Optimizing Neurobehavioral Assessment for Patients with Disorders of Consciousness: Proposal of a Comprehensive Pre-Assessment Checklist for Clinicians
Abstract
1. Introduction
2. Methods
2.1. Study Design
- (a)
- Checklist development. A group of 12 members from the Special Interest Group on DoC of the International Brain Injury Association (IBIA DoC-SIG) developed the checklist. The members of this group are international experts on DoC including physicians, neuropsychologists, occupational therapists, and physical therapists practicing in various locations mostly around the United States and Europe. The members were asked to review existing validated tools recommended by relevant professional association [17], as well as recent guidelines regarding the assessment of DoC [15,16]. The material was reviewed and discussed over recurrent virtual meetings (between April 2022 and July 2024) to guide the development and inclusion of the checklist items. A series of items were identified as essential for assessing the potential impact of patient- and environment-related biases on assessment results. Table 1 highlights the Category Domains of the Checklist. Pre-assessment checklist items included patient’s personal information, medical conditions and confounds, assessment location and scale used, patient and environmental considerations prior to starting the assessment, and patient testing position. The finalized checklist was three pages in length. A draft for the checklist was submitted to the group and a consensus among experts was reached for the final version (Supplemental Material S1)
- (b)
- Survey development. A survey was created on Google forms by the same panel of experts to investigate clinicians’ perception of using the checklist. Survey items were agreed upon through panel consensus over additional virtual meetings. The final survey included nineteen questions to elicit feedback on the feasibility and use of the checklist (Supplemental Material S2).
2.2. Sample
2.3. Data Analysis
3. Results
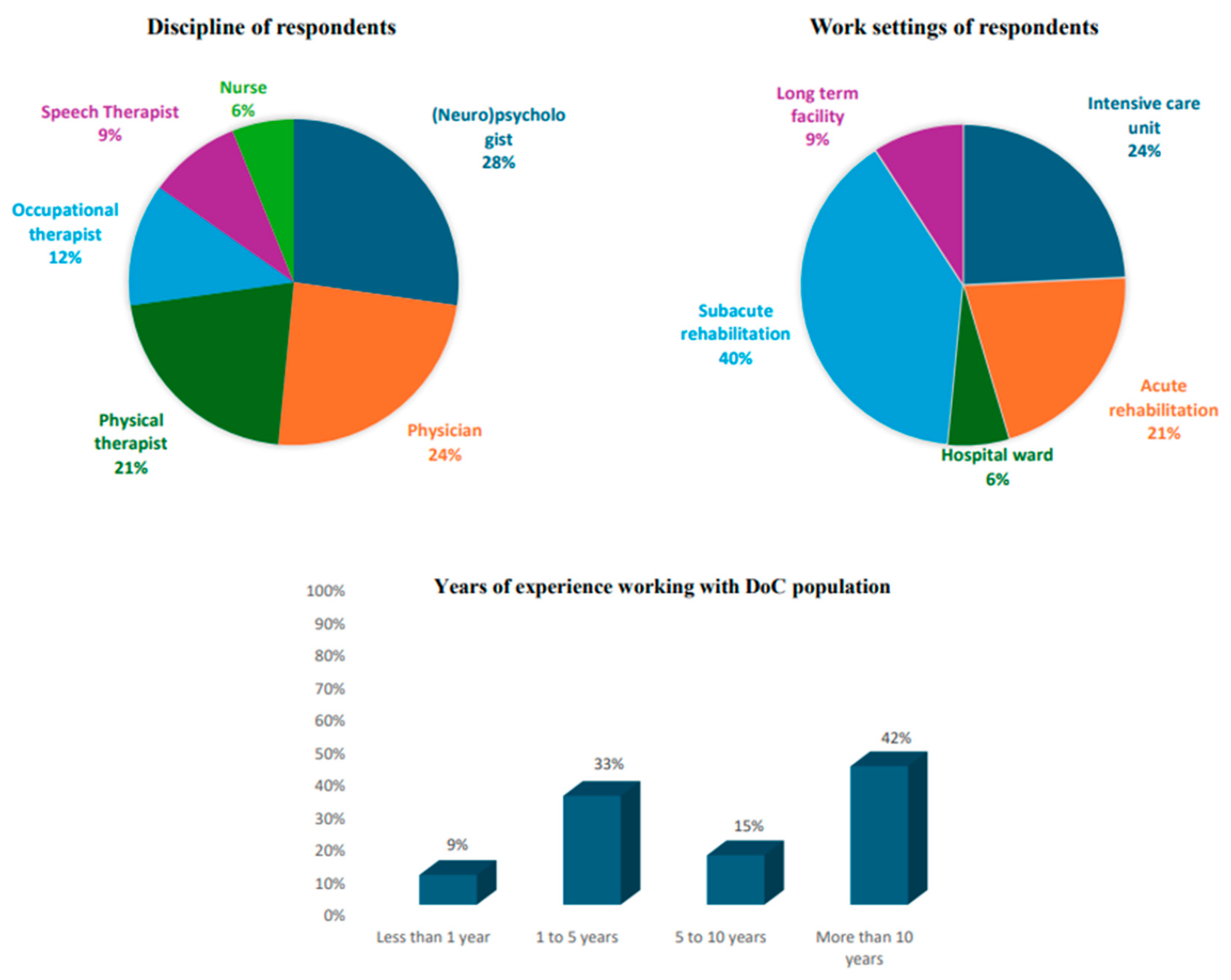
3.1. Respondent Characteristics
3.2. Qualitative Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giacino, J.T. The vegetative and minimally conscious states: Consensus-based criteria for establishing diagnosis and prognosis. NeuroRehabilitation 2005, 19, 293–298. [Google Scholar] [CrossRef]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Jennett, B. The vegetative state. J. Neurol. Neurosurg. Psychiatry 2002, 73, 355–357. [Google Scholar] [CrossRef]
- Thibaut, A.; Bodien, Y.G.; Laureys, S.; Giacino, J.T. Minimally conscious state “plus”: Diagnostic criteria and relation to functional recovery. J. Neurol. 2020, 267, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Llorens, R.; Ippoliti, C.; Navarro, M.D.; Colomer, C.; Maza, A.; Goizueta, S.; Olaya, J.; Moliner, B.; Ferri, J.; Noé, E. Minimally conscious state plus versus minus: Likelihood of emergence and long-term functional independence. Ann. Clin. Transl. Neurol. 2024, 11, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Bodien, Y.G.; Katz, D.I.; Schiff, N.D.; Giacino, J.T. Behavioral Assessment of Patients with Disorders of Consciousness. Semin. Neurol. 2022, 42, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Helbok, R.; Rass, V.; Beghi, E.; Bodien, Y.G.; Citerio, G.; Giacino, J.T.; Kondziella, D.; Mayer, S.A.; Menon, D.; Sharshar, T.; et al. The curing coma campaign international survey on coma epidemiology, evaluation and therapy (COME TOGETHER). Neurocritical Care 2022, 37, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, M.K.; Lazaridis, C. Conceptualizing Consciousness: A Change in Perspective: The elephant still surprises those only touching its trunk. Phys. Med. Rehabil. Clin. N. Am. 2024, 35, 1–13. [Google Scholar] [CrossRef] [PubMed]
- O’brien, K.; Zhang, B.; Anderl, E.; Kothari, S. Special Considerations in Behavioral Assessments for Disorders of Consciousness. Phys. Med. Rehabil. Clin. N. Am. 2024, 35, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Schnakers, C.; Vanhaudenhuyse, A.; Giacino, J.; Ventura, M.; Boly, M.; Majerus, S.; Moonen, G.; Laureys, S. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, A.F.; Lauzier, F.; Simard, J.-F.; Scales, D.C.; Burns, K.E.; Moore, L.; Zygun, D.A.; Bernard, F.; Meade, M.O.; Dung, T.C.; et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: A Canadian multicentre cohort study. Can. Med. Assoc. J. 2011, 183, 1581–1588. [Google Scholar] [CrossRef]
- Fins, J.J. Rights Come To Mind: Brain Injury, Ethics, and the Struggle for Consciousness; Cambridge University Press: New York, NY, USA, 2015. [Google Scholar]
- Fins, J.J.; Bernat, J.L. Ethical, palliative, and policy considerations in disorders of consciousness. Neurology 2018, 91, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.R.; Barber, J.K.; Temkin, N.R.; Foreman, B.; Giacino, J.T.; Williamson, T.; Edlow, B.L.; Manley, G.T.; Bodien, Y.G. Recovery Potential in Patients Who Died After Withdrawal of Life-Sustaining Treatment: A TRACK-TBI Propensity Score Analysis. J. Neurotrauma 2024, 41, 2336–2348. [Google Scholar] [CrossRef]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018, 91, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Kondziella, D.; Bender, A.; Diserens, K.; van Erp, W.; Estraneo, A.; Formisano, R.; Laureys, S.; Naccache, L.; Ozturk, S.; Rohaut, B.; et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol. 2020, 27, 741–756. [Google Scholar] [CrossRef]
- American Congress of Rehabilitation Medicine; Brain Injury-Interdisciplinary Special Interest Group; Disorders of Consciousness Task Force; Seel, R.T.; Sherer, M.; Whyte, J.; Katz, D.I.; Giacino, J.T.; Rosenbaum, A.M.; Hammond, F.M.; et al. Assessment Scales for Disorders of Consciousness: Evidence-Based Recommendations for Clinical Practice and Research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813. [Google Scholar] [CrossRef]
- Løvstad, M.; Frøslie, K.F.; Giacino, J.T.; Skandsen, T.; Anke, A.; Schanke, A.-K. Reliability and Diagnostic Characteristics of the JFK Coma Recovery Scale–Revised: Exploring the influence of rater’s level of experience. J. Head Trauma Rehabil. 2010, 25, 349–356. [Google Scholar] [CrossRef]
- Murtaugh, B.; Rosenbaum, A.S. Clinical application of recommendations for neurobehavioral assessment in disorders of consciousness: An interdisciplinary approach. Front. Hum. Neurosci. 2023, 17, 1129466. [Google Scholar] [CrossRef]
- Whyte, J.; Nakase-Richardson, R. Disorders of Consciousness: Outcomes, Comorbidities, and Care Needs. Arch. Phys. Med. Rehabil. 2013, 94, 1851–1854. [Google Scholar] [CrossRef]
- Formisano, R.; Giustini, M.; Aloisi, M.; Contrada, M.; Schnakers, C.; Zasler, N.; Estraneo, A. An International survey on diagnostic and prognostic protocols in patients with disorder of consciousness. Brain Inj. 2019, 33, 974–984. [Google Scholar] [CrossRef]
- Aubinet, C.; Cassol, H.; Bodart, O.; Sanz, L.R.; Wannez, S.; Martial, C.; Thibaut, A.; Martens, G.; Carrière, M.; Gosseries, O.; et al. Simplified evaluation of CONsciousness disorders (SECONDs) in individuals with severe brain injury: A validation study. Ann. Phys. Rehabil. Med. 2021, 64, 101432. [Google Scholar] [CrossRef]
- Farisco, M.; Formisano, R.; Gosseries, O.; Kato, Y.; Koboyashi, S.; Laureys, S.; Lejeune, N.; Martial, C.; Matar, A.; Morrisey, A.-M.; et al. International survey on the implementation of the European and American guidelines on disorders of consciousness. J. Neurol. 2023, 271, 395–407. [Google Scholar] [CrossRef]
- Chaturvedi, J.; Mudgal, S.K.; Venkataram, T.; Gupta, P.; Goyal, N.; Jain, G.; Sharma, A.K.; Sharma, S.K.; Bendok, B.R. Coma recovery scale: Key clinical tool ignored enough in disorders of consciousness. Surg. Neurol. Int. 2021, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Bodien, Y.G.; Vora, I.; Barra, A.; Chiang, K.; Chatelle, C.; Goostrey, K.; Martens, G.; Malone, C.; Mello, J.; Parlman, K.; et al. Feasibility and Validity of the Coma Recovery Scale-Revised for Accelerated Standardized Testing: A Practical Assessment Tool for Detecting Consciousness in the Intensive Care Unit. Ann. Neurol. 2023, 94, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Pape, T.L.-B.; Mallinson, T.; Guernon, A. Psychometric Properties of the Disorders of Consciousness Scale. Arch. Phys. Med. Rehabil. 2014, 95, 1672–1684. [Google Scholar] [CrossRef]
- Bellon, K.; Wright, J.; Jamison, L.; Kolakowsky-Hayner, S. Disability Rating Scale. J. Head Trauma Rehabil. 2012, 27, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Shiel, A.; Horn, S.A.; Wilson, B.A.; Watson, M.J.; Campbell, M.J.; Mclellan, D.L. The Wessex Head Injury Matrix (WHIM) main scale: A preliminary report on a scale to assess and monitor patient recovery after severe head injury. Clin. Rehabil. 2000, 14, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Chatelle, C.; De Val, M.-D.R.; Catano, A.; Chaskis, C.; Seeldrayers, P.; Laureys, S.; Biston, P.; Schnakers, C. Is the Nociception Coma Scale-Revised a Useful Clinical Tool for Managing Pain in Patients With Disorders of Consciousness? Clin. J. Pain 2016, 32, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Boase, K.; Nelson, L.D.; Temkin, N.R.; Giacino, J.T.; Markowitz, A.J.; Maas, A.; Menon, D.K.; Teasdale, G.; Manley, G.T. A Manual for the Glasgow Outcome Scale-Extended Interview. J. Neurotrauma 2021, 38, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, M. The disability rating and Coma/Near-Coma scales in evaluating severe head injury. Neuropsychol. Rehabil. 2005, 15, 442–453. [Google Scholar] [CrossRef]
- Lin, K.; Wroten, M. Ranchos Los Amigos. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK448151/ (accessed on 11 December 2024).
- Wolffbrandt, M.M.; Poulsen, I.; Engberg, A.W.; Hornnes, N. Occurrence and Severity of Agitated Behavior After Severe Traumatic Brain Injury. Rehabil. Nurs. 2013, 38, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Bodien, Y.G.; Giacino, J.T.; Fins, J.J.; Truog, R.D.; Hochberg, L.R.; Edlow, B.L. The neuroethics of disorders of consciousness: A brief history of evolving ideas. Brain 2021, 144, 3291–3310. [Google Scholar] [CrossRef]
| Category | Included Key Items | Purpose |
|---|---|---|
| Patient Information |
| To ensure an individualized approach to assessment |
| Medical Conditions and Confounds |
| To prompt the use to consider factors that may impact patient’s performance. |
| Location and assessment used |
| To identify assessment and potential variations in performance based on location. |
| Patient and Environmental Considerations |
| To document environmental factors that may influence patient performance and determine optimal testing environment for future assessment. |
| Patient testing position |
| To accurately capture testing position and identify optimal position for arousal. |
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | |
|---|---|---|---|---|---|
| I like the format of the tool | 24% | 54.5% | 18.1% | 0% | 0% |
| The tool was easy to use | 45.5% | 42.4% | 12.1% | 0% | 0% |
| The tool can help me in my practice | 33.3% | 48.5% | 15.2% | 3.0% | 0% |
| I would suggest this tool to colleagues/students | 51.5% | 33.3% | 15.2% | 0% | 0% |
| I notice inconsistencies as I use the tool | 3.0% | 0% | 12.1% | 18.2% | 66.7% |
| Theme | Description |
|---|---|
| Comprehensive | Respondents reported the checklist included most if not all considerations prior to an assessment. |
| Easy to use | Respondents indicated the checklist was logical, straight forward, and easy to use prior to completing an assessment. The check boxes made it easy to use. |
| Consolidation | Respondents indicated that some descriptions were too long and felt the checklist would be more efficient and accessible to complete if more compact. |
| Consideration of patient and environmental factors | Respondents noted that the checklist provides clinicians with a guide to promote optimizing of the patient and testing environment to establish a baseline and track recovery. |
| Standardizes approach | Respondents noted that the checklist is anticipated to increase consistency across the various disciplines of the team. |
| Valuable for training | Respondents reported that this tool would be beneficial in training clinicians with less experience in optimal assessment of patients with DoC. |
| Ease of Use | Helpful in Practice | |
|---|---|---|
| Expertise | ||
| <5 years (n = 14) | 78.6% | 78.6% |
| >5 years (n = 19) | 92.9% | 78.6% |
| Profession | ||
| ST/Psych (n = 12) | 91.7% | 100.0% |
| OT/PT (n = 11) | 85.7% | 78.6% |
| Dr/Nurse (n = 10) | 90.0% | 80.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keech, K.; Schnakers, C.; Murtaugh, B.; O’Brien, K.; Slomine, B.; Briand, M.-M.; Formisano, R.; Thibaut, A.; Estraneo, A.; Noé, E.; et al. Optimizing Neurobehavioral Assessment for Patients with Disorders of Consciousness: Proposal of a Comprehensive Pre-Assessment Checklist for Clinicians. Brain Sci. 2025, 15, 71. https://doi.org/10.3390/brainsci15010071
Keech K, Schnakers C, Murtaugh B, O’Brien K, Slomine B, Briand M-M, Formisano R, Thibaut A, Estraneo A, Noé E, et al. Optimizing Neurobehavioral Assessment for Patients with Disorders of Consciousness: Proposal of a Comprehensive Pre-Assessment Checklist for Clinicians. Brain Sciences. 2025; 15(1):71. https://doi.org/10.3390/brainsci15010071
Chicago/Turabian StyleKeech, Kristen, Caroline Schnakers, Brooke Murtaugh, Katherine O’Brien, Beth Slomine, Marie-Michèle Briand, Rita Formisano, Aurore Thibaut, Anna Estraneo, Enrique Noé, and et al. 2025. "Optimizing Neurobehavioral Assessment for Patients with Disorders of Consciousness: Proposal of a Comprehensive Pre-Assessment Checklist for Clinicians" Brain Sciences 15, no. 1: 71. https://doi.org/10.3390/brainsci15010071
APA StyleKeech, K., Schnakers, C., Murtaugh, B., O’Brien, K., Slomine, B., Briand, M.-M., Formisano, R., Thibaut, A., Estraneo, A., Noé, E., Gosseries, O., & da Conceição Teixeira, L. (2025). Optimizing Neurobehavioral Assessment for Patients with Disorders of Consciousness: Proposal of a Comprehensive Pre-Assessment Checklist for Clinicians. Brain Sciences, 15(1), 71. https://doi.org/10.3390/brainsci15010071







