dampens Subjective and Facial Pain Responsiveness" Brain Sciences 15, no. 10: 1082. https://doi.org/10.3390/brainsci15101082
APA StyleLautenbacher, S., Kunz, M., & Bär, K.-J. (2025). Anorexia Nervosa Dampens Subjective and Facial Pain Responsiveness. Brain Sciences, 15(10), 1082. https://doi.org/10.3390/brainsci15101082






