Multivariate Decoding and Drift-Diffusion Modeling Reveal Adaptive Control in Trilingual Comprehension
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Power Analysis
2.1.2. Language Background and Self-Reported Proficiency
2.1.3. Objective Proficiency Verification
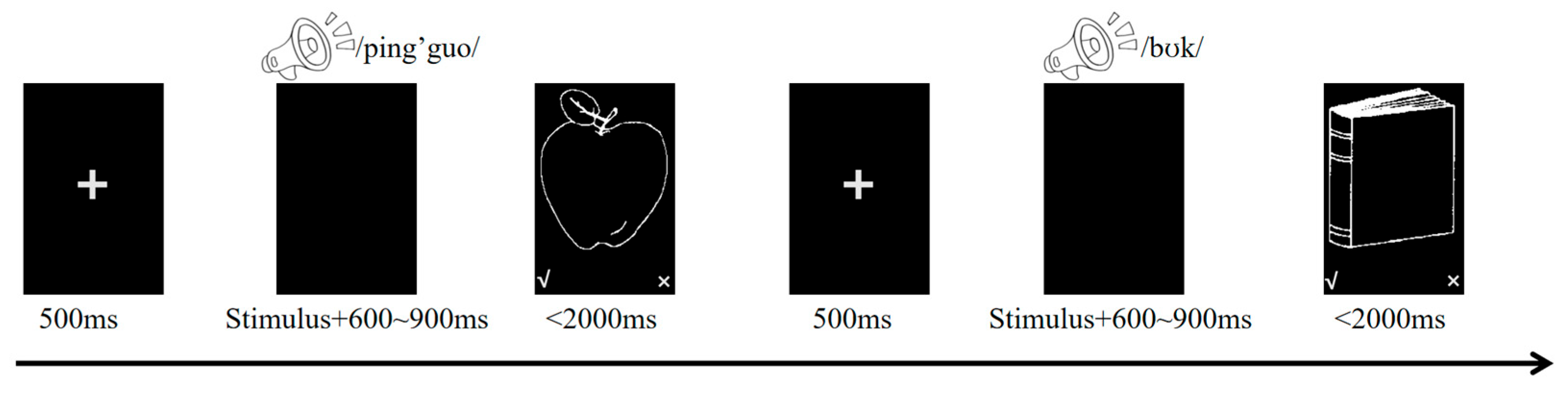
2.2. Materials and Procedure
Experimental Procedure
2.3. Data Acquisition and Preprocessing
2.3.1. EEG Recording
2.3.2. Preprocessing
2.4. Data Analyses
2.4.1. Analytical Framework
2.4.2. Behavioral Analysis
2.4.3. Event-Related Potential (ERP) Analysis
2.4.4. Multivariate Pattern Analysis (MVPA)
2.4.5. Drift-Diffusion Model (DDM) Analysis
2.4.6. Multiple Regression Linking ERPs and DDM
3. Results
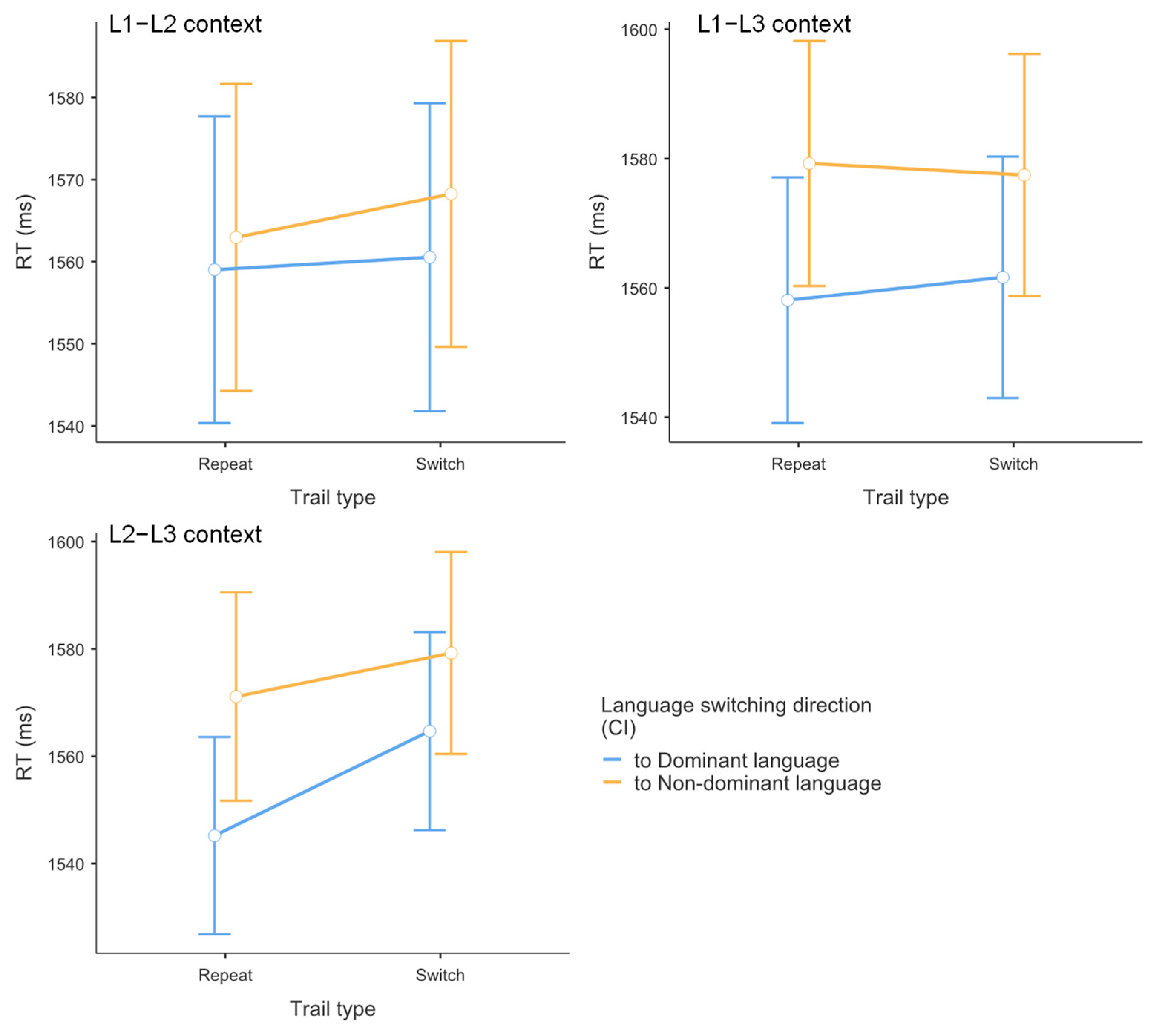
3.1. Results of Reaction Time Data
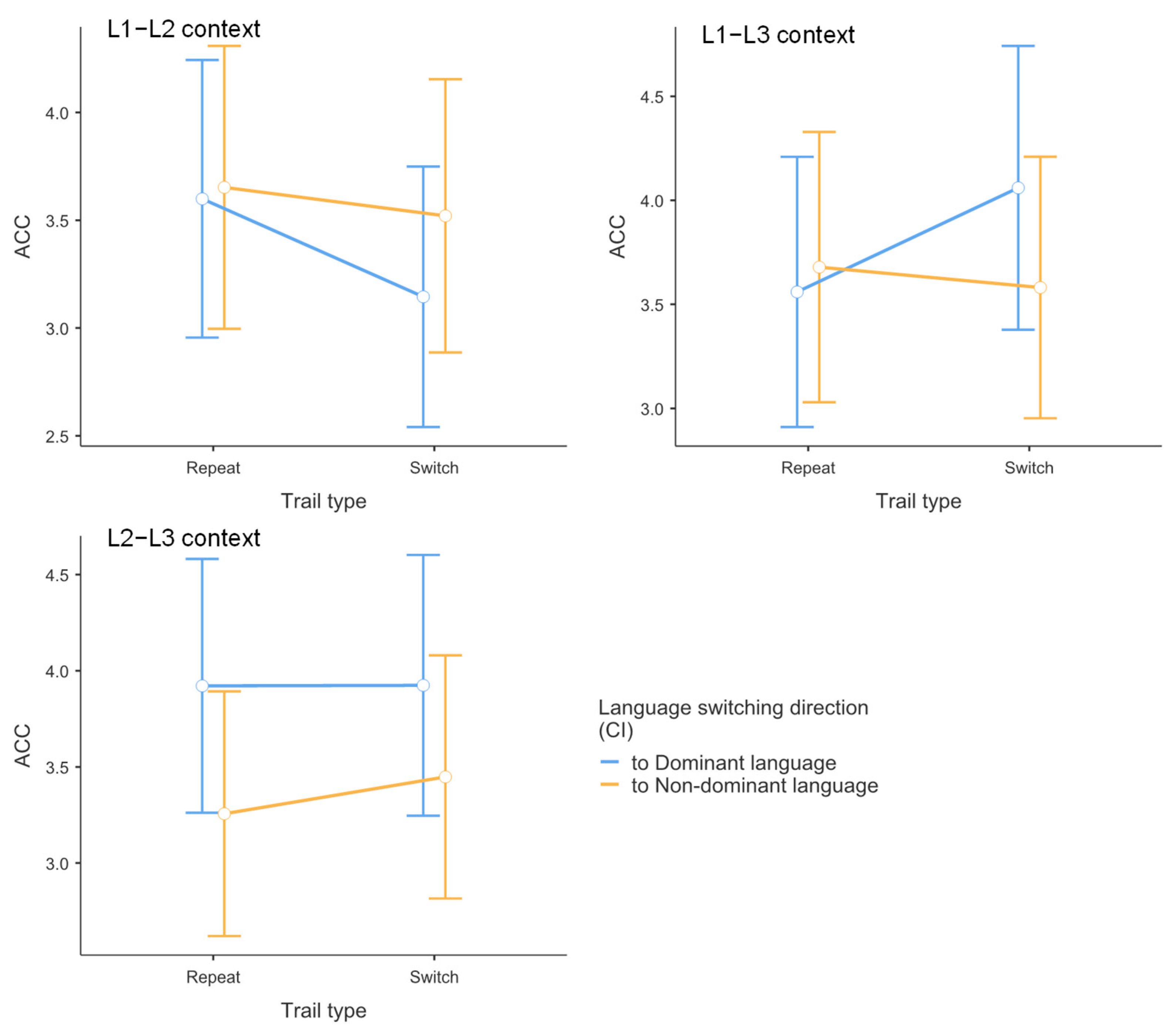
3.2. Results of Accuracy Data
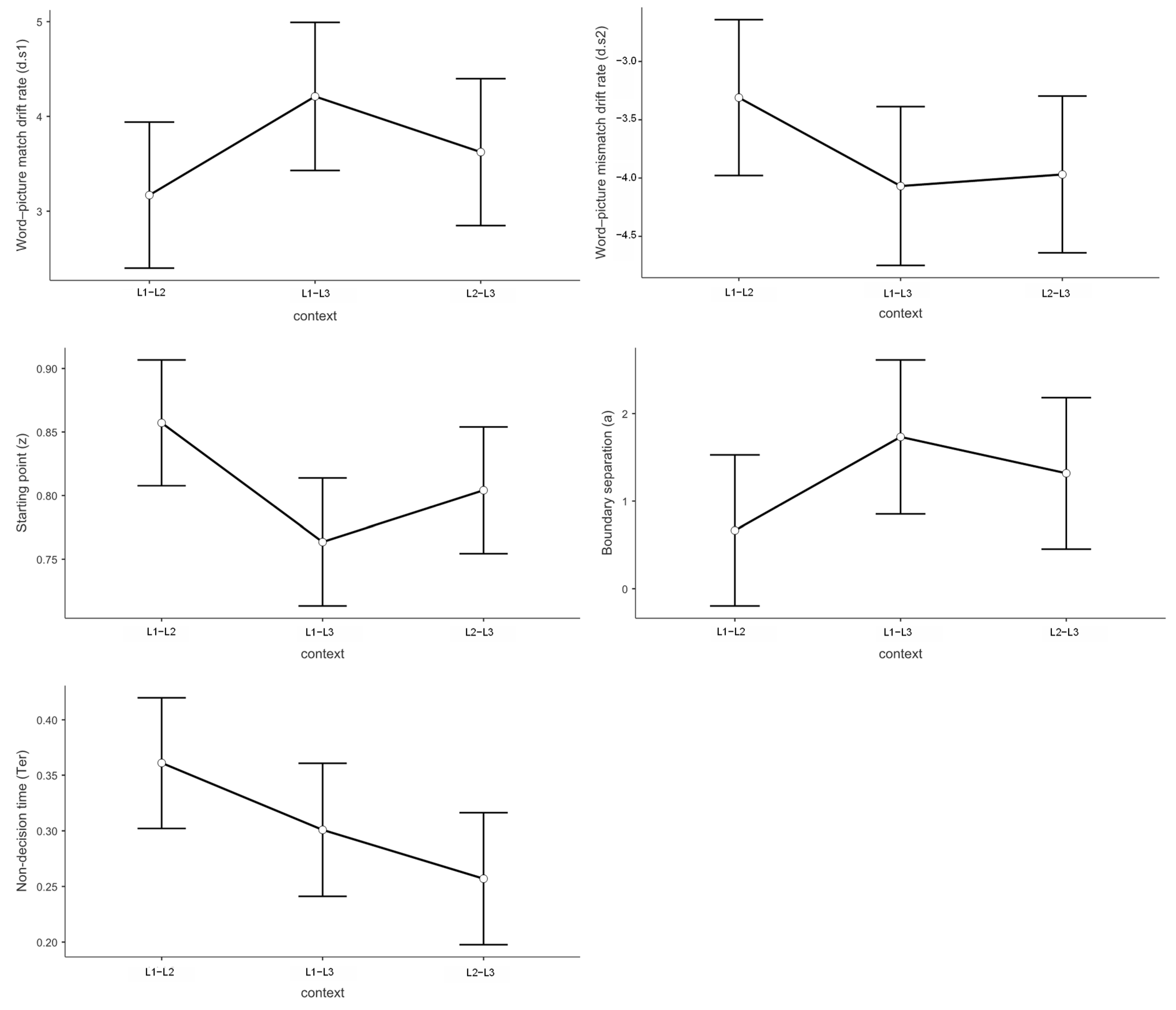
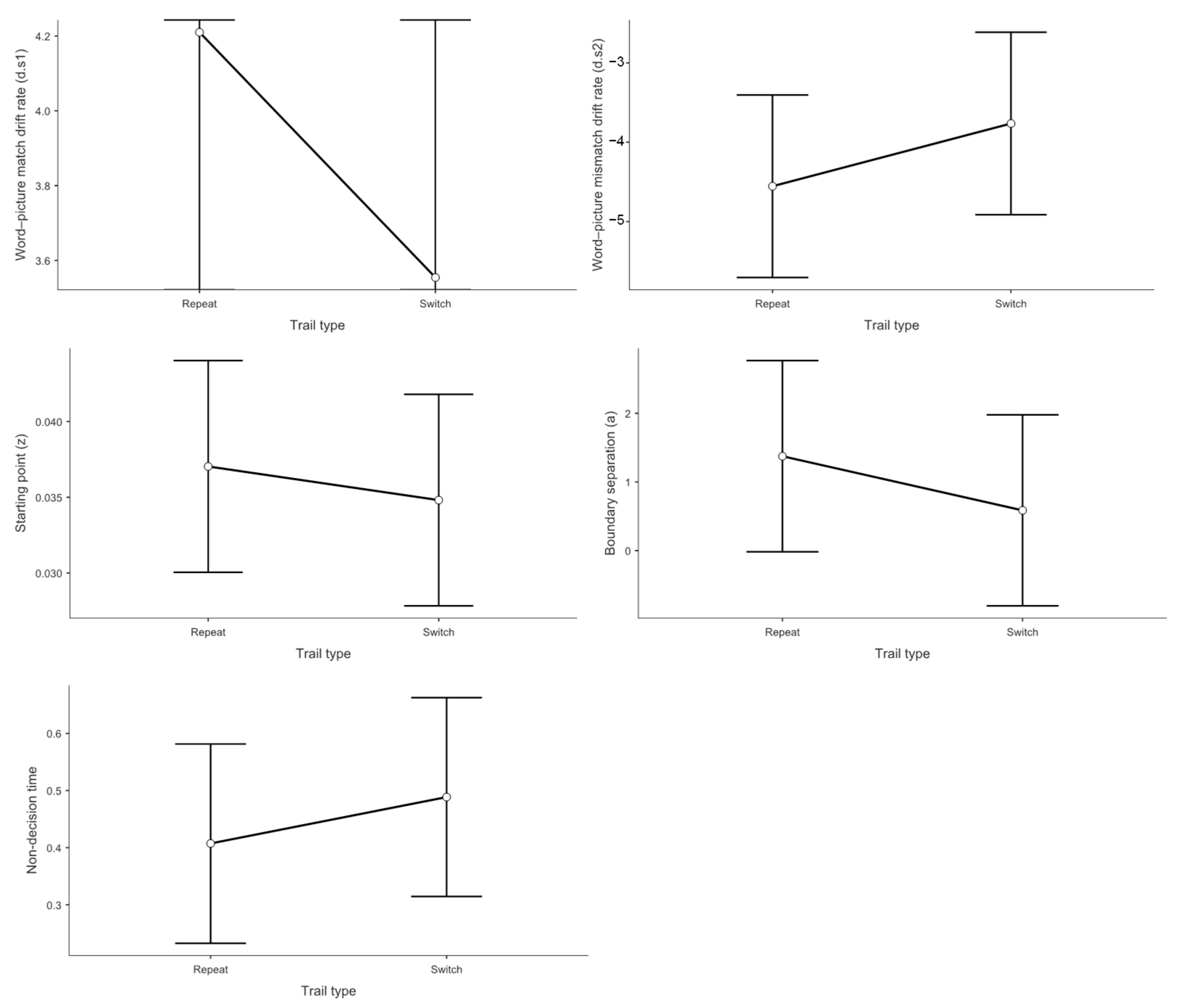
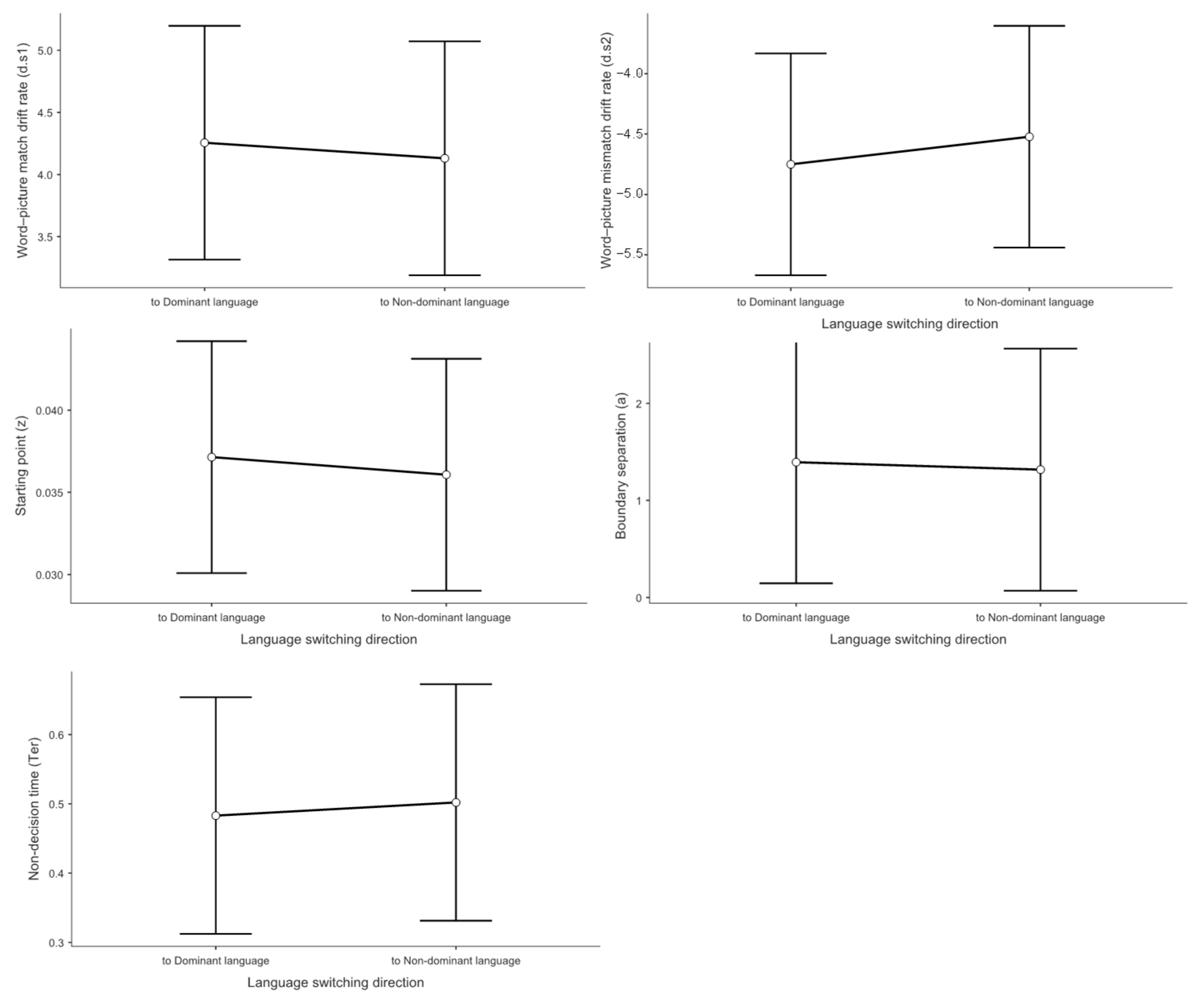
3.3. Drift Diffusion Model Results
3.3.1. Drift Rate Analysis for Match Responses (d.s1)
3.3.2. Drift Rate Analysis for Mismatch Responses (d.s2)
3.3.3. Starting Point Analysis (Response Bias)
3.3.4. Boundary Separation Analysis (Decision Threshold)
3.3.5. Non-Decision Time Analysis (Motor and Encoding Processes)
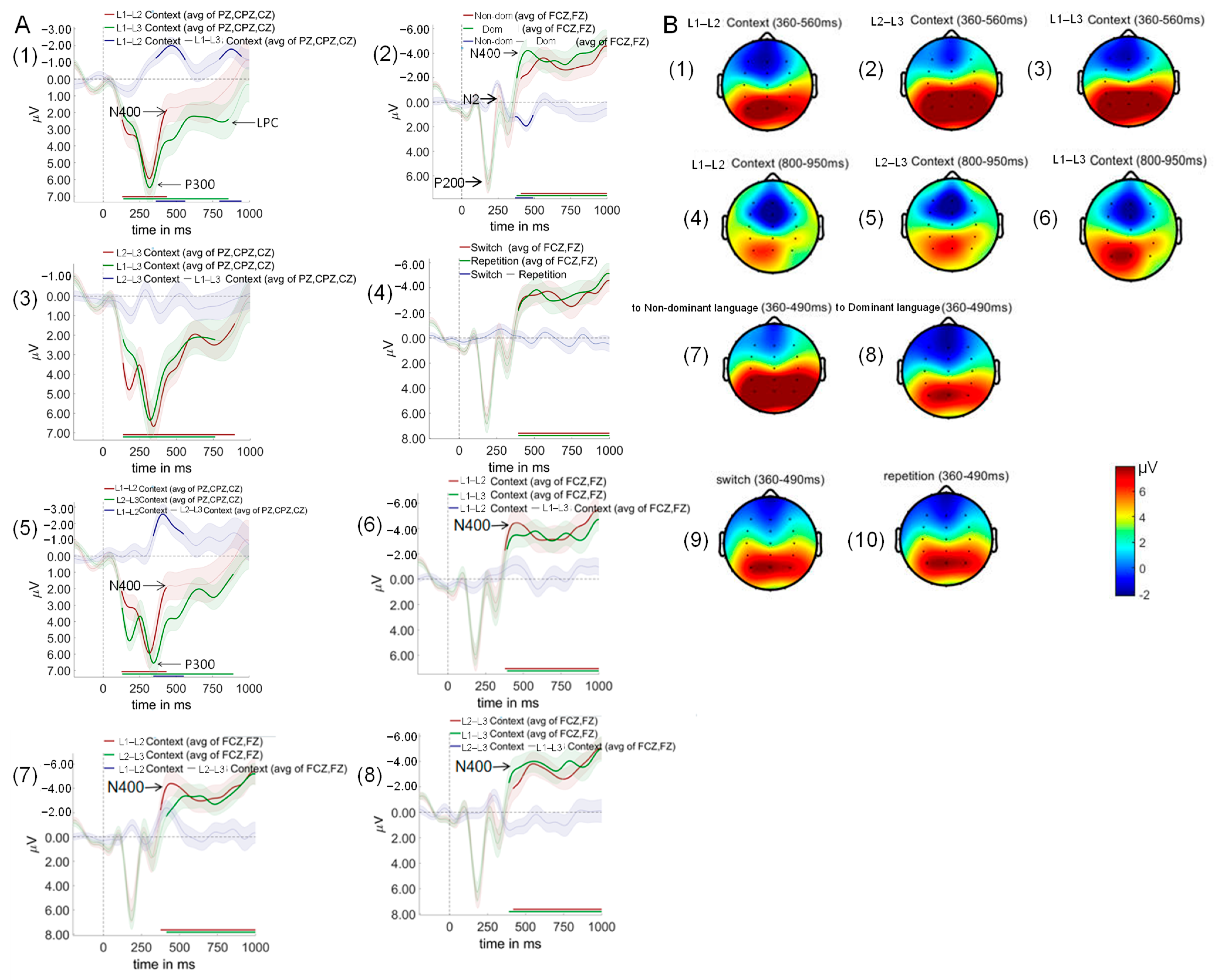
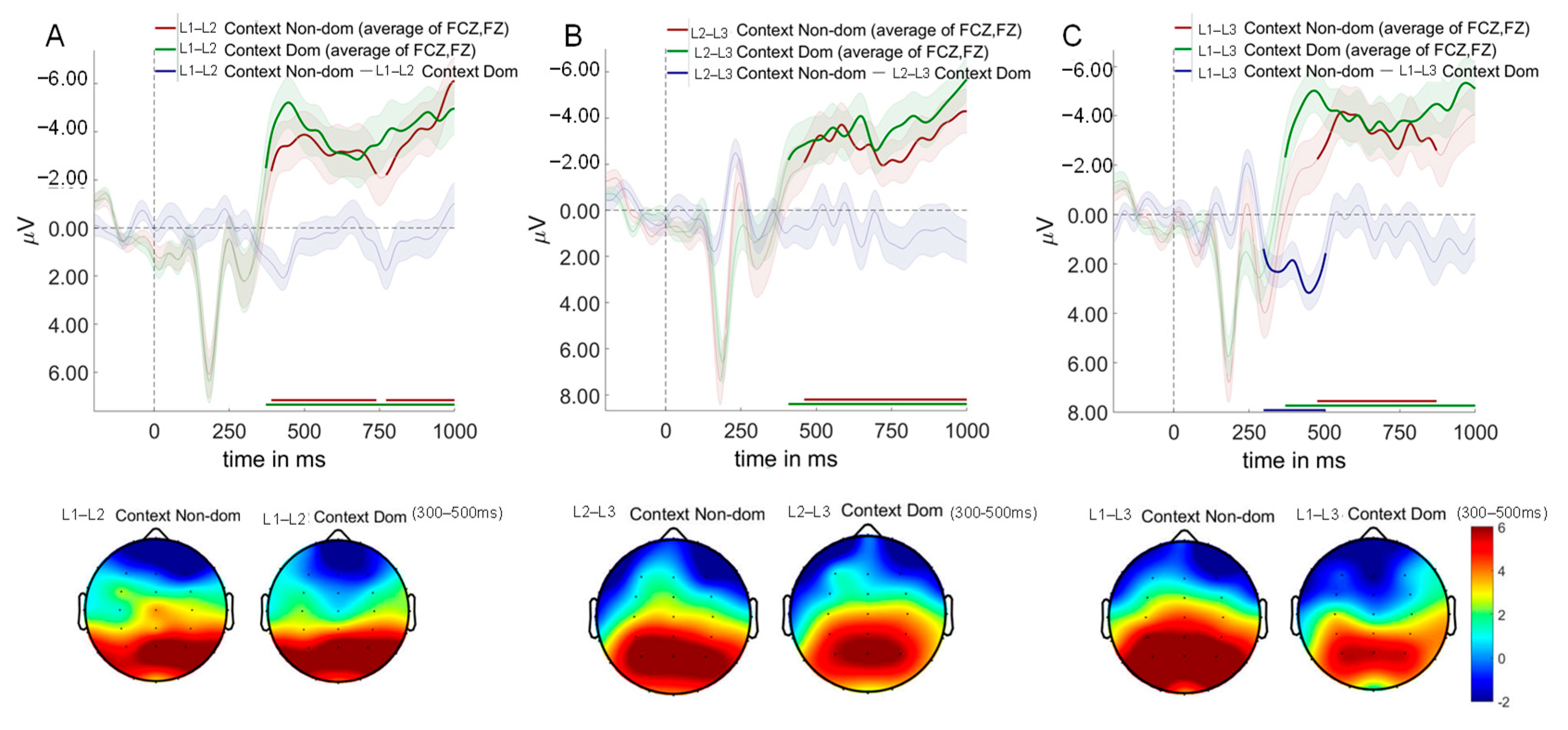
3.4. ERP Results
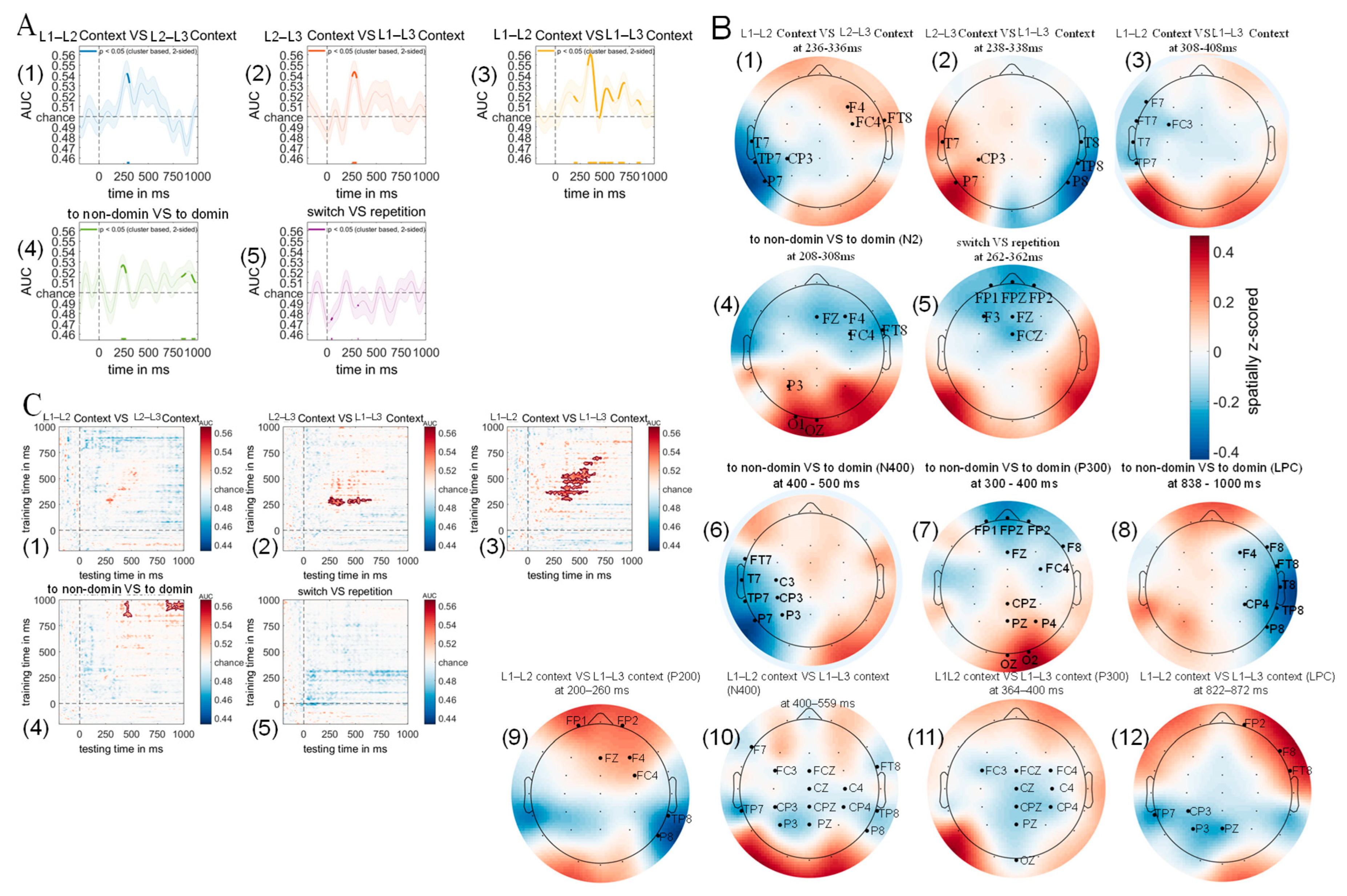
3.5. Multivariate Pattern Analysis Results
3.5.1. Diagonal Decoding Results
3.5.2. Weight Projection Analysis Results
3.5.3. Temporal Generalization Using Classification Across Time Result
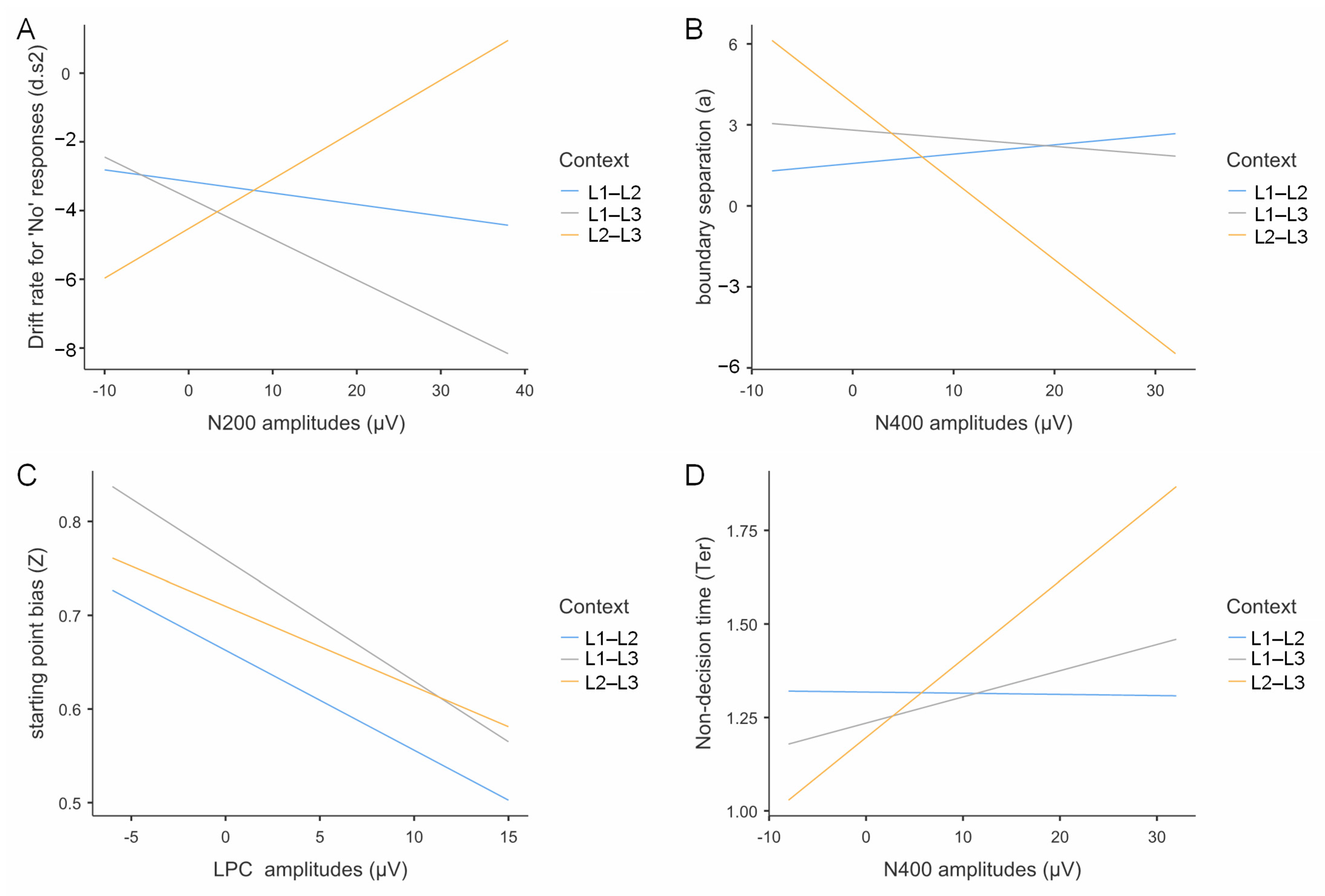
3.5.4. Multiple Regression Analysis of ERP Data and Drift Diffusion Model Parameters
- N200 Amplitudes and Drift Rate for “No” Responses
- N400 Amplitudes and Decision Boundary
- LPC Amplitudes and Response Bias
- N400 Amplitudes and Non-Decision Time
4. Discussion
4.1. Language Comprehension Efficiency Across Dual-Language Contexts
4.2. The Demands of Proactive Control in Different Dual-Language Contexts
4.3. The Demands of Reactive Control in Different Dual-Language Contexts
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilingualism in the US, UK & Global Statistics. Available online: https://preply.com/en/blog/bilingualism-statistics/ (accessed on 12 June 2025).
- Thierry, G.; Wu, Y.J. Brain potentials reveal unconscious translation during foreign-language comprehension. Proc. Natl. Acad. Sci. USA 2007, 104, 12530–12535. [Google Scholar] [CrossRef]
- Chen, J. How do L3 words find conceptual parasitic hosts in typologically distant L1 or L2? Evidence from a cross-linguistic priming effect. Int. J. Biling. Educ. Biling. 2020, 23, 1238–1253. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Xiong, Y.; He, M. Examining sources of language production switch costs amongst Tibetan-Chinese-English trilinguals. Int. J. Biling. Educ. Biling. 2024, 27, 1153–1167. [Google Scholar] [CrossRef]
- Declerck, M.; Koch, I. The concept of inhibition in bilingual control. Psychol. Rev. 2023, 130, 953. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, I.; Rauschecker, J.P. Phoneme and word recognition in the auditory ventral stream. Proc. Natl. Acad. Sci. USA 2012, 109, E505–E514. [Google Scholar] [CrossRef]
- Grainger, J.; Midgley, K.; Holcomb, P. Re-thinking the bilingual interactive-activation model from a developmental perspective (BIA-d). In Language Acquisition Across Linguistic and Cognitive Systems; Kail, M., Hickmann, M., Eds.; John Benjamins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Jiao, L.; Liu, C.; Liang, L.; Plummer, P.; Perfetti, C.A.; Chen, B. The contributions of language control to executive functions: From the perspective of bilingual comprehension. Q. J. Exp. Psychol. 2019, 72, 1984–1997. [Google Scholar] [CrossRef] [PubMed]
- Declerck, M.; Stephan, D.N.; Koch, I.; Philipp, A.M. The other modality: Auditory stimuli in language switching. J. Cogn. Psychol. 2015, 27, 685–691. [Google Scholar] [CrossRef]
- Meyer, M.; Alter, K.; Friederici, A.D.; Lohmann, G.; von Cramon, D.Y. FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum. Brain Mapp. 2002, 17, 73–88. [Google Scholar] [CrossRef]
- Ludersdorfer, P.; Wimmer, H.; Richlan, F.; Schurz, M.; Hutzler, F.; Kronbichler, M. Left ventral occipitotemporal activation during orthographic and semantic processing of auditory words. NeuroImage 2016, 124, 834–842. [Google Scholar] [CrossRef]
- Wu, J.; Cai, C.; Wu, M.; Wang, X.; Yu, M. Distinct language control mechanisms in speech production and comprehension: Evidence from N-2 repetition, switching, and mixing costs. J. Multiling. Multicult. Dev. 2025, 1–17. [Google Scholar] [CrossRef]
- Botvinick, M.M.; Cohen, J.D.; Carter, C.S. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn. Sci. 2004, 8, 539–546. [Google Scholar] [CrossRef]
- Van Heuven, W.J.; Dijkstra, T.; Grainger, J. Orthographic neighborhood effects in bilingual word recognition. J. Mem. Lang. 1998, 39, 458–483. [Google Scholar] [CrossRef]
- Dijkstra, T.; Van Heuven, W.J. The architecture of the bilingual word recognition system: From identification to decision. Biling. Lang. Cogn. 2002, 5, 175–197. [Google Scholar] [CrossRef]
- Declerck, M.; Grainger, J. Inducing asymmetrical switch costs in bilingual language comprehension by language practice. Acta Psychol. 2017, 178, 100–106. [Google Scholar] [CrossRef]
- Orfanidou, E.; Sumner, P. Language switching and the effects of orthographic specificity and response repetition. Mem. Cogn. 2005, 33, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, X.; Lavaur, J.-M. Recognising words in three languages: Effects of language dominance and language switching. Int. J. Multiling. 2014, 11, 164–181. [Google Scholar] [CrossRef]
- Hirsch, P.; Declerck, M.; Koch, I. Exploring the functional locus of language switching: Evidence from a PRP paradigm. Acta Psychol. 2015, 161, 1–6. [Google Scholar] [CrossRef]
- Declerck, M.; Thoma, A.M.; Koch, I.; Philipp, A.M. Highly proficient bilinguals implement inhibition: Evidence from n-2 language repetition costs. J. Exp. Psychol. Learn. Mem. Cogn. 2015, 41, 1911. [Google Scholar] [CrossRef]
- Declerck, M.; Philipp, A.M. Is inhibition implemented during bilingual production and comprehension? n-2 language repetition costs unchained. Lang. Cogn. Neurosci. 2018, 33, 608–617. [Google Scholar] [CrossRef]
- Green, D.W.; Abutalebi, J. Language control in bilinguals: The adaptive control hypothesis. J. Cogn. Psychol. 2013, 25, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Elorrieta, E.; Pylkkänen, L. Bilingual language switching in the laboratory versus in the wild: The spatiotemporal dynamics of adaptive language control. J. Neurosci. 2017, 37, 9022–9036. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Thierry, G. Fast modulation of executive function by language context in bilinguals. J. Neurosci. 2013, 33, 13533–13537. [Google Scholar] [CrossRef] [PubMed]
- Ecke, P. Parasitic vocabulary acquisition, cross-linguistic influence, and lexical retrieval in multilinguals. Biling. Lang. Cogn. 2015, 18, 145–162. [Google Scholar] [CrossRef]
- Ecke, P.; Hall, C.J. The Parasitic Model of L2 and L3 vocabulary acquisition: Evidence from naturalistic and experimental studies. Fórum Linguístico 2014, 11, 360–372. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. The effect of the non-task language when trilingual people use two languages in a language switching experiment. Front. Psychol. 2020, 11, 754. [Google Scholar] [CrossRef]
- Abutalebi, J.; Green, D.W. Neuroimaging of language control in bilinguals: Neural adaptation and reserve. Biling. Lang. Cogn. 2016, 19, 689–698. [Google Scholar] [CrossRef]
- Declerck, M.; Kleinman, D.; Gollan, T.H. Which bilinguals reverse language dominance and why? Cognition 2020, 204, 104384. [Google Scholar] [CrossRef]
- Peeters, D.; Dijkstra, T. Sustained inhibition of the native language in bilingual language production: A virtual reality approach. Biling. Lang. Cogn. 2018, 21, 1035–1061. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, C.; de Bruin, A.; Chen, B. Effects of language context on executive control in unbalanced bilinguals: An ERPs study. Psychophysiology 2020, 57, e13653. [Google Scholar] [CrossRef]
- Jankowiak, K.; Rataj, K. The N400 as a window into lexico-semantic processing in bilingualism. Pozn. Stud. Contemp. Linguist. 2017, 53, 119–156. [Google Scholar] [CrossRef]
- Liu, H.; Rossi, S.; Zhou, H.; Chen, B. Electrophysiological evidence for domain-general inhibitory control during bilingual language switching. PLoS ONE 2014, 9, e110887. [Google Scholar] [CrossRef]
- Ong, G.; McKague, M.; Weekes, B.; Sewell, D.K. Diffusing the bilingual lexicon: Task-based and lexical components of language switch costs. Cogn. Psychol. 2019, 114, 101225. [Google Scholar] [CrossRef]
- Todorova, L.; Neville, D.A.; Piai, V. Lexical-semantic and executive deficits revealed by computational modelling: A drift diffusion model perspective. Neuropsychologia 2020, 146, 107560. [Google Scholar] [CrossRef]
- Todorova, L.; Neville, D.A. Associative and identity words promote the speed of visual categorization: A hierarchical drift diffusion account. Front. Psychol. 2020, 11, 955. [Google Scholar] [CrossRef]
- Shen, C.; Calvin, O.L.; Rawls, E.; Redish, A.D.; Sponheim, S.R. Clarifying cognitive control deficits in psychosis via drift diffusion modeling and attractor dynamics. Schizophr. Bull. 2024, 50, 1357–1370. [Google Scholar] [CrossRef]
- Langford, Z.D.; Krebs, R.M.; Talsma, D.; Woldorff, M.G.; Boehler, C. Strategic down—Regulation of attentional resources as a mechanism of proactive response inhibition. Eur. J. Neurosci. 2016, 44, 2095–2103. [Google Scholar] [CrossRef]
- Wu, R.; Struys, E.; Lochtman, K. Relationship between language dominance and stimulus-stimulus or stimulus-response inhibition in Uyghur-Chinese bilinguals with an investigation of speed-accuracy trade-offs. Behav. Sci. 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Tusun, A.; Wang, Y.; Abulajiang, A. Moving in L2 Chinese from childhood to adulthood: Developmental and crosslinguistic factors in bilingual event construal. Int. J. Biling. 2024. [Google Scholar] [CrossRef]
- Brysbaert, M. How many participants do we have to include in properly powered experiments? A tutorial of power analysis with reference tables. J. Cogn. 2019, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Anderson, J.A.; Mak, L.; Keyvani Chahi, A.; Bialystok, E. The language and social background questionnaire: Assessing degree of bilingualism in a diverse population. Behav. Res. Methods 2018, 50, 250–263. [Google Scholar] [CrossRef]
- Tomoschuk, B.; Ferreira, V.S.; Gollan, T.H. When a seven is not a seven: Self-ratings of bilingual language proficiency differ between and within language populations. Biling. Lang. Cogn. 2019, 22, 516–536. [Google Scholar] [CrossRef]
- Gollan, T.H.; Weissberger, G.H.; Runnqvist, E.; Montoya, R.I.; Cera, C.M. Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish–English bilinguals. Biling. Lang. Cogn. 2012, 15, 594–615. [Google Scholar] [CrossRef]
- Szekely, A.; Jacobsen, T.; D’Amico, S.; Devescovi, A.; Andonova, E.; Herron, D.; Lu, C.C.; Pechmann, T.; Pléh, C.; Wicha, N.; et al. A new on-line resource for psycholinguistic studies. J. Mem. Lang. 2004, 51, 247–250. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P.; Bolker, M.B. Package ‘lme4’. Convergence 2015, 12, 2. [Google Scholar]
- Kwon, Y.; Lee, Y.; Nam, K. The different P200 effects of phonological and orthographic syllable frequency in visual word recognition in Korean. Neurosci. Lett. 2011, 501, 117–121. [Google Scholar] [CrossRef]
- Cheng, X.; Schafer, G.; Riddell, P.M. Immediate auditory repetition of words and nonwords: An ERP study of lexical and sublexical processing. PLoS ONE 2014, 9, e91988. [Google Scholar] [CrossRef] [PubMed]
- Maurer, U.; Brandeis, D.; McCandliss, B.D. Fast, visual specialization for reading in English revealed by the topography of the N170 ERP response. Behav. Brain Funct. 2005, 1, 13. [Google Scholar] [CrossRef]
- Kroll, J.F.; Bobb, S.C.; Misra, M.; Guo, T. Language selection in bilingual speech: Evidence for inhibitory processes. Acta Psychol. 2008, 128, 416–430. [Google Scholar] [CrossRef]
- Donkers, F.C.; Van Boxtel, G.J. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004, 56, 165–176. [Google Scholar] [CrossRef]
- Evans, J.L.; Selinger, C.; Pollak, S.D. P300 as a measure of processing capacity in auditory and visual domains in specific language impairment. Brain Res. 2011, 1389, 93–102. [Google Scholar] [CrossRef]
- Holcomb, P.J.; Neville, H.J. Auditory and visual semantic priming in lexical decision: A comparison using event-related brain potentials. Lang. Cogn. Process. 1990, 5, 281–312. [Google Scholar] [CrossRef]
- Van Petten, C.; Kutas, M. Interactions between sentence context and word frequency in event-related brain potentials. Mem. Cogn. 1990, 18, 380–393. [Google Scholar] [CrossRef]
- Ortu, D.; Allan, K.; Donaldson, D.I. Is the N400 effect a neurophysiological index of associative relationships? Neuropsychologia 2013, 51, 1742–1748. [Google Scholar] [CrossRef]
- Tiedt, H.O.; Ehlen, F.; Klostermann, F. Age-related dissociation of N400 effect and lexical priming. Sci. Rep. 2020, 10, 20291. [Google Scholar] [CrossRef]
- Moreno, E.M.; Rodríguez-Fornells, A.; Laine, M. Event-related potentials (ERPs) in the study of bilingual language processing. J. Neurolinguistics 2008, 21, 477–508. [Google Scholar] [CrossRef]
- Yang, H.; Laforge, G.; Stojanoski, B.; Nichols, E.S.; McRae, K.; Köhler, S. Late positive complex in event-related potentials tracks memory signals when they are decision relevant. Sci. Rep. 2019, 9, 9469. [Google Scholar] [CrossRef] [PubMed]
- Fahrenfort, J.J.; Van Driel, J.; Van Gaal, S.; Olivers, C.N. From ERPs to MVPA using the Amsterdam decoding and modeling toolbox (ADAM). Front. Neurosci. 2018, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Meinecke, F.; Görgen, K.; Dähne, S.; Haynes, J.D.; Blankertz, B.; Bießmann, F. On the interpretation of weight vectors of linear models in multivariate neuroimaging. NeuroImage 2014, 87, 96–110. [Google Scholar] [CrossRef]
- King, J.-R.; Dehaene, S. Characterizing the dynamics of mental representations: The temporal generalization method. Trends Cogn. Sci. 2014, 18, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, A.; Lin, Y.S.; Reynolds, A.; Strickland, L.; Gretton, M.; Matzke, D. Dynamic models of choice. Behav. Res. Methods 2019, 51, 961–985. [Google Scholar] [CrossRef]
- Turner, B.M.; Sederberg, P.B.; Brown, S.D.; Steyvers, M. A method for efficiently sampling from distributions with correlated dimensions. Psychol. Methods 2013, 18, 368. [Google Scholar] [CrossRef]
- Tran, N.H.; Van Maanen, L.; Heathcote, A.; Matzke, D. Systematic parameter reviews in cognitive modeling: Towards a robust and cumulative characterization of psychological processes in the diffusion decision model. Front. Psychol. 2021, 11, 608287. [Google Scholar] [CrossRef]
- Brooks, S.P.; Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar] [CrossRef]
- Hut, S.C.; Helenius, P.; Leminen, A.; Mäkelä, J.P.; Lehtonen, M. Language control mechanisms differ for native languages: Neuromagnetic evidence from trilingual language switching. Neuropsychologia 2017, 107, 108–120. [Google Scholar] [CrossRef]
- Declerck, M.; Philipp, A.M. A review of control processes and their locus in language switching. Psychon. Bull. Rev. 2015, 22, 1630–1645. [Google Scholar] [CrossRef]
- Dijkstra, T.; Van Heuven, W.J. The BIA model and bilingual word recognition. In Localist Connectionist Approaches to Human Cognition; Psychology Press: London, UK, 2013; pp. 189–225. [Google Scholar]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Green, D.W. Mental control of the bilingual lexico-semantic system. Biling. Lang. Cogn. 1998, 1, 67–81. [Google Scholar] [CrossRef]
- van Steenbergen, H.; Band, G.P.; Hommel, B. Does conflict help or hurt cognitive control? Initial evidence for an inverted U-shape relationship between perceived task difficulty and conflict adaptation. Front. Psychol. 2015, 6, 974. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, L.; Zhang, L.; Lu, Y.; Chen, B. Modulatory role of inhibition during language switching: Evidence from evoked and induced oscillatory activity. Int. J. Biling. 2017, 21, 57–80. [Google Scholar] [CrossRef]
- Wodniecka, Z.; Szewczyk, J.; Kałamała, P.; Mandera, P.; Durlik, J. When a second language hits a native language. What ERPs (do and do not) tell us about language retrieval difficulty in bilingual language production. Neuropsychologia 2020, 141, 107390. [Google Scholar] [CrossRef] [PubMed]
- Kałamała, P.; Walther, J.; Zhang, H.; Diaz, M.; Senderecka, M.; Wodniecka, Z. The use of a second language enhances the neural efficiency of inhibitory control: An ERP study. Biling. Lang. Cogn. 2022, 25, 163–180. [Google Scholar] [CrossRef]
- Heidlmayr, K.; Kihlstedt, M.; Isel, F. A review on the electroencephalography markers of Stroop executive control processes. Brain Cogn. 2020, 146, 105637. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ji, Y.; Qu, H.; Zuo, S.; Liang, J.; Su, J.; Wang, Q.; Yan, G.; Ding, G. Transcranial magnetic stimulation of the right inferior frontal gyrus impairs bilinguals’ performance in language-switching tasks. Cognition 2025, 254, 105963. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Chen, W.-W.; Zhang, X. The neurophysiology of P300—An integrated review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1480–1488. [Google Scholar]
- Jiao, L.; Gao, Y.; Schwieter, J.W.; Li, L.; Zhu, M.; Liu, C. Control mechanisms in voluntary versus mandatory language switching: Evidence from ERPs. Int. J. Psychophysiol. 2022, 178, 43–50. [Google Scholar] [CrossRef]
- Declerck, M.; Koch, I.; Duñabeitia, J.A.; Grainger, J.; Stephan, D.N. What absent switch costs and mixing costs during bilingual language comprehension can tell us about language control. J. Exp. Psychol. Hum. Percept. Perform. 2019, 45, 771. [Google Scholar] [CrossRef]
- Palmer, S.D.; van Hooff, J.C.; Havelka, J. Language representation and processing in fluent bilinguals: Electrophysiological evidence for asymmetric mapping in bilingual memory. Neuropsychologia 2010, 48, 1426–1437. [Google Scholar] [CrossRef]

| Characteristic | L1 Uyghur | L2 Chinese | L3 English |
|---|---|---|---|
| Age of acquisition (years) M (SD) | 0.68 (1.80) | 5.7 (3.26) | 11.87 (3.40) |
| Exposure (years) M (SD) | 13.46 (5.70) | 6.61 (5.41) | 0 (0) |
| Usage (years) M (SD) | 19.02 (2.60) | 13.94 (3.27) | 8.00 (3.75) |
| Home Use M (SD) | 2.63 (0.76) | 1.45 (0.83) | 0.92 (0.68) |
| Social Use M (SD) | 0.84 (0.79) | 3.12 (0.78) | 1.04 (0.80) |
| Self-ratings of proficiency | |||
| Speaking M (SD) | 9.03 (1.24) | 8.86 (1.20) | 5.48 (1.62) |
| Listening M (SD) | 9.00 (1.22) | 9.10 (1.07) | 5.86 (1.20) |
| Reading M (SD) | 7.65 (2.61) | 9.02 (1.11) | 6.59 (1.69) |
| Writing M (SD) | 6.95 (3.21) | 9.05 (1.12) | 5.97 (1.99) |
| MINT score M (SD) | 63.0 (1.92) | 62.8 (2.10) | 49.8 (3.80) |
| Learning contexts | |||
| Home-only Learning N (%) | 22 (61.11%) | 0 (0%) | 0 (0%) |
| School-only Learning N (%) | 0 (0%) | 30 (83.33%) | 36 (100%) |
| both N (%) | 14 (38.89%) | 6 (16.67%) | 0 (0%) |
| Medium-of-instruction | |||
| Uyghur N (%) | n/a | 0 (0%) | 0 (0%) |
| Chinese N (%) | n/a | 36 (100%) | 36 (100%) |
| L1–L2 Context | L1–L3 Context | L2–L3 Context | |
|---|---|---|---|
| to Dominant language | |||
| Repeat M (SE) | 1559 (9.39) | 1558 (9.56) | 1545 (9.23) |
| Switch M (SE) | 1561 (9.43) | 1562 (9.39) | 1565 (9.28) |
| Switching cost M (SE) | 2 (9.41) | 4 (9.47) | 20 (9.25) |
| to Non-dominant language | |||
| Repeat M (SE) | 1563 (9.40) | 1579 (9.53) | 1571 (9.79) |
| Switch M (SE) | 1568 (9.36) | 1577 (9.41) | 1579 (9.46) |
| Switching cost M (SE) | 5 (9.38) | −2 (9.47) | 8 (9.62) |
| Language dominance effect M (SE) | 11 (9.40) | 36 (9.47) | 40 (9.44) |
| L1–L2 Context | L1–L3 Context | L2–L3 Context | |
|---|---|---|---|
| to Dominant language | |||
| Repeat M (SE) | 0.973 (0.0085) | 0.972 (0.008) | 0.981 (0.006) |
| Switch M (SE) | 0.959 (0.012) | 0.983 (0.005) | 0.981 (0.006) |
| Switching cost M (SE) | −0.014 (0.010) | −0.011 (0.007) | 0 (0.006) |
| to Non-dominant language | |||
| Repeat M (SE) | 0.975 (0.008) | 0.975 (0.007) | 0.963 (0.011) |
| Switch M (SE) | 0.971 (0.009) | 0.973 (0.008) | 0.969 (0.009) |
| Switching cost M (SE) | −0.004 (0.008) | −0.002 (0.008) | 0.006 (0.010) |
| Language dominance effect M (SE) | 0.014 (0.009) | −0.007 (0.007) | −0.03 (0.008) |
| Outcome | d.s1 | d.s2 | a | ter | z | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | b | t | b | t | b | t | b | t | b | t |
| LPC | −0.023 | −0.433, p = 0.667 | 0.0457 | 1.0543, p = 0.295 | −0.0606 | −1.02, p = 0.1 | 0.01102 | 1.649, p = 0.104 | −0.0107 | −3.41, p = 0.001 |
| Context (L1–L3 vs. L1–L2) | 0.958 | 1.745, p = 0.352 | −0.9875 | −2.05, p = 0.047 | 0.993 | 1.607, p = 0.112 | −0.08316 | −1.855, p = 0.068 | 0.0923 | 2.6107, p = 0.011 |
| Context (L2–L3 vs. L1–L2) | 0.5197 | 0.937, p = 0.085 | −1.374 | −2.52, p = 0.014 | 1.5658 | 2.194, p = 0.032 | −0.12167 | −2.4862, p = 0.015 | 0.05 | 1.4019, p = 0.165 |
| Context (L1–L3 vs. L2–L3) | 0.4383 | 0.79, p = 0.432 | 0.891 | 1.59, p = 0.116 | 0.891 | 1.59, p = 0.116 | −0.01189 | −0.235, p = 0.815 | 0.01766 | −1.964, p = 0.685 |
| N200 | 0.0365 | 1.03, p = 0.304 | −0.0154 | −0.5, p = 0.619 | −0.0199 | −0.502, p = 0.617 | 0.00376 | 1.409, p = 0.163 | −0.0789 | −0.0347, p = 0.972 |
| N200 × Context (L1–L3 vs. L1–L2) | 0.00941 | 0.1043, p = 0.917 | −0.0857 | −1.147, p = 0.256 | 0.0425 | 0.435, p = 0.665 | 0.00364 | 0.539, p = 0.592 | −0.00163 | −0.282, p = 0.778 |
| N200 × Context (L2–L3 vs. L1–L2) | −0.10026 | −1.101, p = 0.275 | 0.178 | 2.35, p = 0.021 | −0.2414 | −2.446, p = 0.017 | 0.01108 | 1.622, p = 0.109 | −0.00854 | −1.461, p = 0.149 |
| N200 × Context (L1–L3 vs. L2–L3) | 0.10966 | 0.9944, p = 0.324 | −0.263 | −2.88, p = 0.005 | 0.284 | 2.376, p = 0.02 | −0.00744 | −0.899, p = 0.372 | 0.0069 | 0.975, p = 0.333 |
| N400 | 0.0534 | 1.018, p = 0.312 | 0.0185 | 0.564, p = 0.575 | −0.0541 | −1.284, p = 0.203 | 0.00627 | 2.24, p = 0.028 | 0.00419 | 1.295, p = 0.2 |
| N400 × Context (L1–L3 vs. L1–L2) | −0.0389 | −0.42, p = 0.676 | 0.00778 | 0.101, p = 0.92 | −0.0647 | −0.669, p = 0.505 | 0.00732 | 1.1387, p = 0.259 | −0.00395 | −0.693, p = 0.491 |
| N400 × Context (L2–L3 vs. L1–L2) | −0.1157 | −1.203, p = 0.233 | 0.2089 | 2.609, p = 0.011 | −0.3246 | −3.231, p = 0.002 | 0.02127 | 3.1808, p = 0.002 | −0.01599 | −2.697, p = 0.009 |
| N400 × Context (L1–L3 vs. L2–L3) | 0.0769 | 0.6927, p = 0.491 | −0.201 | −2.18, p = 0.033 | 0.26 | 2.24, p = 0.028 | −0.0139 | −1.809, p = 0.075 | 0.012 | 1.761, p = 0.083 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Meng, Y.; Yang, Q.; Wang, R. Multivariate Decoding and Drift-Diffusion Modeling Reveal Adaptive Control in Trilingual Comprehension. Brain Sci. 2025, 15, 1046. https://doi.org/10.3390/brainsci15101046
Wang Y, Meng Y, Yang Q, Wang R. Multivariate Decoding and Drift-Diffusion Modeling Reveal Adaptive Control in Trilingual Comprehension. Brain Sciences. 2025; 15(10):1046. https://doi.org/10.3390/brainsci15101046
Chicago/Turabian StyleWang, Yuanbo, Yingfang Meng, Qiuyue Yang, and Ruiming Wang. 2025. "Multivariate Decoding and Drift-Diffusion Modeling Reveal Adaptive Control in Trilingual Comprehension" Brain Sciences 15, no. 10: 1046. https://doi.org/10.3390/brainsci15101046
APA StyleWang, Y., Meng, Y., Yang, Q., & Wang, R. (2025). Multivariate Decoding and Drift-Diffusion Modeling Reveal Adaptive Control in Trilingual Comprehension. Brain Sciences, 15(10), 1046. https://doi.org/10.3390/brainsci15101046





