Acai Berry Extracts Can Mitigate the L-Glutamate-Induced Neurotoxicity Mediated by N-Methyl-D-Aspartate Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Human Rhabdomyosarcoma TE671 Cell Culture and Differentiation
2.3. Preparation of L-Glutamic Acid (L-Glu), Acai Berry Aqueous and Ethanolic Extracts and Cell Treatments
2.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.5. Lactate Dehydrogenase (LDH) Assay
2.6. Adenosine 5′-Triphosphate (ATP) Assay
2.7. Measurements of Mitochondrial Membrane Potential (MMP)
2.8. 2,7-Dichlorodihydrofluorescein Diacetate Assay
2.9. Whole-Cell Patch-Clamp Assay
2.10. In Silico Studies
2.10.1. Molecular Docking of Acai Berry Extract Compounds to NMDA Receptors
2.10.2. Ligand Selection and Preparation
2.10.3. Molecular Docking
2.10.4. Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) Simulations
2.10.5. Molecular Dynamics (MD) Simulations
2.11. Statistical Analysis
3. Results
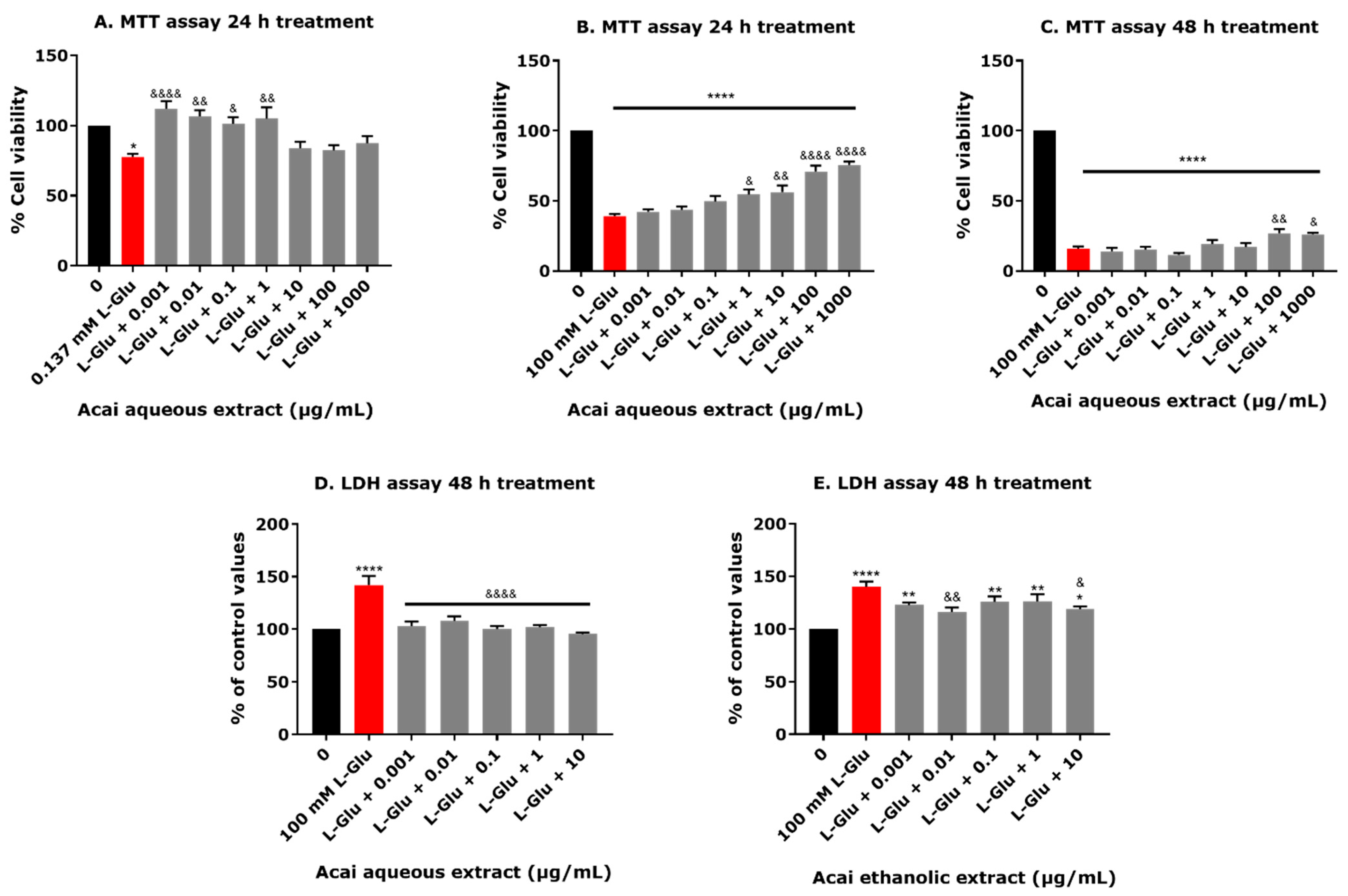
3.1. L-Glu and High Acai Berry Concentrations Reduce the Viability of dTE671 Cells
3.2. Acai Berry Extracts Protect dTE671 Cells from the L-Glu-Induced Loss of Cell Viability
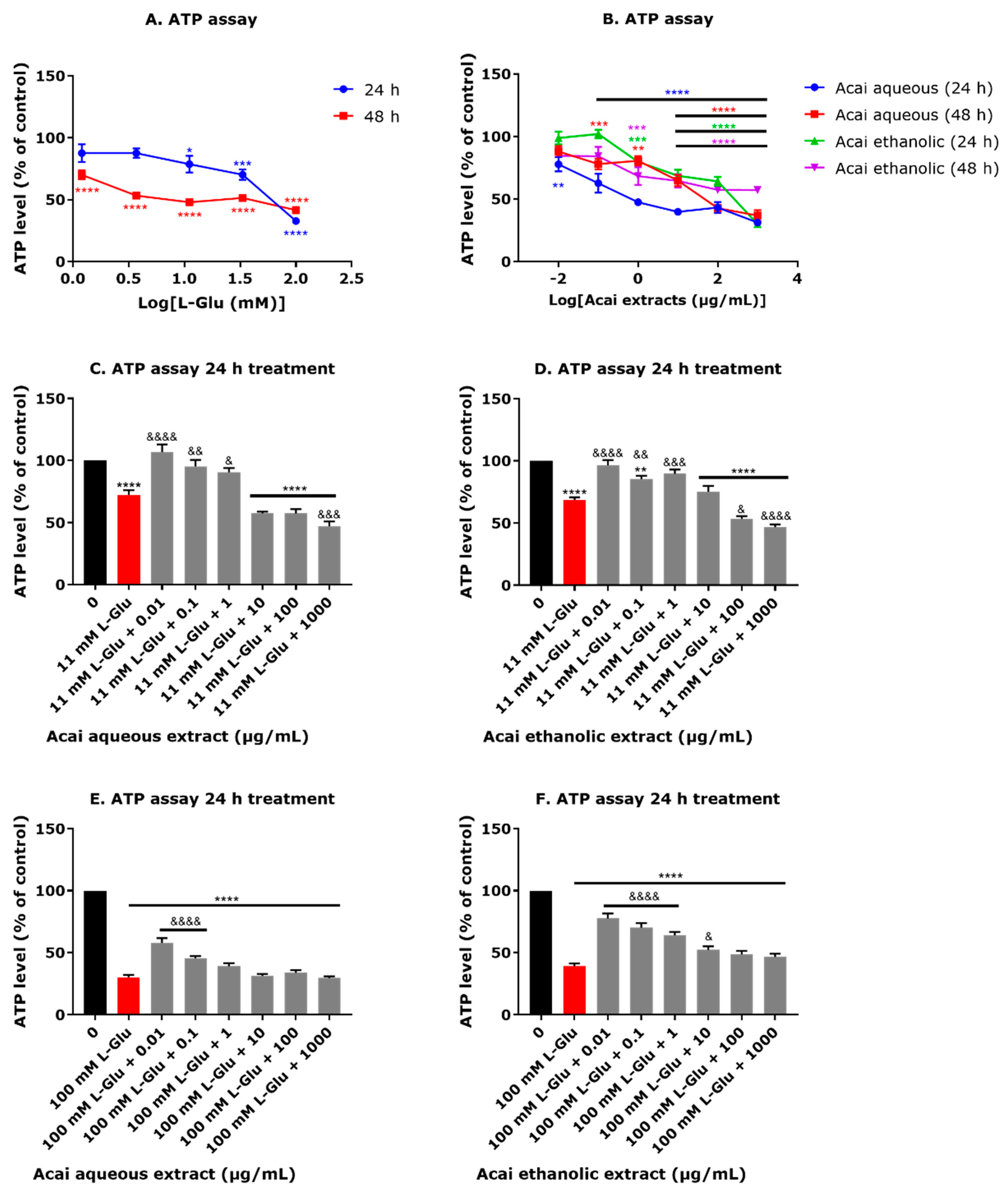
3.3. Acai Berry Extracts Countered the L-Glu-Induced Reduction in ATP Levels in dTE671 Cells
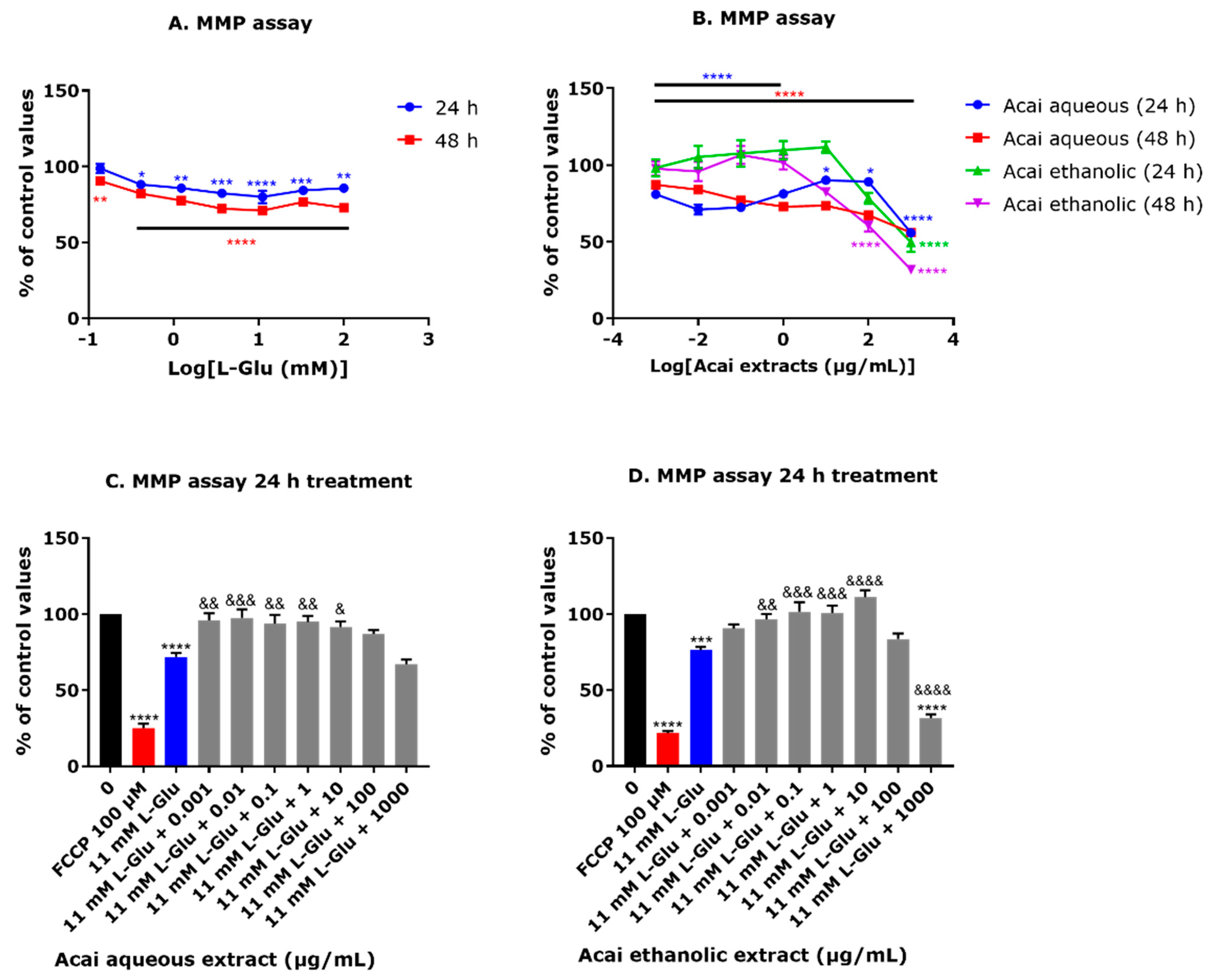
3.4. Acai Berry Extracts Countered the L-Glu-Induced Reduction in the MMP in dTE671 Cells
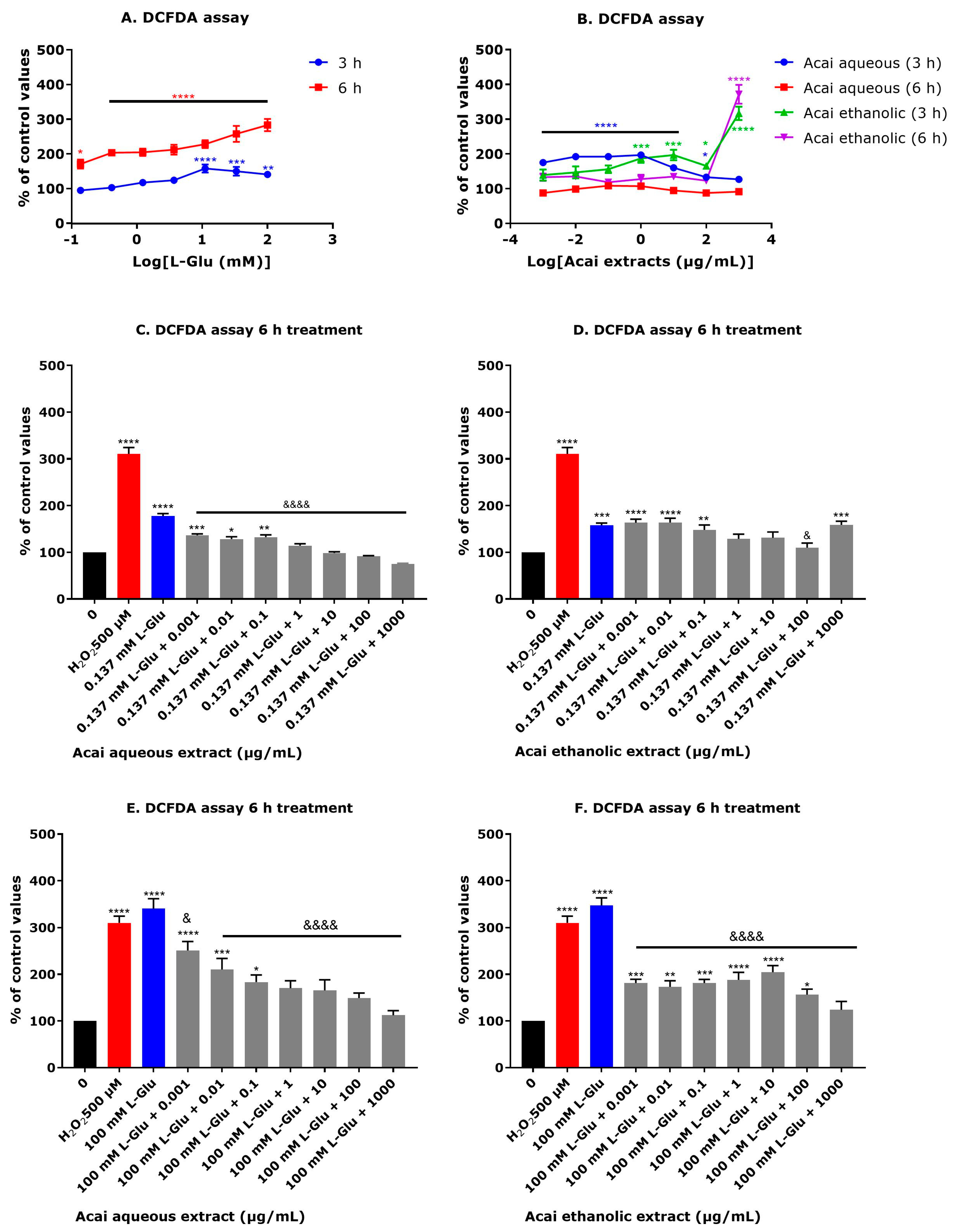
3.5. Acai Berry Extracts Countered the L-Glu-Induced Production of Reactive Oxygen Species in dTE671 Cells
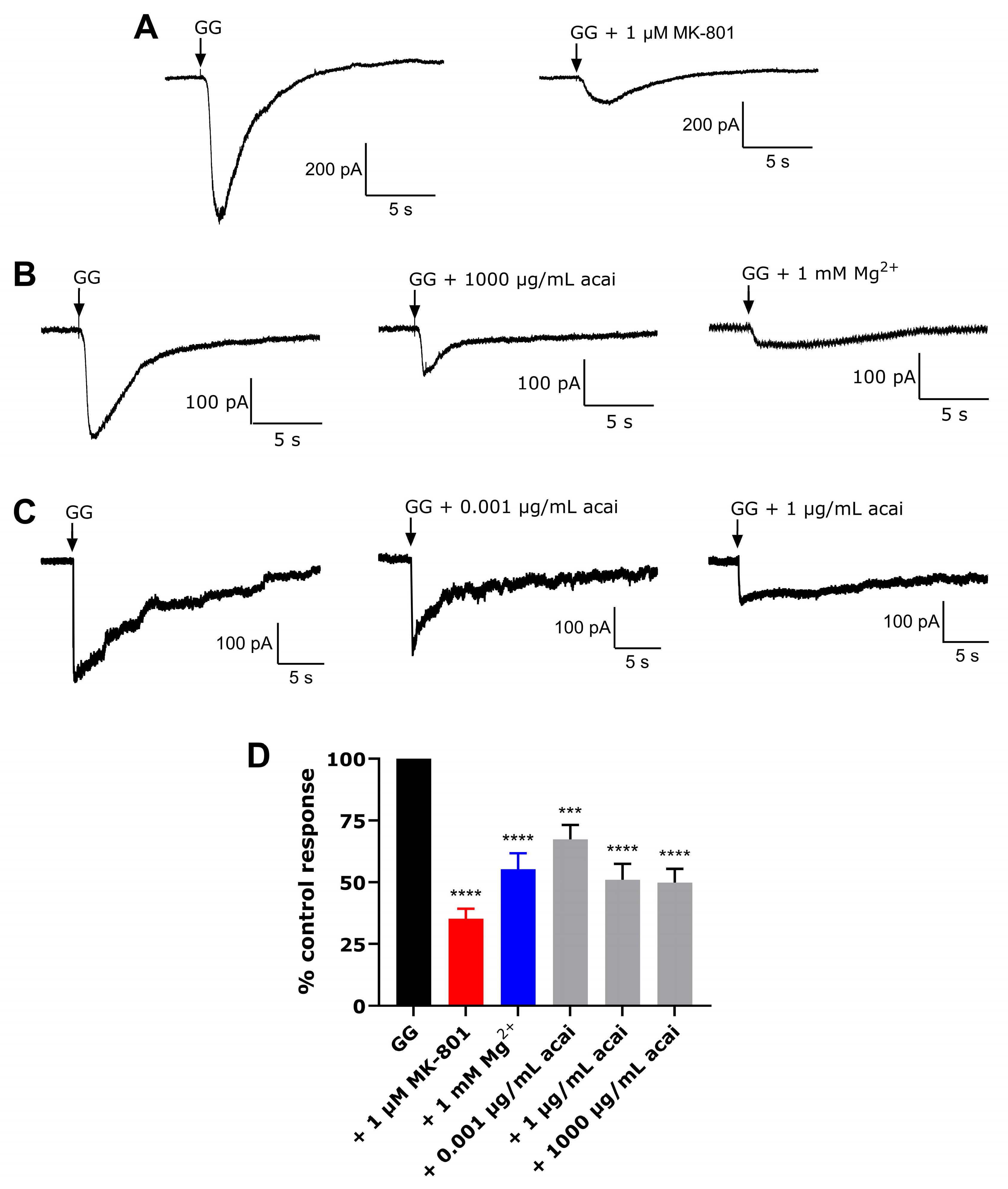
3.6. Acai Berry Extracts Inhibited L-Glu and Gly-Activated Currents in dTE671 Cells
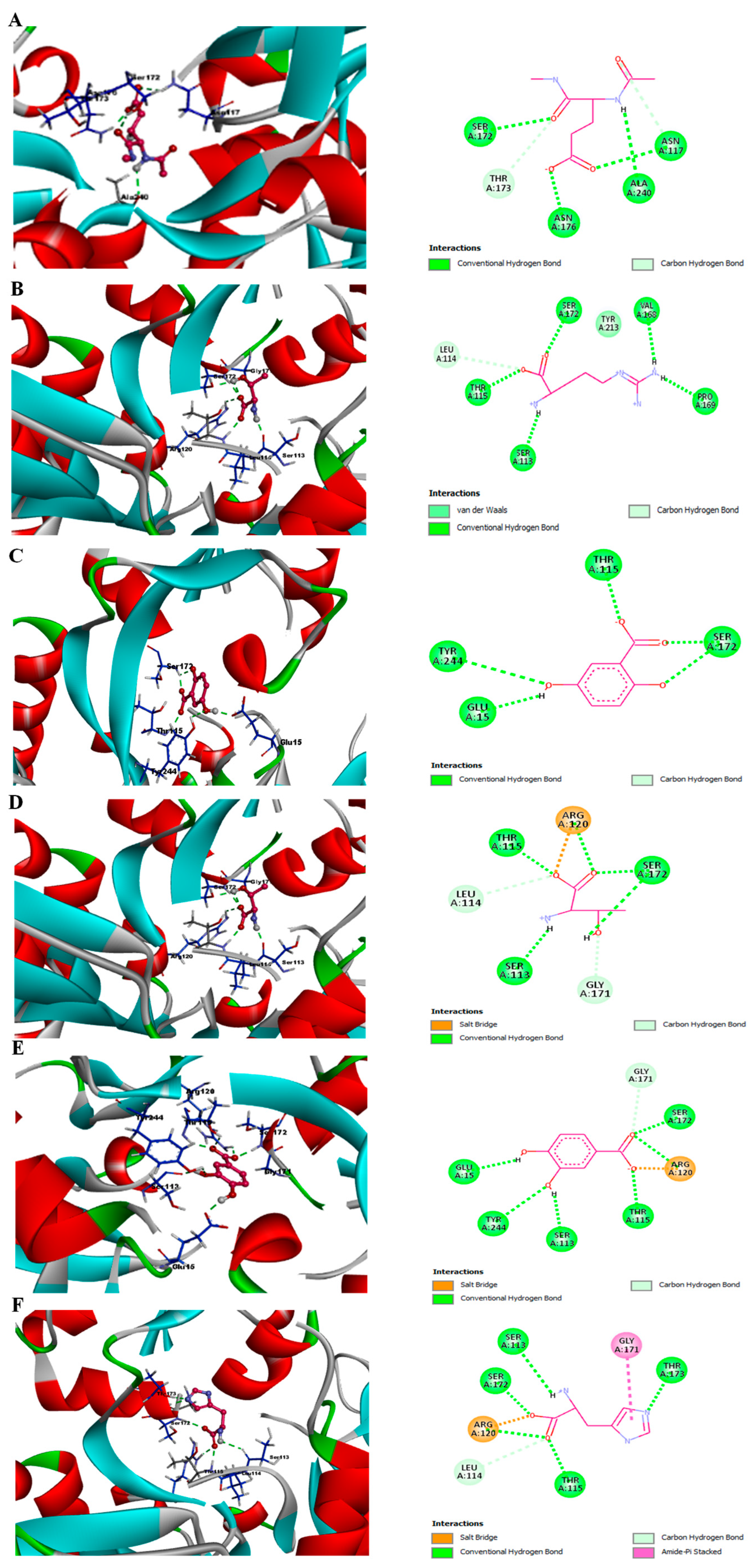
3.7. Phytochemicals from Acai Berry Extracts Display High Affinity Binding to the NMDAR by Molecular Docking
3.8. MM/GBSA Studies
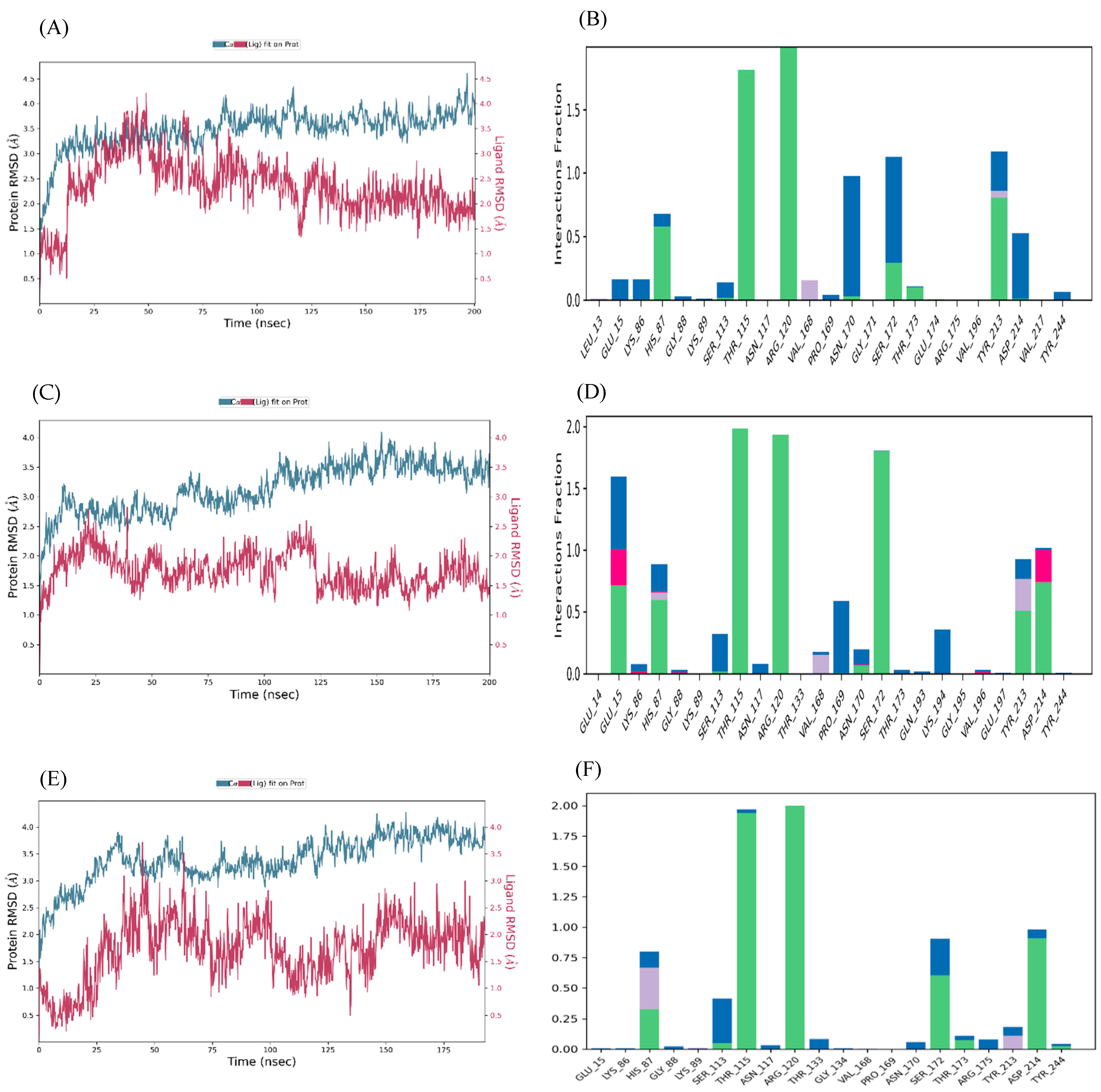
Molecular Dynamics Simulation
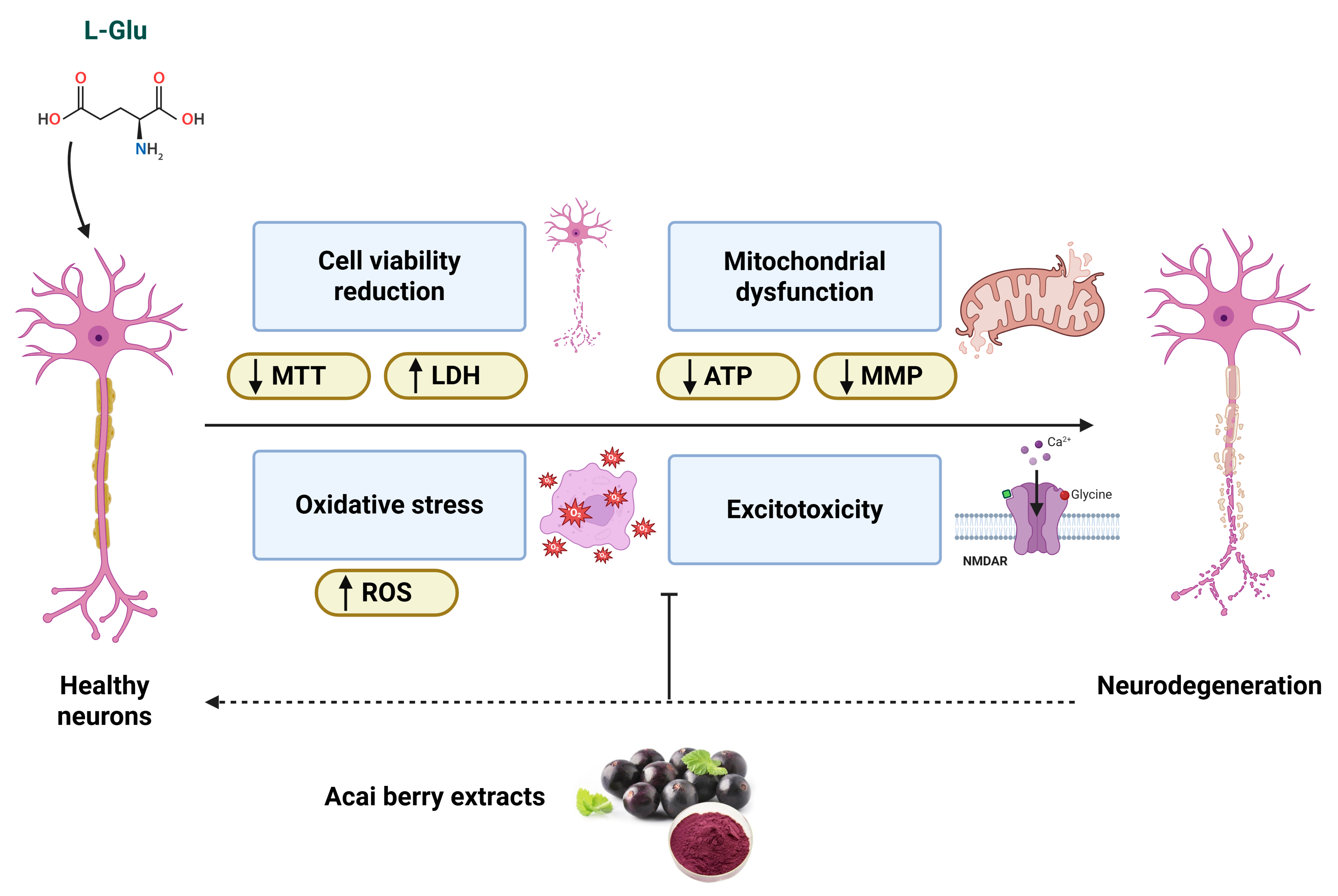
4. Discussion
4.1. Viability of Cells in Response to L-Glu and Acai Berry Extract
4.2. Impact of L-Glu and Acai Berry Extracts on Mitochondrial Function
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuriakose, D.; Xiao, Z. Pathophysiology and treatment of stroke: Present status and future perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- World-Health-Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 8 September 2025).
- King, D.; Wittenberg, R.; Patel, A.; Quayyum, Z.; Berdunov, V.; Knapp, M. The future incidence, prevalence and costs of stroke in the UK. Age Ageing 2020, 49, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Berdunov, V.; Quayyum, Z.; King, D.; Knapp, M.; Wittenberg, R. Estimated societal costs of stroke in the UK based on a discrete event simulation. Age Ageing 2020, 49, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Magid-Bernstein, J.; Girard, R.; Polster, S.; Srinath, A.; Romanos, S.; Awad, I.; Sansing, L.H. Cerebral hemorrhage: Pathophysiology, treatment, and future directions. Circ. Res. 2022, 130, 1204–1229. [Google Scholar] [CrossRef]
- Sacco, R.; Kasner, S.; Broderick, J.; Caplan, L.; Connors, J.; Culebras, A.; Elkind, M.; George, M.; Hamdan, A.; Higashida, R.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef]
- Martínez-Coria, H.; Arrieta-Cruz, I.; Cruz, M.-E.; López-Valdés, H. Physiopathology of ischemic stroke and its modulation using memantine: Evidence from preclinical stroke. Neural Regen. Res. 2021, 16, 433–439. [Google Scholar] [CrossRef]
- Hurford, R.; Sekhar, A.; Hughes, T.; Muir, K. Diagnosis and management of acute ischaemic stroke. Pract. Neurol. 2020, 20, 304–316. [Google Scholar] [CrossRef]
- Yunyun, X.; Ajay, W.; Marc, F. Advances in acute ischemic stroke therapy. Circ. Res. 2022, 130, 1230–1251. [Google Scholar] [CrossRef]
- Hurd, M.; Goel, I.; Sakai, Y.; Teramura, Y. Current status of ischemic stroke treatment: From thrombolysis to potential regenerative medicine. Regen. Ther. 2021, 18, 408–417. [Google Scholar] [CrossRef]
- Saenger, A.; Christenson, R. Stroke biomarkers: Progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin. Chem. 2010, 56, 21–33. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Shen, Z.; Xiang, M.; Chen, C.; Ding, F.; Wang, Y.; Shang, C.; Xin, L.; Zhang, Y.; Cui, X. Glutamate excitotoxicity: Potential therapeutic target for ischemic stroke. Biomed. Pharmacother. 2022, 151, 113125. [Google Scholar] [CrossRef]
- Choi, D. Excitotoxicity: Still hammering the ischemic brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Zhang, S.; Wang, Y. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Chen, X.; Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 2022, 49, 15. [Google Scholar] [CrossRef] [PubMed]
- Ghozy, S.; Reda, A.; Varney, J.; Elhawary, A.S.; Shah, J.; Murry, K.; Sobeeh, M.G.; Nayak, S.S.; Azzam, A.Y.; Brinjikji, W.; et al. Neuroprotection in Acute Ischemic Stroke: A Battle Against the Biology of Nature. Front. Neurol. 2022, 13, 870141. [Google Scholar] [CrossRef]
- ALNasser, M.; Mellor, I. Neuroprotective activities of acai berries (Euterpe sp.): A review. J. Herbmed Pharmacol. 2022, 11, 166–181. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Percival, S.; Talcott, S. Açai (Euterpe oleracea Mart.) polyphenolics in their glycoside and aglycone forms induce apoptosis of HL-60 leukemia cells. J. Agric. Food Chem. 2006, 54, 1222–1229. [Google Scholar] [CrossRef]
- Kang, J.; Li, Z.; Wu, T.; Jensen, G.; Schauss, A.; Wu, X. Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.). Food Chem. 2010, 122, 610–617. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.; Mertens-Talcott, S.; Talcott, S. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Schauss, A.; Clewell, A.; Balogh, L.; Szakonyi, I.; Financsek, I.; Horváth, J.; Thuroczy, J.; Béres, E.; Vértesi, A.; Hirka, G. Safety evaluation of an açai-fortified fruit and berry functional juice beverage (MonaVie Active(®)). Toxicology 2010, 278, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Spada, P.; Dani, C.; Bortolini, G.; Funchal, C.; Henriques, J.; Salvador, M. Frozen fruit pulp of Euterpe oleraceae Mart. (acai) prevents hydrogen peroxide-induced damage in the cerebral cortex, cerebellum, and hippocampus of rats. J. Med. Food 2009, 12, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, H.; Morris, V.; Stewart, D.; Labhasetwar, V. Advances in stroke therapy. Drug Deliv. Transl. Res. 2011, 1, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, E.; Chen, F.; Xiao, J.; Wang, M. Neuroprotective phytochemicals in experimental ischemic stroke: Mechanisms and potential clinical applications. Oxidative Med. Cell. Longev. 2021, 2021, 6687386. [Google Scholar] [CrossRef]
- ALNasser, M.; AlSaadi, A.; Whitby, A.; Kim, D.-H.; Mellor, I.; Carter, W. Acai berry (Euterpe sp.) extracts are neuroprotective against L-glutamate-induced toxicity by limiting mitochondrial dysfunction and cellular redox stress. Life 2023, 13, 1019. [Google Scholar] [CrossRef]
- Brocke, K.; Staufner, C.; Luksch, H.; Geiger, K.; Stepulak, A.; Marzahn, J.; Schackert, G.; Temme, A.; Ikonomidou, C. Glutamate receptors in pediatric tumors of the central nervous system. Cancer Biol. Ther. 2010, 9, 455–468. [Google Scholar] [CrossRef]
- Luksch, H.; Uckermann, O.; Stepulak, A.; Hendruschk, S.; Marzahn, J.; Bastian, S.; Staufner, C.; Temme, A.; Ikonomidou, C. Silencing of selected glutamate receptor subunits modulates cancer growth. Anticancer Res. 2011, 31, 3181–3192. [Google Scholar]
- Stepulak, A.; Luksch, H.; Gebhardt, C.; Uckermann, O.; Marzahn, J.; Sifringer, M.; Rzeski, W.; Staufner, C.; Brocke, K.; Turski, L.; et al. Expression of glutamate receptor subunits in human cancers. Histochem. Cell Biol. 2009, 132, 435–445. [Google Scholar] [CrossRef]
- Wojciech, R.; Turski, L.; Ikonomidou, C. Glutamate antagonists limit tumor growth. Proc. Natl. Acad. Sci. USA 2001, 98, 6372–6377. [Google Scholar] [CrossRef]
- Siegel, H.; Lukas, R. Morphological and biochemical differentiation of the human medulloblastoma cell line TE671. Dev. Brain Res. 1988, 44, 269–280. [Google Scholar] [CrossRef]
- ALNasser, M.; Mellor, I.; Carter, W. A preliminary assessment of the nutraceutical potential of acai berry (Euterpe sp.) as a potential natural treatment for Alzheimer’s disease. Molecules 2022, 27, 4891. [Google Scholar] [CrossRef] [PubMed]
- Crouch, S.; Kozlowski, R.; Slater, K.; Fletcher, J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 1993, 160, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.; Moravec, R.; Niles, A.; Duellman, S.; Benink, H.; Worzella, T.; Minor, L. Cell viability assays. In Assay Guidance Manual [Internet]; Eli Lilly and Company and the National Center for Advancing Translational Sciences: Bethesda, MA, USA, 2016. [Google Scholar]
- Lee, M.-S.; Park, W.-S.; Kim, Y.; Ahn, W.; Kwon, S.-H.; Her, S. Intracellular ATP assay of live cells using PTD-conjugated luciferase. Sensors 2012, 12, 15628–15637. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xue, X. Detection of total reactive oxygen species in adherent cells by 2′,7′-Dichlorodihydrofluorescein diacetate staining. J. Vis. Exp. 2020, 23, 10–3791. [Google Scholar] [CrossRef]
- Nova, Z.; Skovierova, H.; Strnadel, J.; Halasova, E.; Calkovska, A. Short-term versus long-term culture of A549 cells for evaluating the effects of lipopolysaccharide on oxidative stress, surfactant proteins and cathelicidin LL-37. Int. J. Mol. Sci. 2020, 21, 1148. [Google Scholar] [CrossRef]
- Ashraf, A.; Ahmed, A.; Juffer, A.H.; Carter, W.G. An In Vivo and In Silico Approach Reveals Possible Sodium Channel Nav1.2 Inhibitors from Ficus religiosa as a Novel Treatment for Epilepsy. Brain Sci. 2024, 14, 545. [Google Scholar] [CrossRef]
- Naseem, A.; Rasool, F.; Ahmed, A.; Carter, W.G. The Potential of Stilbene Compounds to Inhibit Mpro Protease as a Natural Treatment Strategy for Coronavirus Disease-2019. Curr. Issues Mol. Biol. 2023, 45, 12–32. [Google Scholar]
- El Sharazly, B.M.; Ahmed, A.; Elsheikha, H.M.; Carter, W.G. An In Silico and In Vitro Assessment of the Neurotoxicity of Mefloquine. Biomedicines 2024, 12, 505. [Google Scholar] [CrossRef]
- Zhang, X.; Perez-Sanchez, H.; Lightstone, F.C. A comprehensive docking and MM/GBSA rescoring study of ligand recognition upon binding antithrombin. Curr. Top. Med. Chem. 2017, 17, 1631–1639. [Google Scholar] [CrossRef]
- Brizi, C.; Santulli, C.; Micucci, M.; Budriesi, R.; Chiarini, A.; Aldinucci, C.; Frosini, M. Neuroprotective effects of Castanea sativa Mill. bark extract in human neuroblastoma cells subjected to oxidative stress. J. Cell Biochem. 2016, 117, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Cui, Y.; An, Z.; Yang, Q.; Zou, X.; Yu, N. Attenuated glutamate induced ROS production by antioxidative compounds in neural cell lines. RSC Adv. 2019, 9, 34735–34743. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Cadoná, F.; de Souza, D.; Fontana, T.; Bodenstein, D.; Ramos, A.; Sagrillo, M.; Salvador, M.; Mota, K.; Davidson, C.; Ribeiro, E.; et al. Açaí (Euterpe oleracea Mart.) as a potential anti-neuroinflammatory agent: NLRP3 priming and activating signal pathway modulation. Mol. Neurobiol. 2021, 58, 4460–4476. [Google Scholar] [CrossRef]
- Machado, A.; Andreazza, A.; da Silva, T.; Boligon, A.; do Nascimento, V.; Scola, G.; Duong, A.; Cadona, F.; Ribeiro, E.; da Cruz, I. Neuroprotective effects of acai (Euterpe oleracea Mart.) against rotenone in vitro exposure. Oxidative Med. Cell Longev. 2016, 2016, 8940850. [Google Scholar] [CrossRef]
- Arrifano, G.; Lichtenstein, M.; Souza-Monteiro, J.; Farina, M.; Rogez, H.; Carvalho, J.; Suñol, C.; Crespo-López, M. Clarified açaí (Euterpe oleracea) juice as an anticonvulsant agent: In vitro mechanistic study of GABAergic targets. Oxidative Med. Cell Longev. 2018, 2018, 2678089. [Google Scholar] [CrossRef]
- De Souza, D.; Pappis, L.; Bandeira, T.; Sangoi, G.; Fontana, T.; Rissi, V.; Sagrillo, M.; Duarte, M.; Duarte, T.; Bodenstein, D.; et al. Açaí (Euterpe oleracea Mart.) presents anti-neuroinflammatory capacity in LPS-activated microglia cells. Nutr. Neurosci. 2020, 25, 1188–1199. [Google Scholar] [CrossRef]
- Poulose, S.; Fisher, D.; Larson, J.; Bielinski, D.; Rimando, A.; Carey, A.; Schauss, A.; Shukitt-Hale, B. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef]
- Torma, P.; Brasil, A.; Carvalho, A.; Jablonski, A.; Rabelo, T.; Moreira, J.; Gelain, D.; Flôres, S.; Augusti, P.; Rios, A. Hydroethanolic extracts from different genotypes of açaí (Euterpe oleracea) presented antioxidant potential and protected human neuron-like cells (SH-SY5Y). Food Chem. 2017, 222, 94–104. [Google Scholar] [CrossRef]
- Foo, K.; Blumenthal, L.; Man, H. Regulation of neuronal bioenergy homeostasis by glutamate. Neurochem. Int. 2012, 61, 389–396. [Google Scholar] [CrossRef]
- Selivanov, V.; Zagubnaya, O.; Nartsissov, Y.; Cascante, M. Unveiling a key role of oxaloacetate-glutamate interaction in regulation of respiration and ROS generation in nonsynaptic brain mitochondria using a kinetic model. PLoS ONE 2021, 16, e0255164. [Google Scholar] [CrossRef]
- De Oliveira, M.; Duarte, A.; Chenet, A.; de Almeida, F.; Andrade, C. Carnosic acid pretreatment attenuates mitochondrial dysfunction in SH-SY5Y Cells in an experimental model of glutamate-induced excitotoxicity. Neurotox. Res. 2019, 36, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, W.; Wang, H.; Ding, F.; Xiao, L.; Shi, R.; Ai, L.; Huang, Z. Tanshinone IIA inhibits glutamate-induced oxidative toxicity through prevention of mitochondrial dysfunction and suppression of MAPK activation in SH-SY5Y human neuroblastoma cells. Oxidative Med. Cell Longev. 2017, 2017, 4517486. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, A.; Kim, E.; Yang, J.; Ahn, J.; Na, J.; Cho, S. KHG21834 attenuates glutamate-induced mitochondrial damage, apoptosis, and NLRP3 inflammasome activation in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2019, 856, 172412. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-d.; Jin, M.-f.; Zhao, D.-j.; Ni, H. Reduction of mitophagy-related oxidative stress and preservation of mitochondria function using melatonin therapy in an HT22 hippocampal neuronal cell model of glutamate-induced excitotoxicity. Front. Endocrinol. 2019, 10, 550. [Google Scholar] [CrossRef]
- Zgodova, A.; Pavlova, S.; Nekrasova, A.; Boyarkin, D.; Pinelis, V.; Surin, A.; Bakaeva, Z. Isoliquiritigenin protects neuronal cells against glutamate excitotoxicity. Membranes 2022, 12, 1052. [Google Scholar] [CrossRef]
- ALNasser, M.; Mellor, I.; Carter, W. Is L-Glutamate toxic to neurons and thereby contributes to neuronal loss and neurodegeneration? A systematic review. Brain Sci. 2022, 12, 577. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Margreiter, R.; Ausserlechner, M.; Hagenbuchner, J. The complex interplay between mitochondria, ROS and entire cellular metabolism. Antioxidants 2022, 11, 1995. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial oxidative stress-A causative factor and therapeutic target in many diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Nampoothiri, M.; Reddy, N.; John, J.; Kumar, N.; Kutty Nampurath, G.; Rao Chamallamudi, M. Insulin blocks glutamate-induced neurotoxicity in differentiated SH-SY5Y neuronal cells. Behav. Neurol. 2014, 2014, 674164–674168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, K.; Zhang, K.; Lin, X.; Zhu, L.; Zhou, F. Puerarin protects human neuroblastoma SH-SY5Y cells against glutamate-induced oxidative stress and mitochondrial dysfunction. J. Biochem. Mol. Toxicol. 2016, 30, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Chen, H.-s.V.; Zhang, D.; Lipton, S.A. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J. Neurosci. 2010, 30, 11246–11250. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.; Barbalho, S.; Araújo, A.; Guiguer, E.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in health and disease: A critical review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef]
- Liang, W.; Lam, W.; Tang, H.; Leung, P.; Yew, D. Current evidence of chinese herbal constituents with effects on NMDA receptor blockade. Pharmaceuticals 2013, 6, 1039–1054. [Google Scholar] [CrossRef]
- Afshari, A.; Fanoudi, S.; Rajabian, A.; Sadeghnia, H.; Mollazadeh, H.; Hosseini, A. Potential protective roles of phytochemicals on glutamate-induced neurotoxicity: A review. Iran. J. Basic Med. Sci. 2020, 23, 1113–1123. [Google Scholar] [CrossRef]
- Marchetti, C.; Gavazzo, P.; Stafford, G.; Van Staden, J. South African plants used in traditional medicine to treat epilepsy have an antagonistic effect on NMDA receptor currents. J. Ethnopharmacol. 2011, 137, 382–388. [Google Scholar] [CrossRef]
- Silva, A.; Grosso, C.; Delerue-Matos, C.; Rocha, J. Comprehensive review on the interaction between natural compounds and brain receptors: Benefits and toxicity. Eur. J. Med. Chem. 2019, 174, 87–115. [Google Scholar] [CrossRef]
- Babiaka, S.; Nia, R.; Abuga, K.; Mbah, J.; Nziko, V.; Paper, D.; Ntie-Kang, F. Antioxidant potential of flavonoid glycosides from Manniophyton fulvum Müll. (Euphorbiaceae): Identification and molecular modeling. Sci. Afr. 2020, 8, e00423. [Google Scholar] [CrossRef]
- Bolouri-Moghaddam, M.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef]
- Chunhakant, S.; Chaicharoenpong, C. Antityrosinase, antioxidant, and cytotoxic activities of phytochemical constituents from Manilkara zapota L. Bark. Molecules 2019, 24, 2798. [Google Scholar] [CrossRef]
- Ercan, L.; Dogru, M. Antioxidant and antimicrobial capacity of quinic acid. BEU J. Sci. Technol. 2022, 11, 1018–1025. [Google Scholar] [CrossRef]
- Mardani-Ghahfarokhi, A.; Farhoosh, R. Antioxidant activity and mechanism of inhibitory action of gentisic and α-resorcylic acids. Sci. Rep. 2020, 10, 19487. [Google Scholar] [CrossRef] [PubMed]
- Veselova, M.; Fedoreev, S.; Vasilevskaya, N.; Denisenko, V.; Gerasimenko, A. Antioxidant activity of polyphenols from the far-east plant Taxus cuspidata. Pharm. Chem. J. 2007, 41, 88–93. [Google Scholar] [CrossRef]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Zhou, X.-J.; Mei, R.-Q.; Zhang, L.; Lu, Q.; Zhao, J.; Adebayo, A.H.; Cheng, Y.-X. Antioxidant phenolics from Broussonetia papyrifera fruits. J. Asian Nat. Prod. Res. 2010, 12, 399–406. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Szeto, S.; Chong, C.; Quan, Q.; Huang, C.; Cui, W.; Guo, B.; Wang, Y.; Han, Y.; et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015, 84, 331–343. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective effects of phenolic acids: Molecular mechanism of action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Tan, X.; Liu, J.; Zhi, Y.; Yi, L.; Bai, S.; Du, Q.; Li, Q.X.; Dong, Y. Isoorientin inhibits inflammation in macrophages and endotoxemia mice by regulating glycogen synthase kinase 3β. Mediat. Inflamm. 2020, 2020, 8704146. [Google Scholar] [CrossRef]
- Ahmad, I.; Kumar, D.; Patel, H. Computational investigation of phytochemicals from Withania somnifera (Indian ginseng/ashwagandha) as plausible inhibitors of GluN2B-containing NMDA receptors. J. Biomol. Struct. Dyn. 2022, 40, 7991–8003. [Google Scholar] [CrossRef]
- David, T.; Omotuyi, O.; Agboola, O.; Okonkwo, D.; Adelakun, N. Identification of Gly/NMDAR antagonist from Chromolaena odorata’s derived phytoconstituents using induced fit docking approach. bioRxiv 2019. [Google Scholar] [CrossRef]
- Noh, J.; Lee, E.-s.; Chung, J.-m. The novel NMDA receptor antagonist, 2-hydroxy-5-(2,3,5,6-tetrafluoro-4-trifluoromethyl-benzylamino)-benzoic acid, is a gating modifier in cultured mouse cortical neurons. J. Neurochem. 2009, 109, 1261–1271. [Google Scholar] [CrossRef]
- Wu, Q.; Tymianski, M. Targeting NMDA receptors in stroke: New hope in neuroprotection. Mol. Brain 2018, 11, 15. [Google Scholar] [CrossRef]
- Li, V.; Wang, Y. Molecular mechanisms of NMDA receptor-mediated excitotoxicity: Implications for neuroprotective therapeutics for stroke. Neural Regen. Res. 2016, 11, 1752–1753. [Google Scholar] [CrossRef]
- Alqurashi, R.; Galante, L.; Rowland, I.; Spencer, J.; Commane, D. Consumption of a flavonoid-rich açai meal is associated with acute improvements in vascular function and a reduction in total oxidative status in healthy overweight men. Am. J. Clin. Nutr. 2016, 104, 1227–1235. [Google Scholar] [CrossRef]
- Myrissa, K.; Chappell, P.; Kelaiditi, E. Effect of acute acai (Euterpe oleracea Mart.) supplementation on postprandial glycaemia in healthy adults: A pilot randomised controlled study. Curr. Dev. Nutr. 2021, 5, 853. [Google Scholar] [CrossRef]
- Talcott, S. Absorption and antioxidant effects of polyphenolics from acai. Clin. Trials; 2016. Available online: https://clinicaltrials.gov/study/NCT02227628 (accessed on 8 September 2025).

| Prime MM/GBSA | ||||||
|---|---|---|---|---|---|---|
| Ligand | Molecular Formula | Molecular Weight | Docking Score | XPG Score | Glide Score | Δ Gbind |
| g/mol | kcal/mol | kcal/mol | kcal/mol | kcal/mol | ||
| Glutamate | C8H13N2O4 | 201.20 | −10.041 | −10.041 | −10.041 | −15.897 |
| Arginine | C6H15N4O2 | 175.21 | −8.423 | −8.423 | −8.423 | −40.208 |
| 2,5-Dihydroxybenzoic acid | C7H5O4 | 153.12 | −8.288 | −8.289 | −8.289 | −11.252 |
| Threonine | C4H9NO3 | 119.12 | −7.320 | −7.320 | −7.320 | −42.676 |
| Protocatechuic acid | C7H5O4 | 153.12 | −7.103 | −7.103 | −7.103 | −14.978 |
| Histidine | C6H9N3O2 | 155.15 | −6.933 | −7.394 | −7.394 | −27.708 |
| Interaction Type | Interacting Residues | |
|---|---|---|
| Glutamate | Polar | Asn-117, Ser-172, Thr-173, Asn-176, Ala-240 |
| Arginine | Polar | Ser-113, Leu-114, Thr-115, Val-168, Pro-169, Ser-172, Tyr-213 |
| 2,5-Dihydroxybenzoic acid | Polar | Glu-15, Thr-115, Ser-172, Tyr-244 |
| Threonine | Polar | Ser-113, Leu-114, Thr-115, Arg-120, Gly-171, Ser-172 |
| Protocatechuic acid | Polar | Glu-15, Ser-113, Thr-115, Arg-120, Gly-171, Ser-172, Tyr-244 |
| Histidine | Polar | Ser-113, Leu-114, Thr-115, Arg-120, Gly-171, Ser-172, Thr-173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ALNasser, M.N.; Malik, N.; Ahmed, A.; Newman, A.; Mellor, I.R.; Carter, W.G. Acai Berry Extracts Can Mitigate the L-Glutamate-Induced Neurotoxicity Mediated by N-Methyl-D-Aspartate Receptors. Brain Sci. 2025, 15, 1073. https://doi.org/10.3390/brainsci15101073
ALNasser MN, Malik N, Ahmed A, Newman A, Mellor IR, Carter WG. Acai Berry Extracts Can Mitigate the L-Glutamate-Induced Neurotoxicity Mediated by N-Methyl-D-Aspartate Receptors. Brain Sciences. 2025; 15(10):1073. https://doi.org/10.3390/brainsci15101073
Chicago/Turabian StyleALNasser, Maryam N., Nirmal Malik, Abrar Ahmed, Amy Newman, Ian R. Mellor, and Wayne G. Carter. 2025. "Acai Berry Extracts Can Mitigate the L-Glutamate-Induced Neurotoxicity Mediated by N-Methyl-D-Aspartate Receptors" Brain Sciences 15, no. 10: 1073. https://doi.org/10.3390/brainsci15101073
APA StyleALNasser, M. N., Malik, N., Ahmed, A., Newman, A., Mellor, I. R., & Carter, W. G. (2025). Acai Berry Extracts Can Mitigate the L-Glutamate-Induced Neurotoxicity Mediated by N-Methyl-D-Aspartate Receptors. Brain Sciences, 15(10), 1073. https://doi.org/10.3390/brainsci15101073







