Increased Growth Differentiation Factor 15 Levels Are Associated with HIV-Associated Neurocognitive Impairment: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immunoblotting
2.3. Immunohistochemistry
2.4. Double-Immunolabeling
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Human Cohort
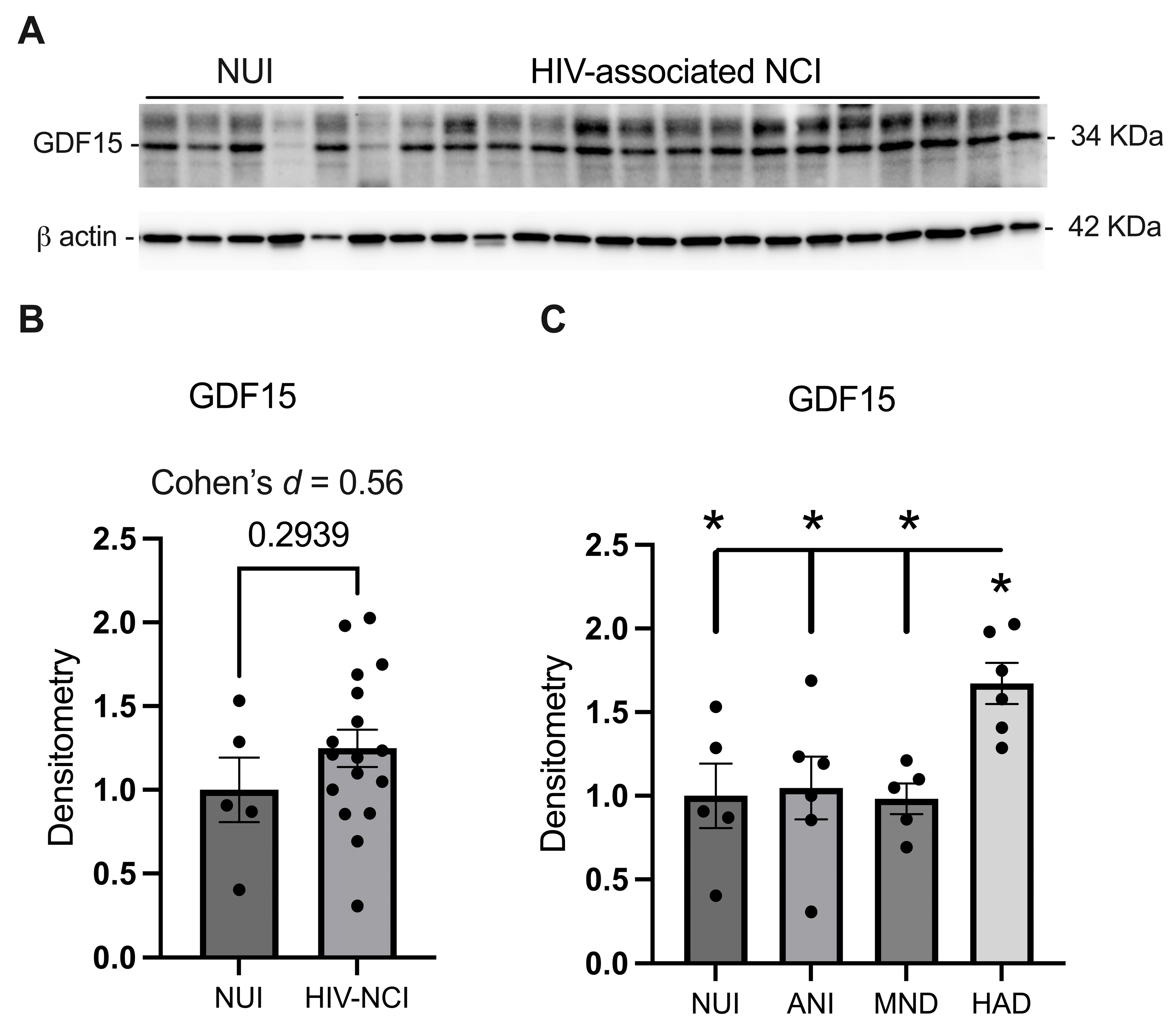
3.2. GDF15 Is Increased in the Frontal Cortex Lysates from PWH with HAD
3.3. GDF15 Is Increased in Gray Matter in the Frontal Cortices of Brain Specimens from PWH with HIV-Associated NCI
3.4. GDF15 Expression Is Increased in Microglia and Neurons in PWH with HIV-Associated NCI
3.5. CSF GDF15 Levels Do Not Show a Statistically Significant Increase in HIV-Associated NCI; However, the Effect Size Analysis Suggests a Moderate Increase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-associated neurocognitive disorder—Pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Killingsworth, L.; Spudich, S. Neuropathogenesis of HIV-1: Insights from across the spectrum of acute through long-term treated infection. Semin. Immunopathol. 2022, 44, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Tavasoli, A.; Gelman, B.B.; Marra, C.M.; Clifford, D.B.; Iudicello, J.E.; Rubin, L.H.; Letendre, S.L.; Tang, B.; Ellis, R.J. Increasing Neuroinflammation Relates to Increasing Neurodegeneration in People with HIV. Viruses 2023, 15, 1835. [Google Scholar] [CrossRef] [PubMed]
- Andalibi, M.S.; Ellis, R.J. The role of immunometabolism in HIV-associated depression and cognitive impairment. In HIV-Associated Neurocognitive Disorders; Elsevier: Amsterdam, The Netherlands, 2024; pp. 161–178. [Google Scholar]
- Bordoni, V.; Sacchi, A.; Casetti, R.; Cimini, E.; Tartaglia, E.; Pinnetti, C.; Mondi, A.; Gruber, C.E.M.; Antinori, A.; Agrati, C. Impact of ART on dynamics of growth factors and cytokines in primary HIV infection. Cytokine 2020, 125, 154839. [Google Scholar] [CrossRef]
- Willig, A.L.; Overton, E.T. Metabolic Complications and Glucose Metabolism in HIV Infection: A Review of the Evidence. Curr. HIV/AIDS Rep. 2016, 13, 289–296. [Google Scholar] [CrossRef]
- McCutchan, J.A.; Marquie-Beck, J.A.; Fitzsimons, C.A.; Letendre, S.L.; Ellis, R.J.; Heaton, R.K.; Wolfson, T.; Rosario, D.; Alexander, T.J.; Marra, C.; et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012, 78, 485–492. [Google Scholar] [CrossRef]
- Sharma, I. Interrogating the impact of combination antiretroviral therapies on HIV-associated neurocognitive disorders. HIV Med. 2021, 22, 783–790. [Google Scholar] [CrossRef]
- Moschopoulos, C.D.; Stanitsa, E.; Protopapas, K.; Kavatha, D.; Papageorgiou, S.G.; Antoniadou, A.; Papadopoulos, A. Multimodal Approach to Neurocognitive Function in People Living with HIV in the cART Era: A Comprehensive Review. Life 2024, 14, 508. [Google Scholar] [CrossRef]
- Elbirt, D.; Mahlab-Guri, K.; Bezalel-Rosenberg, S.; Gill, H.; Attali, M.; Asher, I. HIV-associated neurocognitive disorders (HAND). Isr. Med. Assoc. J. 2015, 17, 54–59. [Google Scholar]
- Carroll, A.; Brew, B. HIV-associated neurocognitive disorders: Recent advances in pathogenesis, biomarkers, and treatment. F1000Res 2017, 6, 312. [Google Scholar] [CrossRef]
- Ruhanya, V.; Jacobs, G.B.; Paul, R.H.; Joska, J.A.; Seedat, S.; Nyandoro, G.; Engelbrecht, S.; Glashoff, R.H. Plasma Cytokine Biomarker Cutoff Values for HIV-Associated Neurocognitive Impairment in Adults. Viral Immunol. 2021, 34, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, J.; Schank, M.; Hill, A.C.; Banik, P.; Zhang, Y.; Wu, X.Y.; Lightner, J.W.; Ning, S.; El Gazzar, M.; et al. Circulating GDF-15: A biomarker for metabolic dysregulation and aging in people living with HIV. Front. Aging 2024, 5, 1414866. [Google Scholar] [CrossRef] [PubMed]
- Domingo, P.; Mateo, M.G.; Villarroya, J.; Cereijo, R.; Torres, F.; Domingo, J.C.; Campderrós, L.; Gallego-Escuredo, J.M.; Gutierrez, M.D.M.; Mur, I.; et al. Increased Circulating Levels of Growth Differentiation Factor 15 in Association with Metabolic Disorders in People Living with HIV Receiving Combined Antiretroviral Therapy. J. Clin. Med. 2022, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Isik, F.I.; Thomson, S.; Cueto, J.F.; Spathos, J.; Breit, S.N.; Tsai, V.W.W.; Brown, D.A.; Finney, C.A. A Systematic Review of the Neuroprotective Role and Biomarker Potential of GDF15 in Neurodegeneration. bioRxiv 2024, 15, 1514518. [Google Scholar] [CrossRef]
- Jiang, W.W.; Zhang, Z.Z.; He, P.P.; Jiang, L.P.; Chen, J.Z.; Zhang, X.T.; Hu, M.; Zhang, Y.K.; Ouyang, X.P. Emerging roles of growth differentiation factor-15 in brain disorders (Review). Exp. Ther. Med. 2021, 22, 1270. [Google Scholar] [CrossRef]
- Xue, X.H.; Tao, L.L.; Su, D.Q.; Guo, C.J.; Liu, H. Diagnostic utility of GDF15 in neurodegenerative diseases: A systematic review and meta-analysis. Brain Behav. 2022, 12, e2502. [Google Scholar] [CrossRef]
- Avdoshina, V.; Fields, J.A.; Castellano, P.; Dedoni, S.; Palchik, G.; Trejo, M.; Adame, A.; Rockenstein, E.; Eugenin, E.; Masliah, E.; et al. The HIV Protein gp120 Alters Mitochondrial Dynamics in Neurons. Neurotox. Res. 2016, 29, 583–593. [Google Scholar] [CrossRef]
- Fields, J.; Dumaop, W.; Eleuteri, S.; Campos, S.; Serger, E.; Trejo, M.; Kosberg, K.; Adame, A.; Spencer, B.; Rockenstein, E.; et al. HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: Implications for HIV-associated neurocognitive disorders. J. Neurosci. 2015, 35, 1921–1938. [Google Scholar] [CrossRef]
- Lockhart, S.M.; Saudek, V.; O’Rahilly, S. GDF15: A Hormone Conveying Somatic Distress to the Brain. Endocr. Rev. 2020, 41, bnaa007. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. Cancer cell immune escape and tumor progression by exploitation of anti-inflammatory and pro-inflammatory responses. Cancer Biol. Ther. 2005, 4, 924–933. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Investig. 2011, 41, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Schmidt-Bleek, K.; Dienelt, A.; Reinke, P.; Volk, H.D. Regulatory T cell-mediated anti-inflammatory effects promote successful tissue repair in both indirect and direct manners. Front. Pharmacol. 2015, 6, 184. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Holzheimer, R.G.; Steinmetz, W. Local and systemic concentrations of pro- and anti-inflammatory cytokines in human wounds. Eur. J. Med. Res. 2000, 5, 347–355. [Google Scholar]
- Yin, C.; Zhao, Q.; Li, W.; Zhao, Z.; Wang, J.; Deng, T.; Zhang, P.; Shen, K.; Li, Z.; Zhang, Y. Biomimetic anti-inflammatory nano-capsule serves as a cytokine blocker and M2 polarization inducer for bone tissue repair. Acta Biomater. 2020, 102, 416–426. [Google Scholar] [CrossRef]
- Luan, H.H.; Wang, A.; Hilliard, B.K.; Carvalho, F.; Rosen, C.E.; Ahasic, A.M.; Herzog, E.L.; Kang, I.; Pisani, M.A.; Yu, S.; et al. GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell 2019, 178, 1231–1244.e1211. [Google Scholar] [CrossRef]
- Herder, C.; Carstensen, M.; Ouwens, D.M. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 39–50. [Google Scholar] [CrossRef]
- Li, H.; Tang, D.; Chen, J.; Hu, Y.; Cai, X.; Zhang, P. The Clinical Value of GDF15 and Its Prospective Mechanism in Sepsis. Front. Immunol. 2021, 12, 710977. [Google Scholar] [CrossRef]
- Conte, M.; Martucci, M.; Mosconi, G.; Chiariello, A.; Cappuccilli, M.; Totti, V.; Santoro, A.; Franceschi, C.; Salvioli, S. GDF15 Plasma Level Is Inversely Associated With Level of Physical Activity and Correlates With Markers of Inflammation and Muscle Weakness. Front. Immunol. 2020, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Insights Into Mechanisms of GDF15 and Receptor GFRAL: Therapeutic Targets. Trends Endocrinol. Metab. 2020, 31, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.; Zahabi, A.; Hart, R.A. GDF15, an update of the physiological and pathological roles it plays: A review. Pflugers Arch. 2020, 472, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.N.; Brown, D.A.; Tsai, V.W. The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe? Annu. Rev. Physiol. 2021, 83, 127–151. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjær, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Crawley, S.; Chen, M.; Ayupova, D.A.; Lindhout, D.A.; Higbee, J.; Kutach, A.; Joo, W.; Gao, Z.; Fu, D.; et al. Erratum: Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 2017, 551, 398. [Google Scholar] [CrossRef]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef]
- Woods, S.P.; Rippeth, J.D.; Frol, A.B.; Levy, J.K.; Ryan, E.; Soukup, V.M.; Hinkin, C.H.; Lazzaretto, D.; Cherner, M.; Marcotte, T.D.; et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J. Clin. Exp. Neuropsychol. 2004, 26, 759–778. [Google Scholar] [CrossRef]
- Ellis, R.J.; Joseph, J.; de Almeida, S.M. NeuroAIDS in Brazil. J. Neurovirol. 2007, 13, 89–96. [Google Scholar] [CrossRef]
- Tu, W.; Chen, P.A.; Koenig, N.; Gomez, D.; Fujiwara, E.; Gill, M.J.; Kong, L.; Power, C. Machine learning models reveal neurocognitive impairment type and prevalence are associated with distinct variables in HIV/AIDS. J. Neurovirol. 2020, 26, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.J. Cohen’s d. In The Corsini Encyclopedia of Psychology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 1. [Google Scholar]
- Siddiqui, J.A.; Pothuraju, R.; Khan, P.; Sharma, G.; Muniyan, S.; Seshacharyulu, P.; Jain, M.; Nasser, M.W.; Batra, S.K. Pathophysiological role of growth differentiation factor 15 (GDF15) in obesity, cancer, and cachexia. Cytokine Growth Factor. Rev. 2022, 64, 71–83. [Google Scholar] [CrossRef]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Silva-Bermudez, L.S.; Klüter, H.; Kzhyshkowska, J.G. Macrophages as a Source and Target of GDF-15. Int. J. Mol. Sci. 2024, 25, 7313. [Google Scholar] [CrossRef]
- Erra Diaz, F.; Mazzitelli, I.; Bleichmar, L.; Melucci, C.; Thibodeau, A.; Dalotto Moreno, T.; Marches, R.; Rabinovich, G.A.; Ucar, D.; Geffner, J. Concomitant inhibition of PPARγ and mTORC1 induces the differentiation of human monocytes into highly immunogenic dendritic cells. Cell Rep. 2023, 42, 112156. [Google Scholar] [CrossRef]
- Reyes, J.; Yap, G.S. Emerging Roles of Growth Differentiation Factor 15 in Immunoregulation and Pathogenesis. J. Immunol. 2023, 210, 5–11. [Google Scholar] [CrossRef]
- Roth, P.; Junker, M.; Tritschler, I.; Mittelbronn, M.; Dombrowski, Y.; Breit, S.N.; Tabatabai, G.; Wick, W.; Weller, M.; Wischhusen, J. GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin. Cancer Res. 2010, 16, 3851–3859. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, Y.; Zhao, S.; Qian, L.; Song, T.; Zheng, J.; Zhang, J.; Chen, B. Growth differentiation factor-15 promotes immune escape of ovarian cancer via targeting CD44 in dendritic cells. Exp. Cell Res. 2021, 402, 112522. [Google Scholar] [CrossRef]
- Han, B.; He, J.; Chen, Q.; Yuan, M.; Zeng, X.; Li, Y.; Zeng, Y.; He, M.; Zhou, Q.; Feng, D.; et al. ELFN1-AS1 promotes GDF15-mediated immune escape of colorectal cancer from NK cells by facilitating GCN5 and SND1 association. Discov. Oncol. 2023, 14, 56. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, D.; Schaefer, N.R.; Harmacek, L.; O’Connor, B.P.; Eling, T.E.; Eickelberg, O.; Chu, H.W. Overproduction of growth differentiation factor 15 promotes human rhinovirus infection and virus-induced inflammation in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L514–L527. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Marjono, B.; Brown, D.A.; Mulvey, S.; Breit, S.N.; Manuelpillai, U.; Wallace, E.M. Serum concentrations of macrophage inhibitory cytokine 1 (MIC 1) as a predictor of miscarriage. Lancet 2004, 363, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Kaitu’u-Lino, T.J.; Bambang, K.; Onwude, J.; Hiscock, R.; Konje, J.; Tong, S. Plasma MIC-1 and PAPP-a levels are decreased among women presenting to an early pregnancy assessment unit, have fetal viability confirmed but later miscarry. PLoS ONE 2013, 8, e72437. [Google Scholar] [CrossRef] [PubMed]
- Skubisz, M.M.; Brown, J.K.; Tong, S.; Kaitu’u-Lino, T.; Horne, A.W. Maternal Serum Macrophage Inhibitory Cytokine-1 as a Biomarker for Ectopic Pregnancy in Women with a Pregnancy of Unknown Location. PLoS ONE 2013, 8, e66339. [Google Scholar] [CrossRef]
- Yi, M.H.; Zhang, E.; Baek, H.; Kim, S.; Shin, N.; Kang, J.W.; Lee, S.; Oh, S.H.; Kim, D.W. Growth Differentiation Factor 15 Expression in Astrocytes After Excitotoxic Lesion in the Mouse Hippocampus. Exp. Neurobiol. 2015, 24, 133–138. [Google Scholar] [CrossRef]
- Chiariello, A.; Valente, S.; Pasquinelli, G.; Baracca, A.; Sgarbi, G.; Solaini, G.; Medici, V.; Fantini, V.; Poloni, T.E.; Tognocchi, M.; et al. The expression pattern of GDF15 in human brain changes during aging and in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1058665. [Google Scholar] [CrossRef]
- Adela, R.; Banerjee, S.K. GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. J. Diabetes Res. 2015, 2015, 490842. [Google Scholar] [CrossRef]



| Sex (f/m) | Age | ART Duration (Months) | Plasma Viral Load | CD4 | |||
|---|---|---|---|---|---|---|---|
| Brain cohort | NUI | 0/5 | 44.4 ± 7.6 | 11 ± 16.3 | 3.5 ± 0.6 | 196.2 ± 156.9 | |
| HIV-associated NCI | 4/12 | 39.5 ± 4.4 | 7.4 ± 10.6 | 5.3 ± 0.8 | 40.3 ± 30.4 | ||
| HAND | ANI | 2/3 | 37 ± 6.7 | 4.1 ± 4.1 | 5.3 ± 0.8 | 29.8 ± 32.8 | |
| MND | 1/4 | 35.6 ± 8.9 | 13.4 ± 17.3 | 5.7 ± 0.9 | 7.2 ± 5.9 | ||
| HAD | 1/5 | 45.2 ± 8.2 | 4.6 ± 6.7 | 5.4 ± 0.8 | 78.4 ± 115.9 | ||
| CSF cohort | NUI | 1/8 | 43.7 ± 7.4 | 4.3 ± 4.1 | 5.6 ± 1.7 | 54.2 ± 102.1 | |
| HIV-associated NCI | 4/16 | 43.7 ± 9.8 | 5.9 ± 6.4 | 5.5 ± 1.6 | 146.5 ± 243.7 | ||
| HAND | ANI | 2/7 | 44.5 ± 13.4 | 6.3 ± 7.3 | 5.0 ± 1.5 | 153.6 ± 216.8 | |
| MND | 1/5 | 42.6 ± 3.8 | 6.6 ± 5.5 | 5.7 ± 0.9 | 183.6 ± 349.1 | ||
| HAD | 1/4 | 43.6 ± 9.0 | 4.2 ± 6.7 | 5.7 ± 1.8 | 75.0 ± 134.7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boustani, A.; Ford, M.K.; Kulbe, J.R.; Laird, A.E.; Shu, L.; Spencer, M.; Avalos, B.; Walter, K.C.; Ellis, R.J.; Fields, J.A. Increased Growth Differentiation Factor 15 Levels Are Associated with HIV-Associated Neurocognitive Impairment: A Pilot Study. Brain Sci. 2025, 15, 49. https://doi.org/10.3390/brainsci15010049
Boustani A, Ford MK, Kulbe JR, Laird AE, Shu L, Spencer M, Avalos B, Walter KC, Ellis RJ, Fields JA. Increased Growth Differentiation Factor 15 Levels Are Associated with HIV-Associated Neurocognitive Impairment: A Pilot Study. Brain Sciences. 2025; 15(1):49. https://doi.org/10.3390/brainsci15010049
Chicago/Turabian StyleBoustani, Ali, Mary K. Ford, Jacqueline R. Kulbe, Anna E. Laird, Leeann Shu, Matthew Spencer, Bryant Avalos, Kyle C. Walter, Ronald J. Ellis, and Jerel Adam Fields. 2025. "Increased Growth Differentiation Factor 15 Levels Are Associated with HIV-Associated Neurocognitive Impairment: A Pilot Study" Brain Sciences 15, no. 1: 49. https://doi.org/10.3390/brainsci15010049
APA StyleBoustani, A., Ford, M. K., Kulbe, J. R., Laird, A. E., Shu, L., Spencer, M., Avalos, B., Walter, K. C., Ellis, R. J., & Fields, J. A. (2025). Increased Growth Differentiation Factor 15 Levels Are Associated with HIV-Associated Neurocognitive Impairment: A Pilot Study. Brain Sciences, 15(1), 49. https://doi.org/10.3390/brainsci15010049







