Abstract
Obsessive–compulsive disorder (OCD) is a prevalent, chronic, and severe neuropsychiatric disorder that leads to illness-related disability. Despite the availability of several treatments, many OCD patients respond inadequately, because the underlying neural mechanisms remain unclear, necessitating the establishment of many animal models, particularly mouse models, to elucidate disease mechanisms and therapeutic strategies better. Although the development of animal models is ongoing, there remain many comprehensive summaries and updates in recent research, hampering efforts to develop novel treatments and enhance existing interventions. This review summarizes the phenotypes of several commonly used models and mechanistic insights from transgenic models of OCD, such as knockout mouse models. In addition, we present the advantages and limitations of these models and discuss their future in helping further understand the pathophysiology and advanced treatment. Here, we highlight current frontline treatment approaches for OCD, including neuromodulation and surgical interventions, and propose potential future directions. By studying gene mutations and observing phenotypes from available OCD animal models, researchers have classified the molecular signatures of each model reminiscent of changes in brain areas and neural pathways, with the hope of guiding the future selection of the most appropriate models for specific research in the OCD field.
1. Introduction
Obsessive–compulsive disorder (OCD) is a prevalent neuropsychiatric condition, with a lifetime prevalence estimated to range from 1% to 3% [1,2]. This prevalence remains consistent across various countries. During childhood and adolescence, about half of patients experience symptoms [3]. Moreover, nearly 90% of individuals with OCD also have comorbid psychiatric conditions, with anxiety disorders being the most prevalent [4]. The primary clinical features of this disorder include conscious compulsive behaviors and obsessive thoughts. Patients frequently encounter repetitive intrusive thoughts or behaviors, some of which may be meaningless or contrary to their intentions. These experiences result in distress, anxiety and aversion, and put a substantial burden on their families and society [5].
As the primary treatment option, OCD patients typically undergo cognitive–behavioral therapy (CBT) and receive medication, particularly, selective serotonin reuptake inhibitors (SSRIs) [6,7,8]. However, initially, these interventions yield limited efficacy, with almost 50% of OCD patients not responding adequately [9]. This underscores the urgent need to develop novel treatments or more efficacious disease-modifying therapies for OCD. Although gene therapy is a promising treatment approach, the identification of risk genes for OCD lags significantly behind that for other mental disorders [10,11], due in part to the lack of comprehensive whole-genome studies and the need for a deeper understanding of OCD’s neurobiological and genetic foundations [12].
Given the urgency for more efficient and complementary therapeutic strategies, the validation of animal models with translational relevance is crucial in the field [12]. In recent years, animal models, specifically mouse models, mainly because of their applications in gene mutation studies, have played a crucial role in genomics research. These models are important for exploring the complex pathophysiological basis of various diseases and elucidate the genetic and neural circuit mechanisms.
In OCD research, these models have shed light on previously obscure aspects of the disorder, thereby substantiating their critical importance. They are independent of both environmental influences and genetic variations [13], thus facilitating targeted exploration of the neural circuits associated with OCD. By parsing OCD-related behaviors, such as compulsions and anxiety, into specific neurobiological components, these models become invaluable tools for analysis. At the same time, they reveal molecular pathways associated with the cortico-striato-thalamo-cortical (CSTC) circuit—an area implicated in OCD. In addition, some relevant animal models have been corroborated by both genetic and imaging evidence, confirming their efficacy in investigating pathogenesis. This has led to the development of multiple investigative strategies using gene knockout animal models, significantly enriching our comprehension of the origins and progression of OCD, and potentially paving the way for innovative treatments for it and similar disorders.
Without attempting to review in detail all of the facets of OCD, we have summarized a few well-established and commonly used transgenic models, such as knockout (KO) models. For transgenic mouse models, Hoxb8-KO, Slc1a1-KO, Sapap3-KO, Slitrk5-KO, and Spred2-KO mouse models have been recently studied. We performed a meticulous comparison among the prevalent OCD mouse models, including phenotypic characterization and underlying mechanisms. In addition, advancements in neuromodulatory and surgical techniques, such as deep brain stimulation (DBS), have shown promise in addressing treatment-resistant OCD. By targeting some specific brain areas, these approaches help patients to go into remission and enhance the quality of life for affected individuals.
Given the interactions with genetic, neural, and environmental factors in OCD, researchers have focused on the future needs of developing novel model systems for OCD. Therefore, understanding the phenotypic characteristics and underlying mechanisms of OCD mouse models helps us translate findings from animal research to human clinical contexts, and contributes to diagnostic and therapeutic outcomes for OCD patients.
2. Biological Basis of OCD
2.1. Clinical Features of OCD
Obsessive–compulsive disorder (OCD), like other neuropsychiatric disorders, necessitates additional research to elucidate its underlying causes. OCD is primarily characterized by obsessions: intrusive, persistent, and unwanted thoughts or urges linked to heightened anxiety and compulsions, which are repetitive behaviors or mental acts often performed rigidly and ritualistically [14]. Generally, based on the CSTC circuit, a complex imbalance between the direct and indirect pathways can elucidate most OCD-related symptoms [15], and we will review the clinical manifestations of OCD and associated molecular mechanisms (Table 1). Contemporary research posits that various factors, encompassing genetics and the environment, exert intricate effects on the evolution of OCD [5]. For instance, there is evidence demonstrating that dopaminergic agents such as amphetamine (AMPH) and L-DOPA can trigger repetitive behaviors in OCD patients [16,17,18]. Family and twin studies show that OCD is a multifactorial condition strongly linked to genetic factors [14]. Furthermore, these studies indicate a genetic predisposition to OCD, with the identification of genes correlated with the disorder, such as glutamatergic-related genes in humans (such as PTPRD, GRID2, DLGAP1, and DLGAP3 (SAPAP3)), alongside genes consistently affecting neurotransmission (such as GRIN2B and SLC1A1) [19,20]. Some see that the interaction between the brain and environment and neuroplasticity might modulate the compulsive-like behavior related to protein regulation [21]. To summarize, we all know that the origin of OCD is complex and shaped by a nexus of genetic and environmental determinants. Our understanding of the disorder can be enhanced by continuing investigation, leading to more efficacious treatment modalities.

Table 1.
Clinical features and related mechanisms.
Although we cannot fully understand the pathogenesis of OCD, the hypothesis that it involves several interrelated mechanisms is proposed: cognitive–motional abnormalities, neural circuitry dysregulation, functional and structural neural alterations, and molecular irregularities.
2.2. Current Research on Animal Models of OCD
The genetic basis of OCD is multifactorial and polygenic, presenting challenges in identifying a single gene correlation. Analysis of genetic associations in thousands of cases has shown that approximately 85% of these associations have odds ratios of less than 1.5 [30]. Due to OCD’s complexity, translating biological knowledge into practical applications such as clinical trials can be daunting. Therefore, the use of animal models for large-scale genetic studies is essential. Some researches enable the exploration of numerous hypothesized genetic factors, revealing their functional relevance and ultimately contributing to the development of enhanced diagnostic and therapeutic approaches for OCD [31]. For the last few years, our research has been dedicated to developing dependable animal models for OCD and using these models to look into the mechanisms of the disorder and evaluate the efficacy of new treatments. Although primarily utilizing rodents, these models only partially replicate the complexities of OCD but demonstrate validity to some extent.
There are currently three primary types of animal models for OCD: genetic models, pharmacological models, and behavioral models. Genetic models involve limited research but can replicate genetic changes found in patients, furthering the understanding of disease mechanisms and demonstrating good face validity. However, these models cannot fully meet all established validity criteria [32], such as the Slc1a1 and Sapap3 models, because such models primarily exhibit face validity since no single gene knockout has been definitively linked to OCD in humans. Pharmacological models induce OCD-like behaviors using drugs and are essential for drug testing, albeit with potential face and construct validity. Pharmacological models that induce stereotyped behavior have been documented in the literature. Scheel-Krüger et al. reported that the specific GABA agonist muscimol elicited strong amphetamine-like stimulation and stereotyped behavior following intracranial injection [33]. Additionally, glutamate receptor agonists, such as NMDA agonists, are known to induce stereotyped behavior [34]. Furthermore, administration of apomorphine has been shown to activate dopamine receptors in the neostriatum and to induce stereotypical behavior in numerous studies across various species [35,36]. Moreover, pharmacological stimulation of postsynaptic serotonin receptors can also induce stereotyped behavior, which is associated with hypoactivity in serotonin pathways [33,37]. Behavioral models are suitable for situations where compulsive behaviors arise spontaneously. While the behavioral models demonstrate good face validity, they do not consider the genetic information underlying the origin of OCD.
Finding effective animal models of OCD needs critical evaluation rather than blindly trusting study conclusions. Animal models have the advantage of the ability to perform repetitive procedures, which are not easily accessible in clinical experiments. But it is controversial for us to use these models, due to the translation of these models into clinical practice. Preclinical operations, such as genetic, pharmacological, network, or other approaches, in animal models provide the opportunity to obtain high-quality and biologically grounded data for studying compulsive behaviors. It is important to note that implementing environmental control methods in a clinical research setting is impractical [5]. So, we will review several transgenic models that have provided intriguing findings related to anxiety-like behavior and unusual repetitive and compulsive behaviors.
2.3. Transgenic Mouse Models of OCD
In recent years, we have carried out extensive research on the genetic basis of OCD. This research necessitates significant time and resources and currently should focus on translating genetic discoveries into practical applications. However, it is challenging to demonstrate relevant cognitive constructs as they may apply to these mouse models [38]. Knockout mouse models enable researchers to effectively study genes associated with OCD and assess their impact on behavior and the nervous system, with the aim to gain a deeper understanding of the underlying mechanisms of OCD and provide a theoretical foundation for the development of new therapies.
Additionally, the use of transgenic models has resulted in significant findings in investigating OCD mechanisms. While transgenic models are commonly used for various scientific purposes, they have proven valuable in exploring potential mechanisms of OCD-related behaviors. In the following sections, we will review several transgenic models that have currently provided intriguing findings related to anxiety-like behaviors as well as unusual repetitive and compulsive behaviors, such as compulsive grooming behavior, perseverative responses in reverse learning processes, and perseveration in excessive locomotion behaviors (Table 2).

Table 2.
Animal models of OCD and related disorders.
2.3.1. Hoxb8-Knockout Mouse
HOXB8 is a component of the mammalian Hox complex, which consists of 39 transcription factors that govern anterior–posterior axis patterning. In a study, a Hoxb8-knockout mouse model was established to explore the potential mechanisms associated with compulsive grooming behavior in OCD patients [39]. The inactivation or mutation of the Hoxb8 gene leads to persistent anxiety, OCD-like behaviors, and prolonged self-grooming in mice, resulting in extensive hair loss and severe skin damage [40].
Importantly, we have to know that Hoxb8, despite its transcription factor nature, plays a crucial role in the development of multicellular organisms by accurate reflections of structural changes. Although the Hoxb8 mutant mouse model did not exhibit morphological changes, it showed noticeable behavioral alterations [39]. Furthermore, studies have indicated that Hoxb8 is expressed not in neurons but in a specific subset of cells called microglia, which originate from myeloid progenitors in the brain [41]. As a result, the knockout of the Hoxb8 gene resulted in a 15% reduction in the overall population of microglia in a mouse model [42].
The transplantation of bone marrow from wild-type mice into Hoxb8-knockout mice produced some very interesting effects. This process not only stimulates the proliferation of donor wild-type microglia in the brains of recipient Hoxb8 gene knockout mice, but also significantly improves their abnormal behaviors [42]. These findings suggest a causal link between the reduction in this specific subset of microglia and the occurrence of excessive grooming behavior.
The expression of the Hoxb8 gene varies among different species. Hoxb8 is widely expressed in mouse central nervous system, including the olfactory bulb, brainstem, hippocampus, frontal cortex, and caudate–putamen. Conversely, in humans, Hoxb8 is primarily expressed in the brainstem, olfactory bulb, cortex, and striatum [39,42], with a particular emphasis on cortical regions associated with the mechanisms of OCD, such as the orbital frontal cortex and anterior cingulate cortex [12].
Nagarajan and colleagues revealed that optogenetic stimulation of Hoxb8 microglia in specific brain regions can trigger anxiety-like and self-grooming behaviors [40]. Additionally, they demonstrated that by inducing the early response of the c-Fos protein and optogenetic stimulation of Hoxb8 microglia in vitro, these microglia can activate neighboring neural activity and induce behaviors akin to OCD. These findings imply that Hoxb8 microglia likely play a significant role in eliciting these behaviors [40]. Furthermore, these studies reveal an association between the Hoxb8 gene, the immune system, and OCD, improving new potential treatment for OCD by examining the involvement of Hoxb8 microglia in the development of excessive grooming behavior.
2.3.2. Slc1a1-Knockout Mouse
The SLC1A1 gene, located on chromosome 9p24, is expressed in brain regions associated with OCD, such as the cerebral cortex, striatum, and thalamus [43]. As research on the genetic mechanisms associated with OCD has increased, more studies have focused on the role of the glutamate transporter gene SLC1A1/EAAC1 in pathogenesis and proposed mechanisms [44,45,46,47]. This gene primarily encodes excitatory amino acid transporter-3 (EAAT3), which plays a crucial role in neurotransmission in mammals, affecting approximately 30–40% of synapses in the mammalian brain [48]. Notably, the alleles of SLC1A1 most strongly associated with OCD lead to an increase in the levels of EAAT3 protein [44].
This study is in line with the initial findings of Zike et al. [25], indicating that the conditional overexpression of EAAT3 in the forebrain (EAAT3glo/CMKII mice, including the frontal cortex, hippocampus, and striatum) leads to an increase in specific OCD-related behaviors. These include heightened grooming and anxiety-related behaviors observed in open field, light–dark box, and marble-burying tests [49].
Furthermore, Gonzalez et al. [50] demonstrated that reducing EAAT3 can prevent drug-induced repetitive behaviors, suggesting that reducing EAAT3 is a potential therapeutic target for OCD. However, studies on EAAT3 heterozygous mice revealed that despite reduced expression of the Slc1a1 gene, there was no impact on anxiety-related or compulsive behaviors or neurotransmitter levels in the cortico-striatal circuit [50]. Nevertheless, inducing the overexpression of this gene in the striatum led to an increase in repetitive or stereotyped behaviors in mice [25]. Therefore, it may be necessary to block the activity of this transporter protein for effective treatment.
Bellini et al. reported that mice deficient in EAAT3 exhibited heightened anxiety-like behaviors and aberrant grooming patterns [51]. This led to the elucidation of a new molecular mechanism whereby Eaac1 moderates the activity of metabotropic glutamate receptor 1 (mGluR1) within the striatum, in turn enhancing the expression of D1 dopamine receptors (D1Rs) and modulating repetitive movements. Furthermore, EAAT3 deficiency disrupts the processes of synaptic excitatory transmission. These data indicate that SLC1A1 gene variants may be implicated in the glutamatergic dysregulation observed in OCD patients [52], shedding light on the molecular underpinnings and informing the creation of targeted therapies.
2.3.3. Sapap3-Knockout Mouse
The SAPAP (SAP90/PSD-95-associated protein, also called discs-large-associated proteins (DLGAPs)) family is composed of crucial postsynaptic scaffold proteins that regulate the trafficking of AMPA- and NMDA-type glutamate receptors to the postsynaptic membrane of excitatory synapse density proteins [53]. Sapap3 (also known as Dlgap3) is highly expressed in the mouse striatum and enriched within specific astrocyte subcompartments to modulate actin cytoskeleton organization [54,55]. While studying cellular and neural circuit mechanisms in human subjects is challenging, transgenic animal models can be instrumental in demonstrating alterations in the cortico-striatal circuit activity that contribute to compulsive behavior. This discussion will centre on the utilization of Sapap3-knockout mouse models in research related to OCD.
The SAPAP protein interacts with the GK domain of postsynaptic density protein-95 (PSD-95), also known as SAP-90 or synapse-associated protein 90. It is a member of the membrane-associated guanylate kinase (MAGUK) family. PSD-95 and PSD-93 form a synaptic scaffold at postsynaptic sites [5], promoting the clustering of ion channels and crucially anchoring the molecular structure of NMDA/AMPA receptor channels [56]. Clinical genetic studies have established a connection between allelic gene variations in SAPAP1 and SAPAP3 and OCD [57,58]. In addition, researchers conducted an initial investigation into the distribution of Sapap family proteins in the mouse brain [54], revealing predominant dendritic expression of Sapap3 in the hippocampus, striatum, and cortex, with the highest expression observed in the striatum. These findings strongly suggest a potentially significant association between Sapap3 and OCD, providing a basis for utilizing Sapap3-knockout mouse models to study compulsive behavior in rodents.
Sapap3-knockout mice are widely used and extensively studied mouse models for OCD research. Because they lack the postsynaptic scaffolding protein SAP90/PSD95-associated protein 3 (Sapap3) [54,59], these mice exhibit similarity with human OCD symptomatology in many ways, such as anxiety-like behavior, increased self-grooming, and impaired habit learning [53,60,61,62]. Additionally, they show reduced motor abilities (unrelated to grooming) and decreased average body weight [61,63]. Treatment with SSRIs has been shown to alleviate many of these symptoms, and research has established an association with the overactive cortico-striato-thalamo-cortical (CSTC) circuits [53,64,65]. Manning et al. observed an operant reversal of learning impairment in a Sapap3-knockout mouse model, which was linked to reduced activity in the medial prefrontal cortex (mPFC) [66]. Furthermore, recent research has revealed that the deletion of Sapap3 leads to pathway-specific and non-synaptic changes in the functionality of circuits within the dorsolateral striatum [67], suggesting that a series of pathway-specific and intracellular functional changes in this Sapap3-KO model may play a key role in the pathogenesis of OCD.
In general, although DLGAP3/SAPAP3 has not shown findings from genome-wide association studies (GWAS) in OCD research, previous research has confirmed the efficacy of this animal model and established a reliable theoretical foundation for its potential clinical relevance in the treatment of OCD.
2.3.4. Slitrk5-Knockout Mouse
Slitrk5-knockout mouse models are widely utilized as transgenic animal models for investigating central nervous system functions. Its primary function is to encode a transmembrane protein, one of six members of the SLITRK family. This gene is broadly expressed within the central nervous system and plays a crucial role in various processes, including neuronal differentiation, dendritic branching, synaptic growth, synaptogenesis, and signal transduction, among others [68].
The Slitrk family gained prominence following its discovery through differential gene expression screening in mice with neural tube defects in 2001. Subsequent studies confirmed the presence of all six members of the SLITRK family. These genes are located on chromosome 13 (SLITRK1, SLITRK5, and SLITRK6), chromosome 3 (SLITRK3), and the X chromosome (SLITRK2 and SLITRK4) [69]. These proteins are primarily expressed within the developing central nervous system [70,71]. In addition, their expression includes various cell types, such as brain tumors, lymphomas, embryonic stem cells, and hematopoietic stem cells, with variations observed among different family members [72].
Slitrk5 expression is prominent in specific regions, including the CA1 region of the hippocampus [73], the frontal lobe of the brain, the spinal cord, and the medulla. Moreover, Slitrk1 is expressed in mature neurons, Slitrk2 is highly expressed in the ventricular layer of the brain, and Slitrk6 is regionally expressed in the diencephalon. Emerging research suggests potential associations between Slitrk5 and the pathogenesis of various central nervous system disorders, including OCD [74]. Additionally, studies indicate that manipulating Slitrk5 expression may facilitate the restoration of neural system function, making it a potential therapeutic target for central nervous system disorders.
The establishment of a Slitrk5-knockout mouse model is essential for understanding the mechanisms underlying central nervous system development and disorders. Initially, these mice displayed heightened anxiety-like behavior, as assessed through the elevated plus maze, open field, and novel object tests. This behavior is subsequently followed by repetitive and excessive self-grooming, leading to severe facial skin injury and hair loss [74]. These behavioral characteristics closely resemble the phenotype observed in the Sapap3-knockout mouse model [53]. Both models exhibit cortico-striatal abnormalities and show symptom relief after fluoxetine treatment. In conclusion, the use of the Slitrk5-knockout mouse model provides a valuable tool for investigating the link between human genes and OCD, exploring its pathogenesis, and identifying potential targets for targeted interventions.
2.3.5. Spred2-Knockout Mouse
A study conducted in 2018 investigated the effects of Spred2-knockout in mice. The findings demonstrated that these mice exhibited behaviors indicative of OCD and anxiety, which closely resembled those observed in mice lacking the Sapap3 gene [75]. Sprouty-related enabled/vasodilator-stimulated phosphoprotein homology 1 (EVH1) domain-containing 2 (Spred2) inhibits the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway and promotes autophagy in several cancers [76]. This pathway is crucial for controlling various cellular processes, including cell proliferation and differentiation. Despite the understanding of the influence of the Spred family on the Ras/ERK-MAPK pathway, the specific mechanism by which it operates remains incompletely understood. The Spred family comprises three members: Spred1, Spred2, and Spred3. These proteins possess an EVH-1 domain at their N-terminus, a c-KIT binding domain, an SPR domain at their C-terminus, and two uncharacterized domains [77,78].
The Spred2 gene encodes a key regulatory factor protein that oversees the Ras/ERK-MAPK pathway, a cellular cascade activated by brain-derived neurotrophic factor (BDNF). In the Spred2-knockout mouse model, researchers observed changes in neural transmission within the thalamus–amygdala circuit. Furthermore, subsequent research revealed that the Spred2-knockout mouse model exhibited changes similar to those observed in Sapap3-knockout mice, and these changes appeared to be age-related [79]. These changes indicate developmental irregularities, suggesting that Spred2 is involved in the development of the CNS in mice [80].
Researchers also find that changes in the Spred2 and Sapap3 genes are linked to functional alterations in the prefrontal cortex, striatum, and other brain regions. The consistency of these in both rodents and humans indicates the conservation of genetic mechanisms associated with OCD. Currently, there is limited research on the role of the Spred2 gene in OCD. And the similarities between Sapap3 and Spred2 warrant further investigation into the potential relevance of the Spred2 gene to OCD. Overall, these investigations may significantly contribute to a deeper understanding of OCD development and provide new insights into the neural circuitry underlying OCD. Below, we provide a concise comparison between humans and corresponding mouse gene-knockout models (Table 3).

Table 3.
Comparison of findings between humans and mouse models.
3. Treatment Strategies for OCD
3.1. Psychotherapy and Pharmacotherapy
The recommended first-line treatment for OCD is CBT, which includes exposure and response prevention (ERP), as well as pharmacological interventions such as SSRIs [7,86]. Current treatment guidelines in the United States endorse three first-line treatments for OCD (SSRI, CBT, and SSRI + CBT), and propose combination therapy for patients with an inadequate response to single treatments or those with treatment-resistant OCD [87]. CBT, which focuses on understanding the symptoms and treatment process of OCD, involves the formation and elimination of patient fears [88]. Although ERP specifically targets this process, it remains uncertain whether ERP can potentially influence the pathophysiology of OCD [89].
For patients with severe OCD or those unable to comply with CBT requirements, the APA guidelines recommend using SSRIs alone. Some patients have a slow response to treatment, creating potential bias with short-term SSRI therapy [87,90]. The guidelines suggest a minimum duration of 8–12 weeks for SSRI treatment (initially at the maximum tolerated dose for 4–6 weeks) before considering a change in medication strategy. If CBT is not feasible, the use of SSRIs alone is also warranted. Combination therapy with both SSRIs and CBT is an option when patients respond partially to monotherapy or show an SSRI response. A preponderance of evidence supports this combination therapy and demonstrates symptom improvement with these interventions [90,91]. Nevertheless, about two-thirds of OCD patients do not achieve satisfactory outcomes, likely due to adverse drug reactions or poor patient adherence [92,93,94]. In addition, transgenic animal models are valuable for developing and screening anti-compulsive drugs because of their strong validity and capacity to quickly produce large, phenotypically stable cohorts [95].
3.2. Neuromodulation and Neurosurgery
Approximately 20–25% of individuals diagnosed with OCD exhibit resistance to conventional pharmacological and psychological treatments [96]. Therefore, surgical and neuromodulatory treatments are becoming promising alternatives. These methods aim to influence the CSTC circuit, which is pivotal in OCD pathology. Neuromodulation includes invasive and noninvasive techniques, such as stereotactic ablation, transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS) [96]. With recent progress in OCD-related circuitry, these findings may offer therapeutic potential. Over the past decade, neuromodulation strategies have emerged as a promising treatment for psychiatric disorders by targeting circuit function [95]; for example, as a non-invasive brain stimulation treatment for these disorders, the therapeutic effects of tDCS can be maintained for some time [97]. Surgical approaches, in particular, show greater efficacy in OCD management than in other psychiatric conditions but are considered for patients who are unresponsive to traditional treatments [10,98]. Furthermore, optogenetic activation of the lateral orbitofrontal cortex (LOFC)–striatal circuit restored fast-spiking neuron microcircuits, reducing compulsive grooming in Sapap3-KO mice and suggesting a potential therapeutic target in OCD [64]. Additionally, circuit manipulation tools, like optogenetics and chemogenetics in animal models, have advanced our understanding of how altered circuits cause maladaptive behaviors [95].
“Traditional” neurosurgical procedures, such as stereotactic ablation, involve irreversible focal tissue ablation in specific regions of the CSTC circuit. However, these procedures can lead to adverse reactions, including nausea, vomiting, seizures, and cognitive decline. In contrast, DBS offers reversibility and adjustability and is primarily used to treat movement disorders, such as Parkinson’s disease and essential tremors [99,100]. Potential targets for DBS in OCD treatment include the striatal regions, such as the anterior limb of the internal capsule (ALIC)/nucleus accumbens or the subthalamic nucleus (STN) [101]. Recent studies have indicated that DBS is equally effective as ablative surgery [102,103]. However, its usage is recommended for long-term (more than 5 years) patients with OCD [96]. In this context, circuit manipulation in animal models may help optimize DBS protocols for OCD by selecting precise stimulation sites and targeting specific circuit alterations [104]. Although DBS may prove an effective treatment for OCD patients [105], there is currently no consensus on the stimulation site, frequency, intensity, treatment duration, or need for maintenance of each technique. Moreover, no comprehensive review of alternative brain stimulation methods for treating treatment-resistant OCD has been conducted. Further research is required to explore the underlying mechanisms of OCD and identify specific treatment targets. The treatment algorithm for OCD patients is summarized in Figure 1.
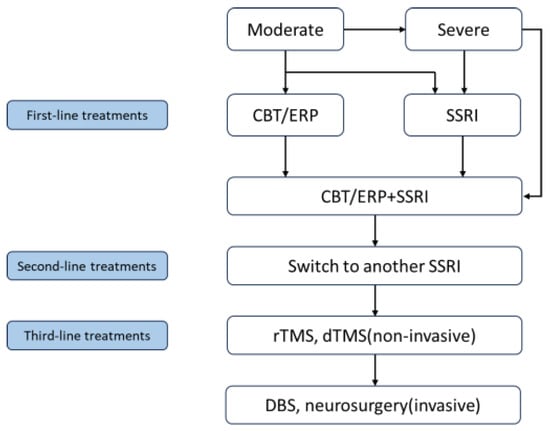
Figure 1.
A Treatment algorithm for OCD patients. Note. CBT, cognitive behavioral therapy; ERP, exposure and response prevention; SSRI, selective serotonin reuptake inhibitor; rTMS, repetitive transcranial magnetic stimulation; dTMS, deep transcranial magnetic stimulation; DBS, deep brain stimulation.
4. Discussion
Recent advancements have helped the development of various gene-knockout animal models that exhibit behaviors similar to those observed in OCD patients, such as repetitive self-grooming and anxiety-like behaviors. These models offer valuable insights into OCD, yet their utility is tempered by species-specific limitations. We need to acknowledge that these existing models do not fully encapsulate the multifaceted clinical manifestations of OCD, highlighting the need for further refinement in their application. Despite these questions, gene knockout models are pivotal in circumventing constraints inherent in human studies—such as environmental and genetic background variability—enabling a more subtlety understanding of the neurobiological mechanisms underpinning gene-related phenotypes, so this advances OCD medication research. However, the reliance on overexpression in these models, which results in behaviors analogous to OCD, limits their capacity to fully mirror the human condition. Consequently, there is a pressing need for innovative research models that more accurately reflect the complex nature of human OCD.
Current transgenic animal models are possibly underdeveloped for OCD research, and some lack strong theoretical underpinning, but they pave the way for innovative research avenues, ideas, and methods. These models facilitate an in-depth examination of the connections among genes, pathways, and circuits, providing us with an effective strategy to refine the modeling process and present a straightforward route for forthcoming OCD studies.
Additionally, in the treatment of OCD, there is growing evidence that neuromodulation and surgical interventions aiming at CSTC circuitry are both safe and effective and are recommended in several clinical guidelines and expert consensuses. DBS and minimally invasive ablative surgeries present potential long-term solutions for refractory OCD, provided that they adhere to treatment guidelines. Advanced research techniques can help us gain an improved understanding of the fundamental pathophysiology of OCD, such as neuroimaging, electrophysiology, comprehensive data analysis, and integrated behavioral models. This is expected to lead to the creation of customized treatment approaches for those affected, marking a significant step forward in the personalized management of OCD.
In summary, this review offers an overview of several frequently used OCD transgenic animal models, including knockout mouse models, and discusses their potential future in OCD research. More importantly, our review updates the literature with the most recent findings on current cutting-edge therapies and treatments for OCD (Table 4), encompassing neuromodulation and surgical interventions. Future directions for advancing more effective therapies, particularly for patients resistant to traditional therapeutic methods, are also highlighted. And there is a need in the realm to develop more diverse transgenic models to explore the mechanisms of OCD. Compared with the changes in the brains of patients and genetic findings in animal models, we aim to find a better selection of the most appropriate models for relevant research questions and help guide future translational research.

Table 4.
Summary of mechanisms, benefits, and limitations of current therapies for OCD.
By comparing genetic findings in these models with observed changes in the brains of individuals with OCD, we aim to better inform the selection of the most appropriate models for relevant research questions and help to guide future translational studies.
5. Conclusions
Transgenic animal models, such as knockout mice, help us make deeper insights into the disorder’s pathophysiology and develop current therapies. Our findings also highlight the potential of advanced treatment modalities, including neuromodulation and neurosurgery (e.g., deep brain stimulation). Because DBS has had emerging success in the treatment of movement disorders, such as Parkinson’s disease, essential tremor, and so on, the usage of these therapies has shown promise in challenging pathologies of different psychiatric disorders. As we are researchers deeply committed to advancing the field, this study aims to provide a comprehensive and rigorous analysis that serves as a foundation for future work. And a multitude of different animal models are under development, and more time will be required to differentiate an optimal path forward for the field.
Author Contributions
Conceptualization, X.H. and L.X.; writing—original draft preparation, X.H. and L.X.; writing—review and editing, Y.W., M.W. and H.D.; supervision, M.W. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sichuan Province Science and Technology Support Program (2024NSFSC1566).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fawcett, E.J.; Power, H.; Fawcett, J.M. Women Are at Greater Risk of OCD Than Men: A Meta-Analytic Review of OCD Prevalence Worldwide. J. Clin. Psychiatry 2020, 81, 19r13085. [Google Scholar] [CrossRef] [PubMed]
- Ruscio, A.M.; Stein, D.J.; Chiu, W.T.; Kessler, R.C. The Epidemiology of Obsessive-Compulsive Disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.K.; Grice, D.E.; Lapidus, K.A.B.; Coffey, B.J. Obsessive-Compulsive Disorder. Psychiatr. Clin. N. Am. 2014, 37, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Fenske, J.N.; Petersen, K. Obsessive-Compulsive Disorder: Diagnosis and Management. Am. Fam. Physician 2015, 92, 896–903. [Google Scholar]
- Wilson, C.; Gattuso, J.J.; Hannan, A.J.; Renoir, T. Mechanisms of Pathogenesis and Environmental Moderators in Preclinical Models of Compulsive-like Behaviours. Neurobiol. Dis. 2023, 185, 106223. [Google Scholar] [CrossRef]
- Öst, L.-G.; Havnen, A.; Hansen, B.; Kvale, G. Cognitive Behavioral Treatments of Obsessive-Compulsive Disorder. A Systematic Review and Meta-Analysis of Studies Published 1993-2014. Clin Psychol Rev 2015, 40, 156–169. [Google Scholar] [CrossRef]
- McGuire, J.F.; Piacentini, J.; Lewin, A.B.; Brennan, E.A.; Murphy, T.K.; Storch, E.A. A meta-analysis of cognitive behavior therapy and medication for child obsessive-compulsive disorder: Moderators of treatment efficacy, response, and remission. Depress. Anxiety 2015, 32, 580–593. [Google Scholar] [CrossRef]
- Soomro, G.M.; Altman, D.; Rajagopal, S.; Oakley-Browne, M. Selective Serotonin Re-Uptake Inhibitors (SSRIs) versus Placebo for Obsessive Compulsive Disorder (OCD). Cochrane Database Syst. Rev. 2008, 2008, CD001765. [Google Scholar] [CrossRef]
- Erzegovesi, S.; Cavallini, M.C.; Cavedini, P.; Diaferia, G.; Locatelli, M.; Bellodi, L. Clinical Predictors of Drug Response in Obsessive-Compulsive Disorder. J. Clin. Psychopharmacol. 2001, 21, 488–492. [Google Scholar] [CrossRef]
- Robbins, T.W.; Vaghi, M.M.; Banca, P. Obsessive-Compulsive Disorder: Puzzles and Prospects. Neuron 2019, 102, 27–47. [Google Scholar] [CrossRef]
- Williams, K.A.; Swedo, S.E. Post-Infectious Autoimmune Disorders: Sydenham’s Chorea, PANDAS and Beyond. Brain Res. 2015, 1617, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, B.L.; Ahmari, S.E. Animal Models for OCD Research. In The Neurobiology and Treatment of OCD: Accelerating Progress; Fineberg, N.A., Robbins, T.W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 55–96. ISBN 978-3-030-75393-1. [Google Scholar]
- Monteiro, P.; Feng, G. Learning From Animal Models of Obsessive-Compulsive Disorder. Biol. Psychiatry 2016, 79, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Pauls, D.L.; Abramovitch, A.; Rauch, S.L.; Geller, D.A. Obsessive–Compulsive Disorder: An Integrative Genetic and Neurobiological Perspective. Nat. Rev. Neurosci. 2014, 15, 410–424. [Google Scholar] [CrossRef]
- Jalal, B.; Chamberlain, S.R.; Sahakian, B.J. Obsessive-Compulsive Disorder: Etiology, Neuropathology, and Cognitive Dysfunction. Brain Behav. 2023, 13, e3000. [Google Scholar] [CrossRef]
- Chohan, M.O.; Kopelman, J.M.; Yueh, H.; Fazlali, Z.; Greene, N.; Harris, A.Z.; Balsam, P.D.; Leonardo, E.D.; Kramer, E.R.; Veenstra-VanderWeele, J.; et al. Developmental Impact of Glutamate Transporter Overexpression on Dopaminergic Neuron Activity and Stereotypic Behavior. Mol. Psychiatry 2022, 27, 1515–1526. [Google Scholar] [CrossRef]
- Shakeri, J.; Farnia, V.; Karimi, A.R.; Tatari, F.; Juibari, T.A.; Alikhani, M.; Bajoghli, H.; Holsboer-Trachsler, E.; Brand, S. The Prevalence and Clinical Features of Amphetamine-Induced Obsessive Compulsive Disorder. Drug Alcohol. Depend. 2016, 160, 157–162. [Google Scholar] [CrossRef]
- Baldan, L.C.; Williams, K.A.; Gallezot, J.-D.; Pogorelov, V.; Rapanelli, M.; Crowley, M.; Anderson, G.M.; Loring, E.; Gorczyca, R.; Billingslea, E.; et al. Histidine Decarboxylase Deficiency Causes Tourette Syndrome: Parallel Findings in Humans and Mice. Neuron 2014, 81, 77–90. [Google Scholar] [CrossRef]
- Mattheisen, M.; Samuels, J.F.; Wang, Y.; Greenberg, B.D.; Fyer, A.J.; McCracken, J.T.; Geller, D.A.; Murphy, D.L.; Knowles, J.A.; Grados, M.A.; et al. Genome-Wide Association Study in Obsessive-Compulsive Disorder: Results from the OCGAS. Mol. Psychiatry 2015, 20, 337–344. [Google Scholar] [CrossRef]
- International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS). Revealing the Complex Genetic Architecture of Obsessive-Compulsive Disorder Using Meta-Analysis. Mol. Psychiatry 2018, 23, 1181–1188. [Google Scholar] [CrossRef]
- Peng, S.; He, C.-Y.; Zhang, Q.; Wang, M.; Sheng, X.; Gao, J.; Ge, L.; Zhang, Z.; Wang, H.; Hu, X.-Z. Acquisition and Extinction of Active Avoidance Compulsive-like Behavior in Mice. J. Psychiatr. Res. 2023, 168, 91–99. [Google Scholar] [CrossRef]
- Voyiaziakis, E.; Evgrafov, O.; Li, D.; Yoon, H.-J.; Tabares, P.; Samuels, J.; Wang, Y.; Riddle, M.A.; Grados, M.A.; Bienvenu, O.J.; et al. Association of SLC6A4 Variants with Obsessive-Compulsive Disorder in a Large Multi-Center US Family Study. Mol. Psychiatry 2011, 16, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Endrass, T.; Ullsperger, M. Specificity of Performance Monitoring Changes in Obsessive-Compulsive Disorder. Neurosci. Biobehav. Rev. 2014, 46, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.E.; Mayerfeld, C.; Arnold, P.D.; Crane, J.R.; O’Dushlaine, C.; Fagerness, J.A.; Yu, D.; Scharf, J.M.; Chan, E.; Kassam, F.; et al. Meta-Analysis of Association between Obsessive-Compulsive Disorder and the 3′ Region of Neuronal Glutamate Transporter Gene SLC1A1. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162B, 367–379. [Google Scholar] [CrossRef]
- Zike, I.D.; Chohan, M.O.; Kopelman, J.M.; Krasnow, E.N.; Flicker, D.; Nautiyal, K.M.; Bubser, M.; Kellendonk, C.; Jones, C.K.; Stanwood, G.; et al. OCD Candidate Gene SLC1A1/EAAT3 Impacts Basal Ganglia-Mediated Activity and Stereotypic Behavior. Proc. Natl. Acad. Sci. USA 2017, 114, 5719–5724. [Google Scholar] [CrossRef]
- van den Heuvel, O.A.; van Wingen, G.; Soriano-Mas, C.; Alonso, P.; Chamberlain, S.R.; Nakamae, T.; Denys, D.; Goudriaan, A.E.; Veltman, D.J. Brain Circuitry of Compulsivity. Eur. Neuropsychopharmacol. 2016, 26, 810–827. [Google Scholar] [CrossRef]
- Furtado, M.; Katzman, M.A. Neuroinflammatory Pathways in Anxiety, Posttraumatic Stress, and Obsessive Compulsive Disorders. Psychiatry Res. 2015, 229, 37–48. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2020, 14, 601939. [Google Scholar] [CrossRef]
- Felix-Ortiz, A.C.; Burgos-Robles, A.; Bhagat, N.D.; Leppla, C.A.; Tye, K.M. Bidirectional Modulation of Anxiety-Related and Social Behaviors by Amygdala Projections to the Medial Prefrontal Cortex. Neuroscience 2016, 321, 197–209. [Google Scholar] [CrossRef]
- Welter, D.; MacArthur, J.; Morales, J.; Burdett, T.; Hall, P.; Junkins, H.; Klemm, A.; Flicek, P.; Manolio, T.; Hindorff, L.; et al. The NHGRI GWAS Catalog, a Curated Resource of SNP-Trait Associations. Nucleic Acids Res. 2014, 42, D1001–D1006. [Google Scholar] [CrossRef]
- D’Amico, D.; Estivill, X.; Terriente, J. Switching to Zebrafish Neurobehavioral Models: The Obsessive–Compulsive Disorder Paradigm. Eur. J. Pharmacol. 2015, 759, 142–150. [Google Scholar] [CrossRef]
- Borba, J.V.; Canzian, J.; Resmim, C.M.; Silva, R.M.; Duarte, M.C.F.; Mohammed, K.A.; Schoenau, W.; Adedara, I.A.; Rosemberg, D.B. Towards Zebrafish Models to Unravel Translational Insights of Obsessive-Compulsive Disorder: A Neurobehavioral Perspective. Neurosci. Biobehav. Rev. 2024, 162, 105715. [Google Scholar] [CrossRef] [PubMed]
- Langen, M.; Kas, M.J.H.; Staal, W.G.; van Engeland, H.; Durston, S. The Neurobiology of Repetitive Behavior: Of Mice…. Neurosci. Biobehav. Rev. 2011, 35, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Bedingfield, J.B.; Calder, L.D.; Thai, D.K.; Karler, R. The Role of the Striatum in the Mouse in Behavioral Sensitization to Amphetamine. Pharmacol. Biochem. Behav. 1997, 56, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.M.; Smelik, P.G. Site of Action of Dopamine and Apomorphine on Compulsive Gnawing Behaviour in Rats. Experientia 1966, 22, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Saka, E.; Goodrich, C.; Harlan, P.; Madras, B.K.; Graybiel, A.M. Repetitive Behaviors in Monkeys Are Linked to Specific Striatal Activation Patterns. J. Neurosci. 2004, 24, 7557–7565. [Google Scholar] [CrossRef]
- Korff, S.; Stein, D.J.; Harvey, B.H. Stereotypic Behaviour in the Deer Mouse: Pharmacological Validation and Relevance for Obsessive Compulsive Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 348–355. [Google Scholar] [CrossRef]
- Theron, V.; Lochner, C.; Stein, D.J.; Harvey, B.H.; Wolmarans, D.W. The Deer Mouse (Peromyscus Maniculatus Bairdii) as a Model Organism to Explore the Naturalistic Psychobiological Mechanisms Contributing to Compulsive-like Rigidity: A Narrative Overview of Advances and Opportunities. Compr. Psychiatry 2025, 136, 152545. [Google Scholar] [CrossRef]
- Greer, J.M.; Capecchi, M.R. Hoxb8 Is Required for Normal Grooming Behavior in Mice. Neuron 2002, 33, 23–34. [Google Scholar] [CrossRef]
- Nagarajan, N.; Capecchi, M.R. Optogenetic Stimulation of Mouse Hoxb8 Microglia in Specific Regions of the Brain Induces Anxiety, Grooming, or Both. Mol. Psychiatry 2023, 29, 1726–1740. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Chen, S.-K.; Tvrdik, P.; Peden, E.; Cho, S.; Wu, S.; Spangrude, G.; Capecchi, M.R. Hematopoietic Origin of Pathological Grooming in Hoxb8 Mutant Mice. Cell 2010, 141, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Hediger, M.A. The Glutamate/Neutral Amino Acid Transporter Family SLC1: Molecular, Physiological and Pharmacological Aspects. Pflug. Arch. 2004, 447, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Wendland, J.R.; Moya, P.R.; Timpano, K.R.; Anavitarte, A.P.; Kruse, M.R.; Wheaton, M.G.; Ren-Patterson, R.F.; Murphy, D.L. A Haplotype Containing Quantitative Trait Loci for SLC1A1 Gene Expression and Its Association with Obsessive-Compulsive Disorder. Arch. Gen. Psychiatry 2009, 66, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Samuels, J.; Wang, Y.; Riddle, M.A.; Greenberg, B.D.; Fyer, A.J.; McCracken, J.T.; Rauch, S.L.; Murphy, D.L.; Grados, M.A.; Knowles, J.A.; et al. Comprehensive Family-Based Association Study of the Glutamate Transporter Gene SLC1A1 in Obsessive-Compulsive Disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156B, 472–477. [Google Scholar] [CrossRef]
- Shugart, Y.Y.; Wang, Y.; Samuels, J.F.; Grados, M.A.; Greenberg, B.D.; Knowles, J.A.; McCracken, J.T.; Rauch, S.L.; Murphy, D.L.; Rasmussen, S.A.; et al. A Family-Based Association Study of the Glutamate Transporter Gene SLC1A1 in Obsessive-Compulsive Disorder in 378 Families. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 886–892. [Google Scholar] [CrossRef]
- Stewart, S.E.; Fagerness, J.A.; Platko, J.; Smoller, J.W.; Scharf, J.M.; Illmann, C.; Jenike, E.; Chabane, N.; Leboyer, M.; Delorme, R.; et al. Association of the SLC1A1 Glutamate Transporter Gene and Obsessive-Compulsive Disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 1027–1033. [Google Scholar] [CrossRef]
- Nieoullon, A.; Canolle, B.; Masmejean, F.; Guillet, B.; Pisano, P.; Lortet, S. The Neuronal Excitatory Amino Acid Transporter EAAC1/EAAT3: Does It Represent a Major Actor at the Brain Excitatory Synapse? J. Neurochem. 2006, 98, 1007–1018. [Google Scholar] [CrossRef]
- Delgado-Acevedo, C.; Estay, S.F.; Radke, A.K.; Sengupta, A.; Escobar, A.P.; Henríquez-Belmar, F.; Reyes, C.A.; Haro-Acuña, V.; Utreras, E.; Sotomayor-Zárate, R.; et al. Behavioral and Synaptic Alterations Relevant to Obsessive-Compulsive Disorder in Mice with Increased EAAT3 Expression. Neuropsychopharmacology 2019, 44, 1163–1173. [Google Scholar] [CrossRef]
- González, L.F.; Henríquez-Belmar, F.; Delgado-Acevedo, C.; Cisternas-Olmedo, M.; Arriagada, G.; Sotomayor-Zárate, R.; Murphy, D.L.; Moya, P.R. Neurochemical and Behavioral Characterization of Neuronal Glutamate Transporter EAAT3 Heterozygous Mice. Biol. Res. 2017, 50, 29. [Google Scholar] [CrossRef]
- Bellini, S.; Fleming, K.E.; De, M.; McCauley, J.P.; Petroccione, M.A.; D’Brant, L.Y.; Tkachenko, A.; Kwon, S.; Jones, L.A.; Scimemi, A. Neuronal Glutamate Transporters Control Dopaminergic Signaling and Compulsive Behaviors. J. Neurosci. 2018, 38, 937–961. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.; Cong, J.; Zhang, X. Association Between the SLC1A1 Glutamate Transporter Gene and Obsessive-Compulsive Disorder in the Chinese Han Population. Neuropsychiatr. Dis. Treat. 2021, 17, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.M.; Lu, J.; Rodriguiz, R.M.; Trotta, N.C.; Peca, J.; Ding, J.-D.; Feliciano, C.; Chen, M.; Adams, J.P.; Luo, J.; et al. Cortico-Striatal Synaptic Defects and OCD-like Behaviours in Sapap3-Mutant Mice. Nature 2007, 448, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.M.; Wang, D.; Feng, G. Differential mRNA Expression and Protein Localization of the SAP90/PSD-95-Associated Proteins (SAPAPs) in the Nervous System of the Mouse. J. Comp. Neurol. 2004, 472, 24–39. [Google Scholar] [CrossRef]
- Soto, J.S.; Jami-Alahmadi, Y.; Chacon, J.; Moye, S.L.; Diaz-Castro, B.; Wohlschlegel, J.A.; Khakh, B.S. Astrocyte-Neuron Subproteomes and Obsessive-Compulsive Disorder Mechanisms. Nature 2023, 616, 764–773. [Google Scholar] [CrossRef]
- Chen, X.; Levy, J.M.; Hou, A.; Winters, C.; Azzam, R.; Sousa, A.A.; Leapman, R.D.; Nicoll, R.A.; Reese, T.S. PSD-95 Family MAGUKs Are Essential for Anchoring AMPA and NMDA Receptor Complexes at the Postsynaptic Density. Proc. Natl. Acad. Sci. USA 2015, 112, E6983–E6992. [Google Scholar] [CrossRef]
- Naaz, S.; Balachander, S.; Srinivasa Murthy, N.; Ms, B.; Sud, R.; Saha, P.; Narayanaswamy, J.C.; Reddy Yc, J.; Jain, S.; Purushottam, M.; et al. Association of SAPAP3 Allelic Variants with Symptom Dimensions and Pharmacological Treatment Response in Obsessive-Compulsive Disorder. Exp. Clin. Psychopharmacol. 2022, 30, 106–112. [Google Scholar] [CrossRef]
- Stewart, S.E.; Yu, D.; Scharf, J.M.; Neale, B.M.; Fagerness, J.A.; Mathews, C.A.; Arnold, P.D.; Evans, P.D.; Gamazon, E.R.; Davis, L.K.; et al. Genome-Wide Association Study of Obsessive-Compulsive Disorder. Mol. Psychiatry 2013, 18, 788–798. [Google Scholar] [CrossRef]
- St. Laurent, R.; Kusche, K.M.; Rein, B.; Raymond, K.B.; Kreitzer, A.C.; Malenka, R.C. St. Laurent, R.; Kusche, K.M.; Rein, B.; Raymond, K.B.; Kreitzer, A.C.; Malenka, R.C. Intercalated Amygdala Dysfunction Drives Avoidance Extinction Deficits in the Sapap3 Mouse Model of Obsessive-Compulsive Disorder. Biol. Psychiatry 2024. [Google Scholar] [CrossRef]
- Ehmer, I.; Feenstra, M.; Willuhn, I.; Denys, D. Instrumental Learning in a Mouse Model for Obsessive-Compulsive Disorder: Impaired Habit Formation in Sapap3 Mutants. Neurobiol. Learn. Mem. 2020, 168, 107162. [Google Scholar] [CrossRef]
- Manning, E.E.; Wang, A.Y.; Saikali, L.M.; Winner, A.S.; Ahmari, S.E. Disruption of Prepulse Inhibition Is Associated with Compulsive Behavior Severity and Nucleus Accumbens Dopamine Receptor Changes in Sapap3 Knockout Mice. Sci. Rep. 2021, 11, 9442. [Google Scholar] [CrossRef]
- Lamothe, H.; Schreiweis, C.; Mondragón-González, L.S.; Rebbah, S.; Lavielle, O.; Mallet, L.; Burguière, E. The Sapap3−/− Mouse Reconsidered as a Comorbid Model Expressing a Spectrum of Pathological Repetitive Behaviours. Transl. Psychiatry 2023, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Hadjas, L.C.; Schartner, M.M.; Cand, J.; Creed, M.C.; Pascoli, V.; Lüscher, C.; Simmler, L.D. Projection-Specific Deficits in Synaptic Transmission in Adult Sapap3-Knockout Mice. Neuropsychopharmacology 2020, 45, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Burguière, E.; Monteiro, P.; Feng, G.; Graybiel, A.M. Optogenetic Stimulation of Lateral Orbitofronto-Striatal Pathway Suppresses Compulsive Behaviors. Science 2013, 340, 1243–1246. [Google Scholar] [CrossRef]
- Mintzopoulos, D.; Gillis, T.E.; Robertson, H.R.; Dalia, T.; Feng, G.; Rauch, S.L.; Kaufman, M.J. Striatal Magnetic Resonance Spectroscopy Abnormalities in Young Adult SAPAP3 Knockout Mice. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 39–48. [Google Scholar] [CrossRef]
- Manning, E.E.; Dombrovski, A.Y.; Torregrossa, M.M.; Ahmari, S.E. Impaired Instrumental Reversal Learning Is Associated with Increased Medial Prefrontal Cortex Activity in Sapap3 Knockout Mouse Model of Compulsive Behavior. Neuropsychopharmacology 2019, 44, 1494–1504. [Google Scholar] [CrossRef]
- Malgady, J.M.; Baez, A.; Hobel, Z.B.; Jimenez, K.; Goldfried, J.; Prager, E.M.; Wilking, J.A.; Zhang, Q.; Feng, G.; Plotkin, J.L. Pathway-Specific Alterations in Striatal Excitability and Cholinergic Modulation in a SAPAP3 Mouse Model of Compulsive Motor Behavior. Cell Rep. 2023, 42, 113384. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Ai, M.; Xia, D.; Chen, H.; Pang, R.; Mei, R.; Zhong, L.; Chen, L. Upregulation of SLITRK5 in Patients with Epilepsy and in a Rat Model. Synapse 2023, 77, e22266. [Google Scholar] [CrossRef]
- Ko, J. The Leucine-Rich Repeat Superfamily of Synaptic Adhesion Molecules: LRRTMs and Slitrks. Mol. Cells 2012, 34, 335–340. [Google Scholar] [CrossRef]
- Round, J.; Ross, B.; Angel, M.; Shields, K.; Lom, B. Slitrk Gene Duplication and Expression in the Developing Zebrafish Nervous System. Dev. Dyn. 2014, 243, 339–349. [Google Scholar] [CrossRef]
- Beaubien, F.; Cloutier, J.-F. Differential Expression of Slitrk Family Members in the Mouse Nervous System. Dev. Dyn. 2009, 238, 3285–3296. [Google Scholar] [CrossRef]
- Aruga, J.; Mikoshiba, K. Identification and Characterization of Slitrk, a Novel Neuronal Transmembrane Protein Family Controlling Neurite Outgrowth. Mol. Cell Neurosci. 2003, 24, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Aruga, J.; Yokota, N.; Mikoshiba, K. Human SLITRK Family Genes: Genomic Organization and Expression Profiling in Normal Brain and Brain Tumor Tissue. Gene 2003, 315, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Shmelkov, S.V.; Hormigo, A.; Jing, D.; Proenca, C.C.; Bath, K.G.; Milde, T.; Shmelkov, E.; Kushner, J.S.; Baljevic, M.; Dincheva, I.; et al. Slitrk5 Deficiency Impairs Corticostriatal Circuitry and Leads to Obsessive-Compulsive-like Behaviors in Mice. Nat. Med. 2010, 16, 598–602. [Google Scholar] [CrossRef]
- Ullrich, M.; Weber, M.; Post, A.M.; Popp, S.; Grein, J.; Zechner, M.; Guerrero González, H.; Kreis, A.; Schmitt, A.G.; Üçeyler, N.; et al. OCD-like Behavior Is Caused by Dysfunction of Thalamo-Amygdala Circuits and Upregulated TrkB/ERK-MAPK Signaling as a Result of SPRED2 Deficiency. Mol. Psychiatry 2018, 23, 444–458. [Google Scholar] [CrossRef]
- Wang, T.; Gao, T.; Fujisawa, M.; Ohara, T.; Sakaguchi, M.; Yoshimura, T.; Matsukawa, A. SPRED2 Is a Novel Regulator of Autophagy in Hepatocellular Carcinoma Cells and Normal Hepatocytes. Int. J. Mol. Sci. 2024, 25, 6269. [Google Scholar] [CrossRef]
- Wakioka, T.; Sasaki, A.; Kato, R.; Shouda, T.; Matsumoto, A.; Miyoshi, K.; Tsuneoka, M.; Komiya, S.; Baron, R.; Yoshimura, A. Spred Is a Sprouty-Related Suppressor of Ras Signalling. Nature 2001, 412, 647–651. [Google Scholar] [CrossRef]
- Kato, R.; Nonami, A.; Taketomi, T.; Wakioka, T.; Kuroiwa, A.; Matsuda, Y.; Yoshimura, A. Molecular Cloning of Mammalian Spred-3 Which Suppresses Tyrosine Kinase-Mediated Erk Activation. Biochem. Biophys. Res. Commun. 2003, 302, 767–772. [Google Scholar] [CrossRef]
- Hepbasli, D.; Gredy, S.; Ullrich, M.; Reigl, A.; Abeßer, M.; Raabe, T.; Schuh, K. Genotype- and Age-Dependent Differences in Ultrasound Vocalizations of SPRED2 Mutant Mice Revealed by Machine Deep Learning. Brain Sci. 2021, 11, 1365. [Google Scholar] [CrossRef]
- Tuduce, I.L.; Schuh, K.; Bundschu, K. Spred2 Expression during Mouse Development. Dev. Dyn. 2010, 239, 3072–3085. [Google Scholar] [CrossRef]
- Tränkner, D.; Boulet, A.; Peden, E.; Focht, R.; Van Deren, D.; Capecchi, M. A Microglia Sublineage Protects from Sex-Linked Anxiety Symptoms and Obsessive Compulsion. Cell Rep. 2019, 29, 791–799.e3. [Google Scholar] [CrossRef]
- Hienert, M.; Gryglewski, G.; Stamenkovic, M.; Kasper, S.; Lanzenberger, R. Striatal Dopaminergic Alterations in Tourette’s Syndrome: A Meta-Analysis Based on 16 PET and SPECT Neuroimaging Studies. Transl. Psychiatry 2018, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Exome Sequencing in Obsessive-Compulsive Disorder Reveals a Burden of Rare Damaging Coding Variants—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34183866/ (accessed on 25 December 2024).
- Song, M.; Mathews, C.A.; Stewart, S.E.; Shmelkov, S.V.; Mezey, J.G.; Rodriguez-Flores, J.L.; Rasmussen, S.A.; Britton, J.C.; Oh, Y.-S.; Walkup, J.T.; et al. Rare Synaptogenesis-Impairing Mutations in SLITRK5 Are Associated with Obsessive Compulsive Disorder. PLoS ONE 2017, 12, e0169994. [Google Scholar] [CrossRef]
- Rajkumar, R.P. SAPAP3, SPRED2, and Obsessive-Compulsive Disorder: The Search for Fundamental Phenotypes. Front. Mol. Neurosci. 2023, 16, 1095455. [Google Scholar] [CrossRef]
- Romanelli, R.J.; Wu, F.M.; Gamba, R.; Mojtabai, R.; Segal, J.B. Behavioral Therapy and Serotonin Reuptake Inhibitor Pharmacotherapy in the Treatment of Obsessive-Compulsive Disorder: A Systematic Review and Meta-Analysis of Head-to-Head Randomized Controlled Trials. Depress. Anxiety 2014, 31, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Nezgovorova, V.; Reid, J.; Fineberg, N.A.; Hollander, E. Optimizing First Line Treatments for Adults with OCD. Compr. Psychiatry 2022, 115, 152305. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.F.; Orr, S.P.; Essoe, J.K.-Y.; McCracken, J.T.; Storch, E.A.; Piacentini, J. Extinction Learning in Childhood Anxiety Disorders, Obsessive Compulsive Disorder and Post-Traumatic Stress Disorder: Implications for Treatment. Expert. Rev. Neurother. 2016, 16, 1155–1174. [Google Scholar] [CrossRef]
- Goodman, W.K.; Storch, E.A.; Sheth, S.A. Harmonizing the Neurobiology and Treatment of Obsessive-Compulsive Disorder. Am. J. Psychiatry 2021, 178, 17–29. [Google Scholar] [CrossRef]
- Del Casale, A.; Sorice, S.; Padovano, A.; Simmaco, M.; Ferracuti, S.; Lamis, D.A.; Rapinesi, C.; Sani, G.; Girardi, P.; Kotzalidis, G.D.; et al. Psychopharmacological Treatment of Obsessive-Compulsive Disorder (OCD). Curr. Neuropharmacol. 2019, 17, 710–736. [Google Scholar] [CrossRef]
- Skapinakis, P.; Caldwell, D.M.; Hollingworth, W.; Bryden, P.; Fineberg, N.A.; Salkovskis, P.; Welton, N.J.; Baxter, H.; Kessler, D.; Churchill, R.; et al. Pharmacological and Psychotherapeutic Interventions for Management of Obsessive-Compulsive Disorder in Adults: A Systematic Review and Network Meta-Analysis. Focus (Am. Psychiatr. Publ.) 2021, 19, 457–467. [Google Scholar] [CrossRef]
- Jenike, M.A. Obsessive–compulsive disorder. N. Eng. J. Med. 2004, 350, 259–265. [Google Scholar] [CrossRef]
- Pallanti, S.; Quercioli, L. Treatment-Refractory Obsessive-Compulsive Disorder: Methodological Issues, Operational Definitions and Therapeutic Lines. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Simpson, H.B.; Huppert, J.D.; Petkova, E.; Foa, E.B.; Liebowitz, M.R. Response versus Remission in Obsessive-Compulsive Disorder. J. Clin. Psychiatry 2006, 67, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-D.; Shi, D.-D.; Wang, Z. Neurobiology of Obsessive-Compulsive Disorder from Genes to Circuits: Insights from Animal Models. Neurosci. Bull. 2024, 40, 1975–1994. [Google Scholar] [CrossRef] [PubMed]
- Roh, D.; Jang, K.W.; Kim, C.-H. Clinical Advances in Treatment Strategies for Obsessive-Compulsive Disorder in Adults. Clin. Psychopharmacol. Neurosci. 2023, 21, 676–685. [Google Scholar] [CrossRef]
- Xie, L.; Hu, P.; Guo, Z.; Chen, M.; Wang, X.; Du, X.; Li, Y.; Chen, B.; Zhang, J.; Zhao, W.; et al. Immediate and Long-Term Efficacy of Transcranial Direct Current Stimulation (tCDS) in Obsessive-Compulsive Disorder, Posttraumatic Stress Disorder and Anxiety Disorders: A Systematic Review and Meta-Analysis. Transl. Psychiatry 2024, 14, 343. [Google Scholar] [CrossRef]
- Greenberg, B.D.; Rauch, S.L.; Haber, S.N. Invasive Circuitry-Based Neurotherapeutics: Stereotactic Ablation and Deep Brain Stimulation for OCD. Neuropsychopharmacology 2010, 35, 317–336. [Google Scholar] [CrossRef]
- Goodman, W.K.; Insel, T.R. Deep Brain Stimulation in Psychiatry: Concentrating on the Road Ahead. Biol. Psychiatry 2009, 65, 263–266. [Google Scholar] [CrossRef]
- Goodman, W.K.; Alterman, R.L. Deep Brain Stimulation for Intractable Psychiatric Disorders. Annu. Rev. Med. 2012, 63, 511–524. [Google Scholar] [CrossRef]
- Alonso, P.; Cuadras, D.; Gabriëls, L.; Denys, D.; Goodman, W.; Greenberg, B.D.; Jimenez-Ponce, F.; Kuhn, J.; Lenartz, D.; Mallet, L.; et al. Deep Brain Stimulation for Obsessive-Compulsive Disorder: A Meta-Analysis of Treatment Outcome and Predictors of Response. PLoS ONE 2015, 10, e0133591. [Google Scholar] [CrossRef]
- Martinho, F.P.; Duarte, G.S.; Couto, F.S. do Efficacy, Effect on Mood Symptoms, and Safety of Deep Brain Stimulation in Refractory Obsessive-Compulsive Disorder: A Systematic Review and Meta-Analysis. J. Clin. Psychiatry 2020, 81, 19r12821. [Google Scholar] [CrossRef]
- Hageman, S.B.; van Rooijen, G.; Bergfeld, I.O.; Schirmbeck, F.; de Koning, P.; Schuurman, P.R.; Denys, D. Deep Brain Stimulation versus Ablative Surgery for Treatment-Refractory Obsessive-Compulsive Disorder: A Meta-Analysis. Acta Psychiatr. Scand. 2021, 143, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, C.; Pollak, P. Optogenetically Inspired Deep Brain Stimulation: Linking Basic with Clinical Research. Swiss Med. Wkly. 2016, 146, w14278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiverstein, J.; Rietveld, E.; Slagter, H.A.; Denys, D. Obsessive Compulsive Disorder: A Pathology of Self-Confidence? Trends Cogn. Sci. 2019, 23, 369–372. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).