Role of Glyoxalase in Astrocytes’ Supportive Function Under Hyperglycemic Conditions: Aminoguanidine and Kir4.1 Channel Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cell Culture
2.2. Aminoguanidine Treatment
2.3. SDS-PAGE and Western Blot Analysis
2.4. Electrophysiology
2.5. Statistical Analysis
3. Results
3.1. Protein Expression of Glyoxalase 1 and 2 Is Downregulated in High-Glucose Conditions When Compared to Control
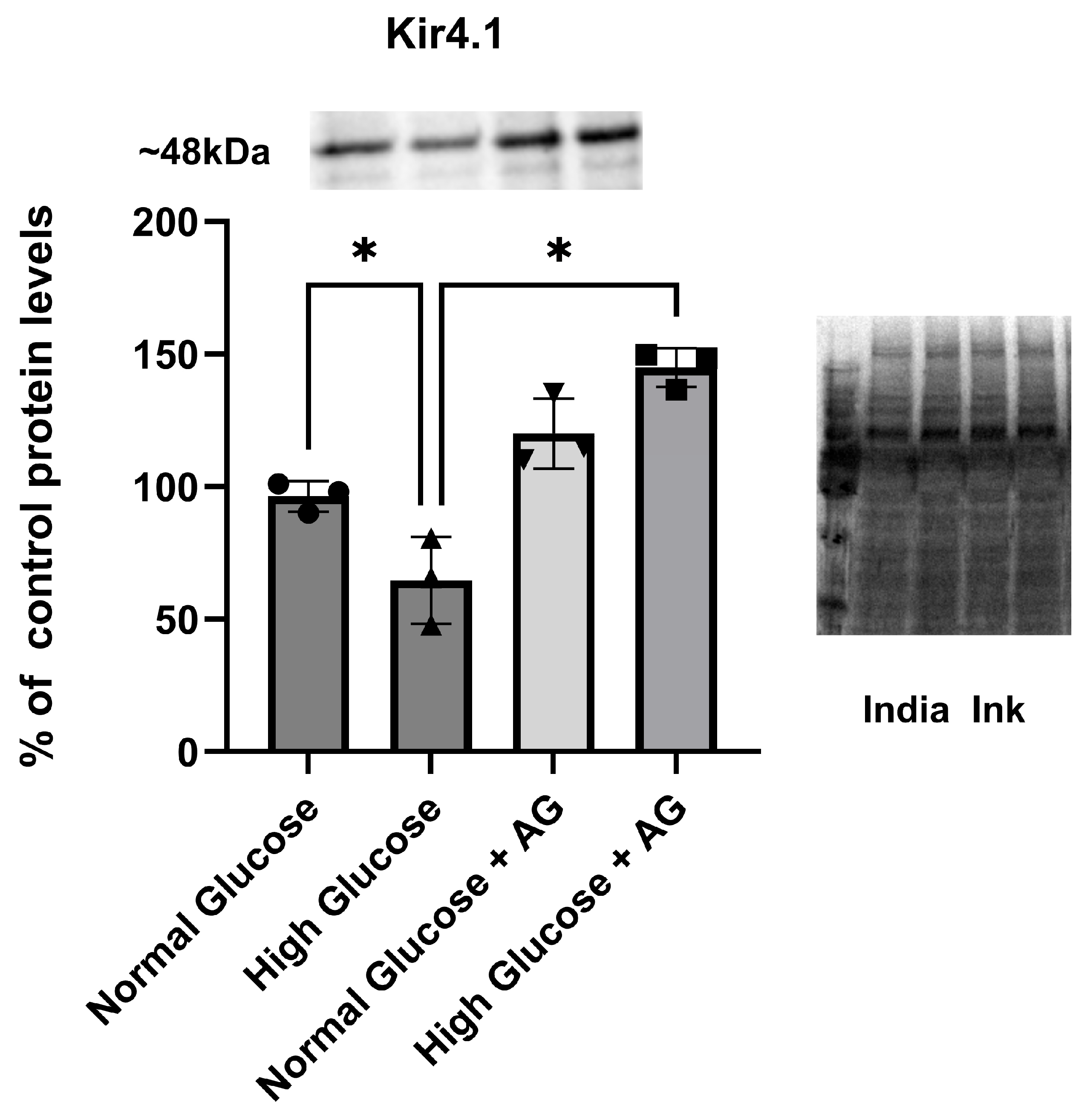
3.2. Kir4.1 Protein Expression Is Downregulated in High-Glucose Conditions When Compared to Control: Role of AG in Recovering Kir4.1 Expression
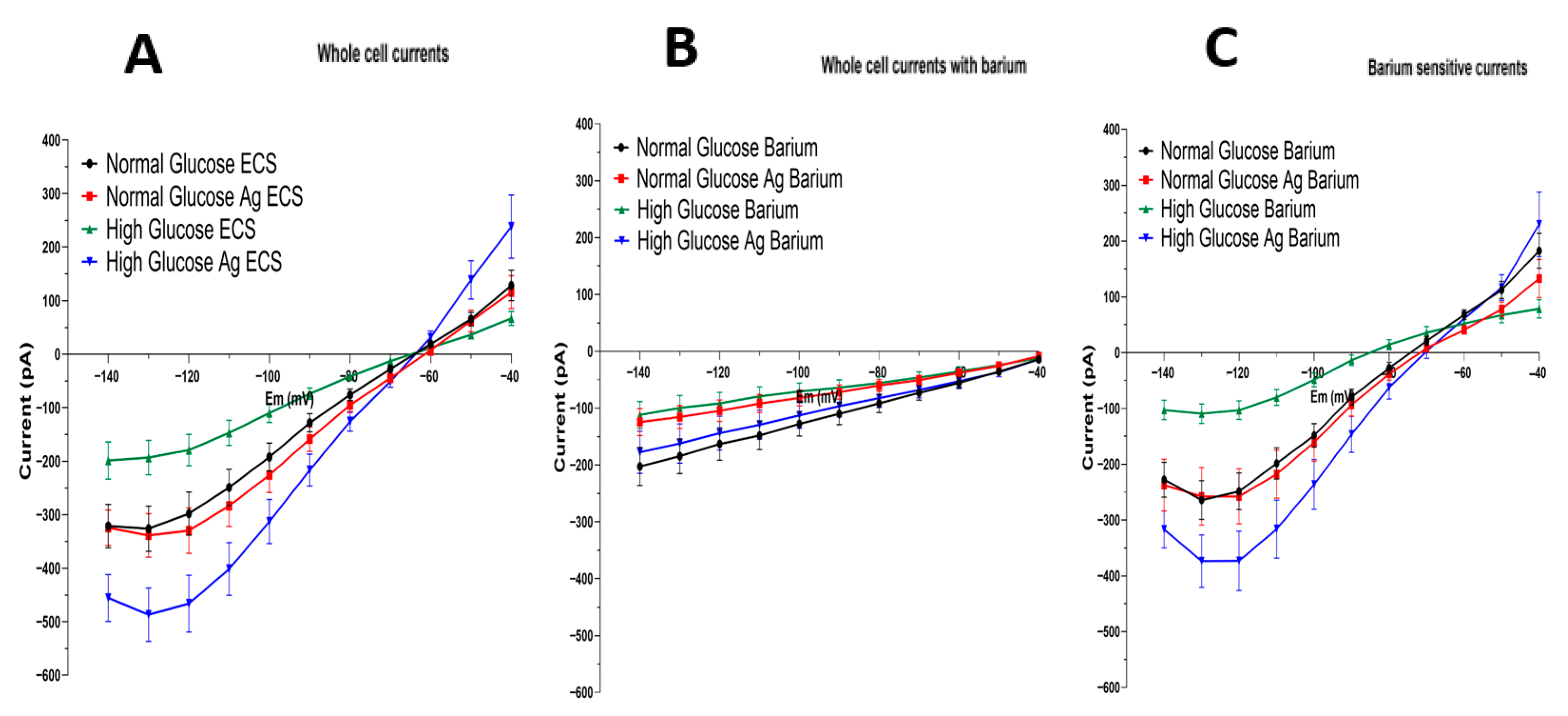
3.3. Barium-Sensitive Kir4.1 Current Is Increased in Astrocytes Grown in High-Glucose Conditions and Treated with Aminoguanidine
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AG | Aminoguanidine |
| PAs | Polyamines |
| AGE | Advance glycation end product |
| CNS | Central nervous system |
| Em, Eh | Membrane and holding potentials, respectively |
| GABA | Gamma amino butiric acid (a gliotransmitter and neurotransmitter) |
| GLAST, GLT1 | Glutamate transporters |
| Glo 1, 2 | Glyoxalases 1 and 2 |
| GSH | Glutathione |
| Kcnj10 | Gene-encoding subunit protein of Kir4.1 channels |
| Kir4.1 | Potassium inward-rectifying channels expressed in glia |
| MG | Methylglyoxal |
| TASK-1 | Two-pore domain acid-sensitive potassium channel, gene KCNK3 |
| TREK-1 | Two-pore domain acid-sensitive potassium channel, gene KCNK2 |
| TREK-2 | Two-pore domain acid-sensitive potassium channel, gene KCNK10 |
| Cx 43 GJs | Connexin-43 gap junctions (pores between astrocytes in the glial syncytium) |
References
- Tomlinson, D.R.; Gardiner, N.J. Glucose neurotoxicity. Nat. Rev. Neurosci. 2008, 9, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Desilles, J.-P.; Meseguer, E.; Labreuche, J.; Lapergue, B.; Sirimarco, G.; Gonzalez-Valcarcel, J.; Lavallée, P.; Cabrejo, L.; Guidoux, C.; Klein, I.; et al. Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: A registry and systematic review. Stroke. 2013, 44, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Lin, C.C.; Hsiao, L.D.; Yang, C.M. High glucose induces reactive oxygen species-dependent matrix metalloproteinase-9 expression and cell migration in brain astrocytes. Mol. Neurobiol. 2013, 48, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Huang, Q.; Gurel, Z.; Sorenson, C.M.; Sheibani, N.; Nagaraj, R. High glucose alters retinal astrocytes phenotype through increased production of inflammatory cytokines and oxidative stress. PLoS ONE 2014, 9, e103148. [Google Scholar] [CrossRef]
- Lent, R.; Azevedo, F.A.C.; Andrade-Moraes, C.H.; Pinto, A.V.O. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur. J. Neurosci. 2012, 35, 1–9. [Google Scholar] [CrossRef]
- Jing, L.; He, Q.; Zhang, J.Z.; Li, P.A. Temporal profile of astrocytes and changes of oligodendrocyte-based myelin following middle cerebral artery occlusion in diabetic and non-diabetic rats. Int. J. Biol. Sci. 2013, 9, 190–199. [Google Scholar] [CrossRef]
- Olsen, M.L.; Khakh, B.S.; Skatchkov, S.N.; Zhou, M.; Lee, C.J.; Rouach, N. New Insights on Astrocyte Ion Channels: Critical for Homeostasis and Neuron-Glia Signaling. J. Neurosci. 2015, 35, 13827–13835. [Google Scholar] [CrossRef]
- Kovács, Z.; Skatchkov, S.N.; Veh, R.W.; Szabó, Z.; Németh, K.; Szabó, P.T.; Kardos, J.; Héja, L. Critical Role of Astrocytic Polyamine and GABA Metabolism in Epileptogenesis. Front. Cell Neurosci. 2022, 15, 787319. [Google Scholar] [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Dityatev, A.; Rusakov, D.A. Molecular signals of plasticity at the tetrapartite synapse. Curr. Opin. Neurobiol. 2011, 21, 353–359. [Google Scholar] [CrossRef]
- Kucheryavykh, Y.V.; Kucheryavykh, L.Y.; Nichols, C.G.; Maldonado, H.M.; Baksi, K.; Reichenbach, A.; Skatchkov, S.N.; Eaton, M.J. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia 2007, 55, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Inyushin, M.; Kucheryavykh, L.Y.; Kucheryavykh, Y.V.; Nichols, C.G.; Buono, R.J.; Ferraro, T.N.; Skatchkov, S.N.; Eaton, M.J. Potassium channel activity and glutamate uptake are impaired in astrocytes of seizure-susceptible DBA/2 mice. Epilepsia 2010, 51, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M. Examining potassium channel function in astrocytes. Methods Mol. Biol. 2012, 814, 265–281. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Woodbury-Fariña, M.A.; Eaton, M. The role of glia in stress: Polyamines and brain disorders. Psychiatr. Clin. N. Am. 2014, 37, 653–678. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Bukauskas, F.F.; Benedikt, J.; Inyushin, M.; Kucheryavykh, Y.V. Intracellular spermine prevents acid-induced uncoupling of Cx43 gap junction channels. Neuroreport 2015, 26, 528–532. [Google Scholar] [CrossRef]
- Steinhäuser, C.; Seifert, G. Glial membrane channels and receptors in epilepsy: Impact for generation and spread of seizure activity. Eur. J. Pharmacol. 2002, 447, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.; Hüttmann, K.; Binder, D.K.; Hartmann, C.; Wyczynski, A.; Neusch, C.; Steinhäuser, C. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 7474–7488. [Google Scholar] [CrossRef]
- Poopalasundaram, S.; Knott, C.; Shamotienko, O.G.; Foran, P.G.; Dolly, J.O.; Ghiani, C.A.; Gallo, V.; Wilkin, G.P. Glial heterogeneity in expression of the inwardly rectifying K(+) channel, Kir4.1, in adult rat CNS. Glia 2000, 30, 362–372. [Google Scholar] [CrossRef]
- Sala-Rabanal, M.; Kucheryavykh, L.Y.; Skatchkov, S.N.; Eaton, M.J.; Nichols, C.G. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10). J. Biol. Chem. 2010, 285, 36040–36048. [Google Scholar] [CrossRef]
- Méndez-González, M.P.; Kucheryavykh, Y.V.; Zayas-Santiago, A.; Vélez-Carrasco, W.; Maldonado-Martínez, G.; Cubano, L.A.; Nichols, C.G.; Skatchkov, S.N.; Eaton, M.J. Novel KCNJ10 Gene Variations Compromise Function of Inwardly Rectifying Potassium Channel 4.1. J. Biol. Chem. 2016, 291, 7716–7726. [Google Scholar] [CrossRef]
- Bockenhauer, D.; Feather, S.; Stanescu, H.C.; Bandulik, S.; Zdebik, A.A.; Reichold, M.; Tobin, J.; Lieberer, E.; Sterner, C.; Landoure, G.; et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N. Engl. J. Med. 2009, 360, 1960–1970. [Google Scholar] [CrossRef]
- Scholl, U.I.; Choi, M.; Liu, T.; Ramaekers, V.T.; Häusler, M.G.; Grimmer, J.; Tobe, S.W.; Farhi, A.; Nelson-Williams, C.; Lifton, R.P. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc. Natl. Acad. Sci. USA 2009, 106, 5842–5847. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.G.; Lee, S.J. Polyamines and potassium channels: A 25-year romance. J. Biol. Chem. 2018, 293, 18779–18788. [Google Scholar] [CrossRef]
- Neusch, C.; Papadopoulos, N.; Müller, M.; Maletzki, I.; Winter, S.M.; Hirrlinger, J.; Handschuh, M.; Bähr, M.; Richter, D.W.; Kirchhoff, F.; et al. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: Impact on extracellular K+ regulation. J. Neurophysiol. 2006, 95, 1843–1852. [Google Scholar] [CrossRef]
- Djukic, B.; Casper, K.B.; Philpot, B.D.; Chin, L.S.; McCarthy, K.D. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 11354–11365. [Google Scholar] [CrossRef]
- Méndez-González, M.P.; Rivera-Aponte, D.E.; Benedikt, J.; Maldonado-Martínez, G.; Tejeda-Bayron, F.; Skatchkov, S.N.; Eaton, M.J. Downregulation of Astrocytic Kir4.1 Potassium Channels Is Associated with Hippocampal Neuronal Hyperexcitability in Type 2 Diabetic Mice. Brain Sci. 2020, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Chever, O.; Djukic, B.; McCarthy, K.D.; Amzica, F. Implication of Kir4.1 channel in excess potassium clearance: An in vivo study on anesthetized glial-conditional Kir4.1 knock-out mice. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 15769–15777. [Google Scholar] [CrossRef]
- Ransom, C.B.; Sontheimer, H. Biophysical and pharmacological characterization of inwardly rectifying K+ currents in rat spinal cord astrocytes. J. Neurophysiol. 1995, 73, 333–346. [Google Scholar] [CrossRef]
- Olsen, M.L.; Campbell, S.L.; Sontheimer, H. Differential distribution of Kir4.1 in spinal cord astrocytes suggests regional differences in K+ homeostasis. J. Neurophysiol. 2007, 98, 786–793. [Google Scholar] [CrossRef]
- Kofuji, P.; Ceelen, P.; Zahs, K.R.; Surbeck, L.W.; Lester, H.A.; Newman, E.A. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: Phenotypic impact in retina. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 5733–5740. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Newman, E.A. Potassium buffering in the central nervous system. Neuroscience 2004, 129, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pinzón, M.A.; Tao, L.; Nicholson, C. Extracellular potassium, volume fraction, and tortuosity in rat hippocampal CA1, CA3, and cortical slices during ischemia. J. Neurophysiol. 1995, 74, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Skatchkov, S.N.; Eaton, M.J.; Shuba, Y.M.; Kucheryavykh, Y.V.; Derst, C.; Veh, R.W.; Wurm, A.; Iandiev, I.; Pannicke, T.; Bringmann, A.; et al. Tandem-pore domain potassium channels are functionally expressed in retinal (Müller) glial cells. Glia 2006, 53, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Benedikt, J.; Malpica-Nieves, C.J.; Rivera, Y.; Méndez-González, M.; Nichols, C.G.; Veh, R.W.; Eaton, M.J.; Skatchkov, S.N. The Polyamine Spermine Potentiates the Propagation of Negatively Charged Molecules through the Astrocytic Syncytium. Biomolecules 2022, 12, 1812. [Google Scholar] [CrossRef]
- Zhong, S.; Kiyoshi, C.M.; Du, Y.; Wang, W.; Luo, Y.; Wu, X.; Taylor, A.T.; Ma, B.; Aten, S.; Liu, X.; et al. Genesis of a functional astrocyte syncytium in the developing mouse hippocampus. Glia 2023, 71, 1081–1098. [Google Scholar] [CrossRef]
- Barbour, B.; Brew, H.; Attwell, D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature 1988, 335, 433–435. [Google Scholar] [CrossRef]
- Billups, B.; Rossi, D.; Oshima, T.; Warr, O.; Takahashi, M.; Sarantis, M.; Szatkowski, M.; Attwell, D. Physiological and pathological operation of glutamate transporters. Prog. Brain Res. 1998, 116, 45–57. [Google Scholar] [CrossRef]
- Hodebourg, R.; Scofield, M.D.; Kalivas, P.W.; Kuhn, B.N. Nonneuronal contributions to synaptic function. Neuron 2025, 113, 2399–2415. [Google Scholar] [CrossRef]
- Rivera-Aponte, D.; Méndez-González, M.; Rivera-Pagán, A.; Kucheryavykh, Y.; Kucheryavykh, L.; Skatchkov, S.; Eaton, M. Hyperglycemia Reduces Functional Expression of Astrocytic Kir4.1 Channels and Glial Glutamate Uptake. Neuroscience 2015, 310, 216–223. [Google Scholar] [CrossRef]
- Rivera-Aponte, D.E.; Melnik-Martínez, K.V.; Malpica-Nieves, C.J.; Tejeda-Bayron, F.; Méndez-González, M.P.; Skatchkov, S.N.; Eaton, M.J. Kir4.1 potassium channel regulation via miR-205 in astrocytes exposed to hyperglycemic conditions. Neuroreport 2020, 31, 450–455. [Google Scholar] [CrossRef]
- Tejeda-Bayron, F.A.; Rivera-Aponte, D.E.; Malpica-Nieves, C.J.; Maldonado-Martínez, G.; Maldonado, H.M.; Skatchkov, S.N.; Eaton, M.J. Activation of Glutamate Transporter-1 (GLT-1) Confers Sex-Dependent Neuroprotection in Brain Ischemia. Brain Sci. 2021, 11, 76. [Google Scholar] [CrossRef]
- Engel, R.; Halberg, F.; Ziegler, M.; McQuarrie, I. Observations on two children with diabetes mellitus and epilepsy. J. Lancet 1952, 72, 242–248. [Google Scholar]
- Yun, C.; Xuefeng, W. Association between seizures and diabetes mellitus: A comprehensive review of literature. Curr. Diabetes Rev. 2013, 9, 350–354. [Google Scholar] [CrossRef]
- Anderson, M.F.; Blomstrand, F.; Blomstrand, C.; Eriksson, P.S.; Nilsson, M. Astrocytes and stroke: Networking for survival? Neurochem. Res. 2003, 28, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Swanson, R.A. Astrocytes and brain injury. J. Cereb. Blood Flow. Metab. Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef]
- Di Loreto, S.; Zimmitti, V.; Sebastiani, P.; Cervelli, C.; Falone, S.; Amicarelli, F. Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int. J. Biochem. Cell Biol. 2008, 40, 245–257. [Google Scholar] [CrossRef]
- Thornalley, P.J. The glyoxalase system in health and disease. Mol. Asp. Med. 1993, 14, 287–371. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.; de Souza, D.F.; Silveira, S.d.L.; Hoefel, A.L.; Fontoura, J.B.; Tramontina, A.C.; Bobermin, L.D.; Leite, M.C.; Perry, M.L.S.; Gonçalves, C.A. Methylglyoxal alters glucose metabolism and increases AGEs content in C6 glioma cells. Metab. Brain Dis. 2012, 27, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Gilad, G.M.; Gilad, V.H. Polyamines affect growth of cultured rat cerebellar neurons in different sera. Int. J. Dev. Neurosci. 1986, 4, 195–208. [Google Scholar] [CrossRef]
- Hansen, F.; Galland, F.; Lirio, F.; de Souza, D.F.; Da Ré, C.; Pacheco, R.F.; Vizuete, A.F.; Quincozes-Santos, A.; Leite, M.C.; Gonçalves, C.-A.; et al. Methylglyoxal Induces Changes in the Glyoxalase System and Impairs Glutamate Uptake Activity in Primary Astrocytes. Oxid. Med. Cell Longev. 2017, 2017, 9574201. [Google Scholar] [CrossRef]
- Gilad, V.H.; Tetzlaff, W.G.; Rabey, J.M.; Gilad, G.M. Accelerated Recovery Following Polyamines and Aminoguanidine Treatment after Facial Nerve Injury in Rats. Brain Res. 1996, 724, 141–144. [Google Scholar] [CrossRef]
- Noro, T.; Namekata, K.; Kimura, A.; Guo, X.; Azuchi, Y.; Harada, C.; Nakano, T.; Tsuneoka, H.; Harada, T. Spermidine promotes retinal ganglion cell survival and optic nerve regeneration in adult mice following optic nerve injury. Cell Death Dis. 2015, 6, e1720. [Google Scholar] [CrossRef] [PubMed]
- Cockroft, K.M.; Meistrell M3rd Zimmerman, G.A.; Risucci, D.; Bloom, O.; Cerami, A.; Tracey, K.J. Cerebroprotective effects of aminoguanidine in a rodent model of stroke. Stroke 1996, 27, 1393–1398. [Google Scholar] [CrossRef]
- Schimchowitsch, S.; Cassel, J.C. Polyamine and aminoguanidine treatments to promote structural and functional recovery in the adult mammalian brain after injury: A brief literature review and preliminary data about their combined administration. J. Physiol. Paris. 2006, 99, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Malpica-Nieves, C.J.; Rivera-Aponte, D.E.; Tejeda-Bayron, F.A.; Mayor, A.M.; Phanstiel, O.; Veh, R.W.; Eaton, M.J.; Skatchkov, S.N. The involvement of polyamine uptake and synthesis pathways in the proliferation of neonatal astrocytes. Amino Acids. 2020, 52, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Herberth, H.; Cosquer, B.; Kelche, C.; Cassel, J.C.; Schimchowitsch, S. Structural and functional recovery elicited by combined putrescine and aminoguanidine treatment after aspirative lesion of the fimbria-fornix and overlying cortex in the adult rat. Eur. J. Neurosci. 2007, 25, 1949–1960. [Google Scholar] [CrossRef]
- Kauppila, T. Polyamines enhance recovery after sciatic nerve trauma in the rat. Brain Res. 1992, 575, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in Aging and Disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef]
- Estrada-Cuzcano, A.; Martin, S.; Chamova, T.; Synofzik, M.; Timmann, D.; Holemans, T.; Andreeva, A.; Reichbauer, J.; De Rycke, R.; Chang, D.-I.; et al. Loss-of-Function Mutations in the ATP13A2/PARK9 Gene Cause Complicated Hereditary Spastic Paraplegia (SPG78). Brain 2017, 140, 287–305. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in Health and Disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef]
- Hofer, S.J.; Simon, A.K.; Bergmann, M.; Eisenberg, T.; Kroemer, G.; Madeo, F. Mechanisms of Spermidine-Induced Autophagy and Geroprotection. Nat. Aging 2022, 2, 1112–1129. [Google Scholar] [CrossRef]
- Tao, X.; Liu, J.; Diaz-Perez, Z.; Foley, J.R.; Nwafor, A.; Stewart, T.M.; Casero, R.A.; Zhai, R.G. Reduction of Spermine Synthase Enhances Autophagy to Suppress Tau Accumulation. Cell Death Dis. 2024, 15, 333. [Google Scholar] [CrossRef]
- Sigrist, S.J.; Carmona-Gutierrez, D.; Gupta, V.K.; Bhukel, A.; Mertel, S.; Eisenberg, T.; Madeo, F. Spermidine-Triggered Autophagy Ameliorates Memory during Aging. Autophagy 2014, 10, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Bhukel, A.; Madeo, F.; Sigrist, S.J. Spermidine Boosts Autophagy to Protect from Synapse Aging. Autophagy 2017, 13, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Buttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of Autophagy by Spermidine Promotes Longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and Lifespan Extension by the Natural Polyamine Spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.J.; Daskalaki, I.; Bergmann, M.; Friščić, J.; Zimmermann, A.; Mueller, M.I.; Abdellatif, M.; Nicastro, R.; Masser, S.; Durand, S.; et al. Spermidine Is Essential for Fasting-Mediated Autophagy and Longevity. Nat. Cell Biol. 2024, 26, 1571–1584. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Wong, T.Y.; Gardner, T.W.; Sun, J.K.; Bressler, N.M. Diabetic retinal disease. Nat. Rev. Dis. Primers. 2025, 11, 62. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colón-Vázquez, J.; Rosado-Rivera, N.M.; Navedo-Jackson, J.J.; Angueira-Laureano, A.A.; Hernandez-Santiago, Y.; Maldonado-Martinez, G.; Méndez-González, M.P.; Eaton, M.J.; Skatchkov, S.N.; Rivera-Aponte, D.E. Role of Glyoxalase in Astrocytes’ Supportive Function Under Hyperglycemic Conditions: Aminoguanidine and Kir4.1 Channel Recovery. Brain Sci. 2025, 15, 1075. https://doi.org/10.3390/brainsci15101075
Colón-Vázquez J, Rosado-Rivera NM, Navedo-Jackson JJ, Angueira-Laureano AA, Hernandez-Santiago Y, Maldonado-Martinez G, Méndez-González MP, Eaton MJ, Skatchkov SN, Rivera-Aponte DE. Role of Glyoxalase in Astrocytes’ Supportive Function Under Hyperglycemic Conditions: Aminoguanidine and Kir4.1 Channel Recovery. Brain Sciences. 2025; 15(10):1075. https://doi.org/10.3390/brainsci15101075
Chicago/Turabian StyleColón-Vázquez, Jadier, Nathaly M. Rosado-Rivera, Joshua J. Navedo-Jackson, Arelys A. Angueira-Laureano, Yanitza Hernandez-Santiago, Geronimo Maldonado-Martinez, Miguel P. Méndez-González, Misty J. Eaton, Serguei N. Skatchkov, and David E. Rivera-Aponte. 2025. "Role of Glyoxalase in Astrocytes’ Supportive Function Under Hyperglycemic Conditions: Aminoguanidine and Kir4.1 Channel Recovery" Brain Sciences 15, no. 10: 1075. https://doi.org/10.3390/brainsci15101075
APA StyleColón-Vázquez, J., Rosado-Rivera, N. M., Navedo-Jackson, J. J., Angueira-Laureano, A. A., Hernandez-Santiago, Y., Maldonado-Martinez, G., Méndez-González, M. P., Eaton, M. J., Skatchkov, S. N., & Rivera-Aponte, D. E. (2025). Role of Glyoxalase in Astrocytes’ Supportive Function Under Hyperglycemic Conditions: Aminoguanidine and Kir4.1 Channel Recovery. Brain Sciences, 15(10), 1075. https://doi.org/10.3390/brainsci15101075








