Regression-Based Classification of the Middle-Latency Auditory-Evoked Potentials in Vestibular Migraine and Concussion Patients with Dizziness
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants
2.2.1. Inclusion Criteria
CCS Group
- Any patient aged 18–65 years old with a confirmed diagnosis of a single concussion, at least 3 months prior to the test, fulfilling the CISG definition of sport-related CCS [56] and the ACRM diagnostic criteria for mild TBI [57]. Accordingly, a mild TBI is defined as a traumatically induced physiological disruption of brain function, evidenced by: (I) at least one clinical sign attributed to the attributable to brain injury or having two (or more) symptoms and one (or more) abnormal clinical examination or laboratory findings; (II) loss of consciousness with maximal duration of 30 min, a Glasgow Coma Scale between 13 and 15 and a post-traumatic amnesia with maximal duration of 24 h; (IV) no evidence of any abnormalities on neuroimaging.
- Cerebral MRI including susceptibility-weighted imaging confirming no signs of brain trauma.
- Normal hearing in the PTA—defined as ≤20 dB nHL for frequencies between 500 and 4000 Hz—and hearing threshold ≤10 dB for click stimuli in both ears. This measure was taken in order to exclude any heterogeneity in hearing function amongst the study groups.
VM Group
- Any patient aged 18–65 years old diagnosed with VM in accordance with the Bárány Society [17].
- Normal hearing in the PTA—defined as ≤20 dB nHL for frequencies between 500 and 4000 Hz—and hearing threshold ≤10 dB for click stimuli in both ears. This measure was taken in order to exclude any heterogeneity in hearing function amongst the study groups.
2.2.2. Exclusion Criteria
CCS Group
- Presence of neurological disorders other than concussion (besides primary headache disorders), central vestibular disorders, ocular disorders, or/and psychiatric (excl. ADHD) disorders. Central vestibular and psychiatric disorders were excluded by (I) taking the patients’ history with special regard to those disorders and respective medication, (II) performing a detailed clinical neurological examination in all patients and (III) studying the patients’ records including brain imaging, when available.
- Patients with acute concussion (<3 months since injury).
- Patients who incurred a CCS that have or did experience one (or more) of the following: (A) a post-traumatic loss of consciousness with a duration exceeding 30 min; (B) a Glasgow Coma Scale below 13; (C) a post-traumatic amnesia lasting more than 24 h; (D) post-traumatic evidence of abnormalities on neuroimaging.
- History of migraine or VM to ensure that the neurophysiological differences observed were attributable selectively to the condition under study.
- History of multiple CCS to reduce data variability and a more homogenous study population, as neurophysiological responses after repeated CCS could differ significantly from those observed after a single CCS.
VM Group
- History of CCS to ensure that the neurophysiological differences observed were attributable selectively to the condition under study.
- Presence of hearing and balance disorders due to other causes. In order to exclude hearing disorders due to other causes, study subjects underwent an otological examination (including otoscopy, Weber, Rinne and Valsalva test) by an ENT specialist and a PTA. In addition, audiological history was taken and patient records including audiological examinations were reviewed. Other vestibular disorders were excluded by patients’ history, clinical neurotological examination and vestibular testing as indicated by the clinical phenotype.
- Subjects who had an attack 1 or 2 days prior to the AMLR test.
2.3. Recording and Stimulatory Parameters
2.4. Procedure
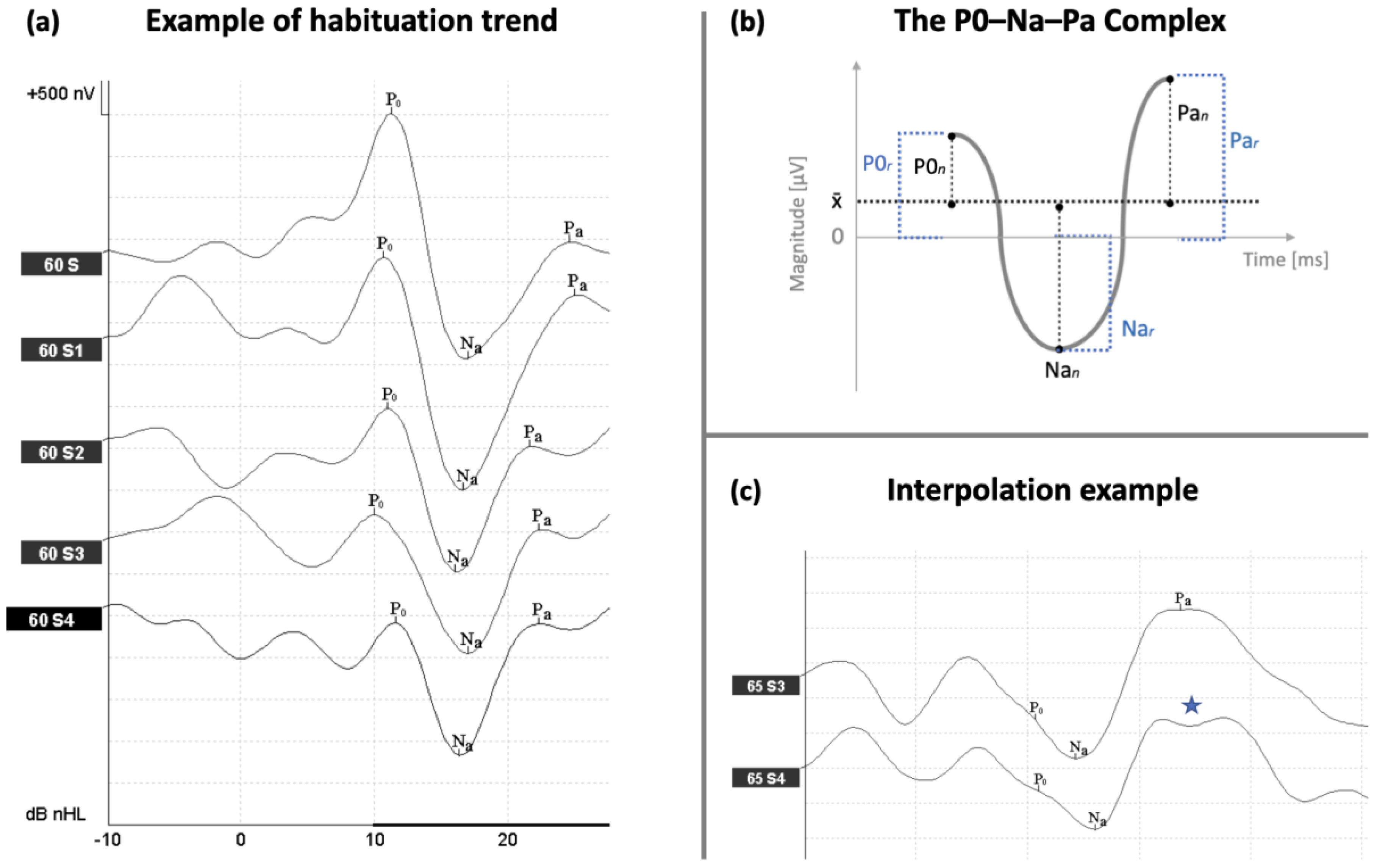
2.5. AMLR Labeling Nomenclature and Corrections
2.6. AMLR Measures
2.7. Statistical Analyses
3. Results
3.1. Age
3.2. AMLR Habituation
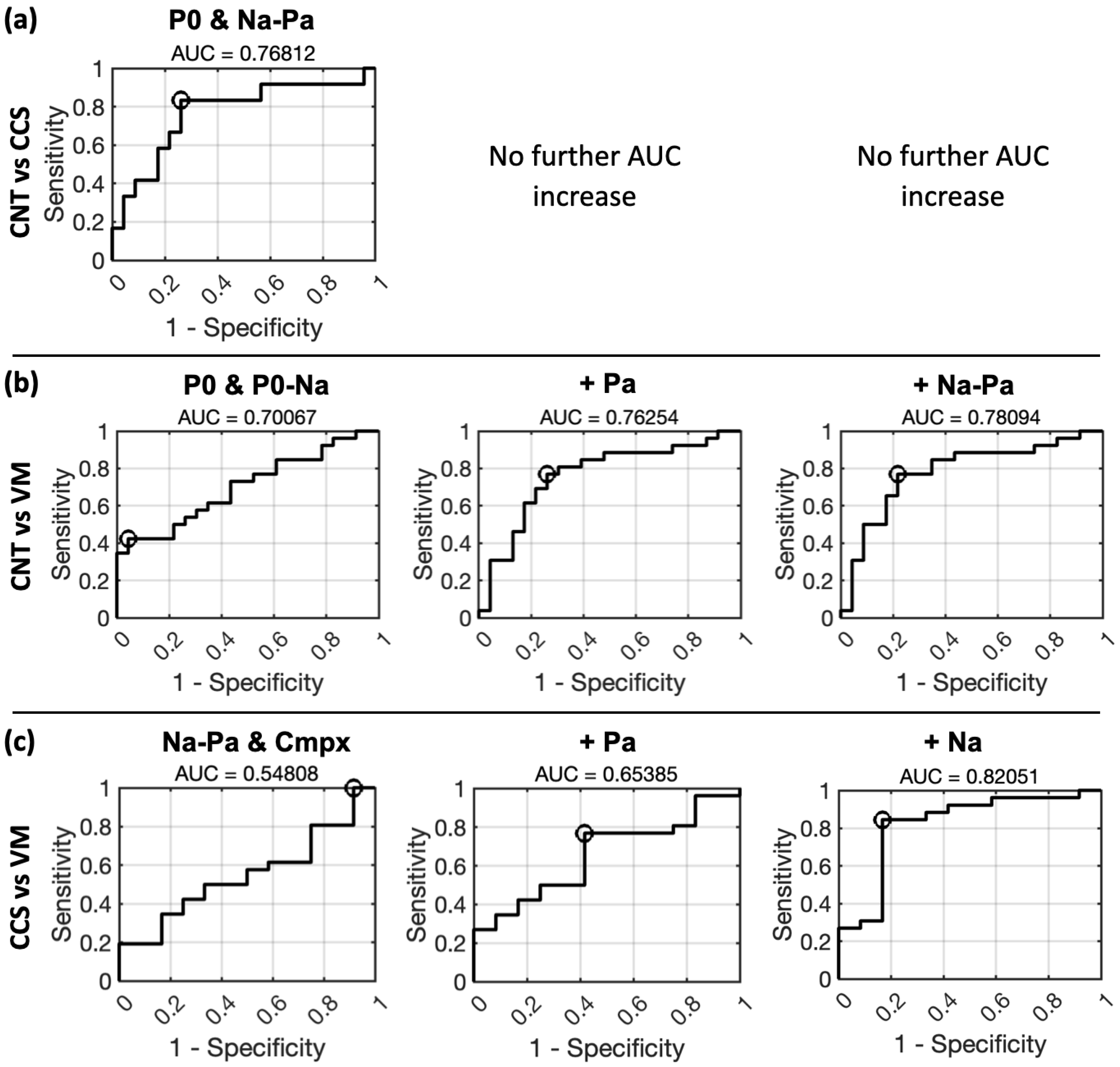
3.3. ROC Curves for 1-by-2 Classification Using Logistic Regression
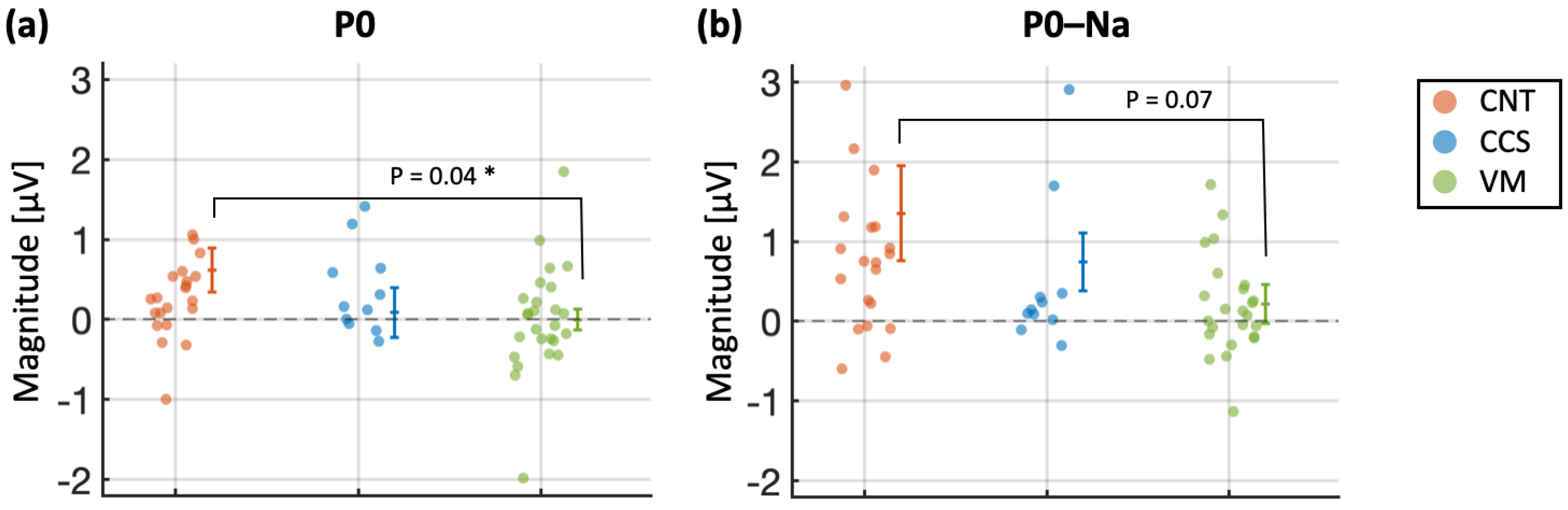
- P0 reaches the highest AUC when distinguishing the CNT group from the rest and was, on a general level, the measure achieving the highest AUCs for all 1-by-2 group comparisons. Statistically significant AUCs for the P0 measure were achieved for the following comparisons: CNT vs. rest (AUC = 0.723, p = 0.0065) and VM vs. rest (AUC = 0.696, p = 0.0118). For the Po–Na measure, statistically significant AUC were instead achieved for the CCS vs. rest comparison (AUC = 0.740, p = 0.04) and for the VM vs. rest comparison (AUC = 0.651, p = 0.05).
- Na was instead the most “disadvantaged” measure, achieving the lowest AUCs. A statistically significant AUC for the Na measure was achieved for the VM vs. rest comparison only (AUC = 0.534, p = 0.045).
- Pa and Na–Pa prevailed in the distinction of CCS patients from the rest. For the Pa measure, only the CCS vs. rest comparison approached statistical significance (AUC = 0.832, p = 0.0007). Statistically significant AUCs for the Na–Pa measure were achieved for the CCS vs. rest comparison (AUC = 0.787, p = 0.0065) and for the CNT vs. rest comparison (AUC = 0.566, p = 0.0071).
- Cmpx showed a balanced class-prediction ability across 1-by-2 group comparisons. Statistically significant AUCs for the cmpx measure included: CCS vs. rest (AUC = 0.781, p = 0.04); CNT vs. rest (AUC = 0.632, p = 0.0261).
3.4. ROC Curves for 1-by-1 Classification Using Multiple Regression
4. Discussion
Further Limitations, Challenges and Future Directions
5. Conclusions
- The results expand the work by Murofushi et al. [12], comparing the performance of VM patients with a different neurological group (i.e., chronic CCS) with related clinical symptoms, providing commonalities and distinguishing AMLR features. Deficits of AMLR habituation are evident in VM patients, whereas CCS patients display alterations—rather than deficits—in sensory processing and integration, possibly due to plasticity processes of functional reorganization that warrant further investigation. Particularly, for cases presenting overlapping symptoms and unclear history, the identification of different neurophysiological patterns could aid the identification of the more dominant underlying pathology. As such, AMLR testing as a complementary tool could contribute to the definition of more targeted therapies.
- AMLR habituation is a helpful quantitative and qualitative measure to interpret and support the regression-based accuracy of group classifications. No AMLR measure can, in general, be thought of as an “absolute best” at distinguishing clinical groups. Each measure shows a relevant advantage depending on the comparison group: P0 for distinguishing CNT and CCS, P0 and P0–Na for distinguishing CNT and VM and finally Na and Pa for distinguishing CCS and VM.
- Despite distinct AMLR patterns having been identified for the VM, chronic concussion and healthy subjects, our results are still premature, and univocal inferences the diagnostic specificity of these measures cannot yet be derived.
- The collection of larger datasets is required to improve statistical power and predictive ability and to control for possibly confounding factors like age.
- Our methodological challenges point to the need for weighting different baseline adjustment and standard normalisation procedures against each other, considering their relative benefits and risks, to minimize potential bias while preserving data quality.
- The inclusion of the acute concussion subgroup—(A) acute (<3 months since injury) and (B) chronic (>3 months) stage—would provide relevant insights into the AMLR investigation, in comparison with the VM group.
- Follow-up studies might consider including patients with comorbid VM and CCS to explore their interaction and potentially beneficial treatments for this clinical subgroup.
- The AMLR assessment could be extended to other neurological and psychiatric disorders sharing aspects of the clinical profile of CCS patients, such as long COVID-19 [82] and cognitive/neurological deficits related to dementia, multiple sclerosis and/or Parkinson’s disease.
- It might also be relevant to try distinguishing groups based on the presence of given symptoms, instead of based on diagnosis. For example, headache and vestibular signs are common in mild TBI and (vestibular) migraine patients [39].
- Future research should not only aim to replicate these findings but rather investigate additional biomarkers that might complement AMLR testing in clinical practice.
- Cell recordings during repeated auditory stimuli could be considered to test whether a peripheral component of sensory adaptation contributes to response habituation. Whether sensory adaptation and central habituation are mutually exclusive processes is in fact still an open research question, and a matter of debate in the existing animal and human literature [83,84,85,86].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Washnik, N.J.; Anjum, J.; Lundgren, K.; Phillips, S.A. A review of the role of auditory evoked potentials in mild traumatic brain injury assessment. Trends Hear. 2019, 23, 2331216519840094. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.; White-Schwoch, T. Unraveling the biology of auditory learning: A cognitive-sensorimotor-reward framework. Trends Cogn. Sci. 2015, 19, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Hoth, S.; Baljić, I. Current audiological diagnostics. GMS Curr. Top. Otorhinolaryngol. Head. Neck Surg. 2017, 16, 18. [Google Scholar]
- Hall, J. New Handbook of Auditory Evoked Responses; Allyn & Bacon: Boston, MA, USA, 2007. [Google Scholar]
- De Siati, R.D.; Rosenzweig, F.; Gersdorff, G.; Gregoire, A.; Rombaux, P.; Deggouj, N. Auditory neuropathy spectrum disorders: From diagnosis to treatment: Literature review and case reports. J. Clin. Med. 2020, 9, 1074. [Google Scholar] [CrossRef]
- Atcherson, S.R.; Kennett, S.W. Early, mid, and late-latency auditory evoked potentials during pregnancy: Two cases. J. Pregnancy Child. Health. 2014, 1, 121. [Google Scholar]
- Musiek, F.; Lee, W. Auditory middle and late potentials. In Contemporary Perspectives on Hearing Assessment; Musiek, F., Rintelmann, W., Eds.; Allyn & Bacon: Boston, MA, USA, 1999; pp. 243–272. [Google Scholar]
- Moore, E.J. Basis of Auditory Brain-Stem Evoked Responses; Grune & Stratton: New York, NY, USA, 1983. [Google Scholar]
- Picton, T.W.; Hillyard, S.A.; Krausz, H.I.; Galambos, R. Human auditory evoked potentials. I: Evaluation of components. Electroencephalogr. Clin. Neurophysiol. 1974, 36, 179–190. [Google Scholar] [CrossRef]
- Pratt, H. Middle-latency response. In Auditory Evoked Potentials: Basic Principles and Clinical Application; Burkard, R.F., Don, M., Eggermont, J.J., Eds.; Williams & Wilkins: Baltimore, MD, USA, 2007; pp. 463–481. [Google Scholar]
- Beppi, C.; Violante, I.R.; Scott, G.; Sandrone, S. EEG, MEG and neuromodulatory approaches to explore cognition: Current status and future directions. Brain Cogn. 2021, 148, 105677. [Google Scholar] [CrossRef]
- Murofushi, T.; Goto, F.; Tsubota, M. Vestibular migraine patients show lack of habituation in auditory middle latency responses to repetitive stimuli: Comparison with Meniere’s disease patients. Front. Neurol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Pelizzone, M.; Hari, R.; Mäkelä, J.P.; Huttunen, J.; Ahlfors, S.; Hämäläinen, M. Cortical origin of middle-latency auditory evoked responses in man. Neurosci. Lett. 1987, 82, 303–307. [Google Scholar] [CrossRef]
- Borgmann, C.; Ross, B.; Draganova, R.; Pantev, C. Human auditory middle latency responses: Influence of stimulus type and intensity. Hear. Res. 2001, 158, 57–64. [Google Scholar] [CrossRef]
- Bell, S.L.; Smith, D.C.; Allen, R.; Lutman, M.E. Recording the middle latency response of the auditory evoked potential as a measure of depth of anaesthesia. A technical note. Br. J. Anaesth. 2004, 92, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.W.; Katz, J.; Chasin, M.; English, K.; Hood, L.J.; Tillery, K.L. Handbook of Clinical Audiology, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2015. [Google Scholar]
- Lempert, T.; Olesen, J.; Furman, J.; Waterston, J.; Seemungal, B.; Carey, J.; Bisdorff, A.; Versino, M.; Evers, S.; Kheradmand, A.; et al. Vestibular migraine: Diagnostic criteria. J. Vestib. Res. 2022, 32, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.; Maihoub, S.; Mavrogeni, P.; Tamás, L.; Szirmai, Á. Depression scores and quality of life of vertiginous patients, suffering from different vestibular disorders. Eur. Arch. Otorhinolaryngol. 2022, 279, 5173–5179. [Google Scholar] [CrossRef] [PubMed]
- Lahmann, C.; Henningsen, P.; Brandt, T.; Strupp, M.; Jahn, K.; Dieterich, M.; Eckhardt-Henn, A.; Feuerecker, R.; Dinkel, A.; Schmid, G. Psychiatric comorbidity and psychosocial impairment among patients with vertigo and dizziness. J. Neurol. Neurosurg. Psychiatry 2015, 86, 302–308. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.H.; Lin, J.H.; Zhou, C.; Pan, Y.H. Research on the relationship between vestibular migraine with/without cognitive impairment and brainstem auditory evoked potential. Front. Neurol. 2020, 11, 159. [Google Scholar] [CrossRef]
- Meaney, D.F.; Smith, D.H. Biomechanics of concussion. Clin. Sports Med. 2011, 30, 19–31. [Google Scholar] [CrossRef]
- Voss, J.D.; Connolly, J.; Schwab, K.A.; Scher, A.I. Update on the epidemiology of concussion/mild traumatic brain injury. Curr. Pain. Headache Rep. 2015, 19, 32. [Google Scholar] [CrossRef]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef]
- Rabinowitz, A.R.; Fisher, A.J. Person-specific methods for characterizing the course and temporal dynamics of concussion symptomatology: A pilot study. Sci. Rep. 2020, 10, 1248. [Google Scholar] [CrossRef]
- Randolph, C.; Millis, S.; Barr, W.B.; McCrea, M.; Guskiewicz, K.M.; Hammeke, T.A.; Kelly, J.P. Concussion symptom inventory: An empirically derived scale for monitoring resolution of symptoms following sport-related concussion. Arch. Clin. Neuropsychol. 2009, 24, 219–229. [Google Scholar] [CrossRef]
- Giza, C.C.; Hovda, D.A. The new neurometabolic cascade of concussion. Neurosurgery 2014, 75, S24–S33. [Google Scholar] [CrossRef] [PubMed]
- McCrea, M.; Barr, W.B.; Guskiewicz, K.; Randolph, C.; Marshall, S.W.; Cantu, R.; Onate, J.A.; Kelly, J.P. Standard regression-based methods for measuring recovery after sport-related concussion. J. Int. Neuropsychol. Soc. 2005, 11, 58–69. [Google Scholar] [CrossRef]
- Lovell, M.R.; Collins, M.W.; Iverson, G.L.; Field, M.; Maroon, J.C.; Cantu, R.; Podell, K.; Powell, J.W.; Belza, M.; Fu, F.H. Recovery from mild concussion in high school athletes. J. Neurosurg. 2003, 98, 296–301. [Google Scholar] [CrossRef]
- O’Keeffe, E.; Kelly, E.; Liu, Y.; Giordano, C.; Wallace, E.; Hynes, M.; Tiernan, S.; Meagher, A.; Greene, C.; Hughes, S.; et al. Dynamic blood–brain barrier regulation in mild traumatic brain injury. J. Neurotrauma 2020, 37, 347–356. [Google Scholar] [CrossRef]
- Rivara, F.P.; Graham, R. Sports-related concussions in youth: Report from the Institute of Medicine and National Research Council. JAMA 2014, 311, 239–240. [Google Scholar] [CrossRef]
- Baillargeon, A.; Lassonde, M.; Leclerc, S.; Ellemberg, D. Neuropsychological and neurophysiological assessment of sport concussion in children, adolescents and adults. Brain Inj. 2012, 26, 211–220. [Google Scholar] [CrossRef]
- Toth, A. Magnetic resonance imaging application in the area of mild and acute traumatic brain injury: Implications for diagnostic markers? In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2015; p. 24. [Google Scholar]
- Byrnes, K.R.; Wilson, C.M.; Brabazon, F.; Von Leden, R.; Jurgens, J.S.; Oakes, T.R.; Selwyn, R.G. FDG-PET imaging in mild traumatic brain injury: A critical review. Front. Neuroenergetics 2014, 5, 13. [Google Scholar] [CrossRef]
- Churchill, N.W.; Hutchison, M.G.; Graham, S.J.; Schweizer, T.A. Long-term changes in the small-world organization of brain networks after concussion. Sci. Rep. 2021, 11, 6862. [Google Scholar] [CrossRef]
- Sharp, D.J.; Jenkins, P.O. Concussion is confusing us all. Pract. Neurol. 2015, 15, 172–186. [Google Scholar] [CrossRef]
- Beppi, C.; Penner, M.; Straumann, D.; Bögli, S.Y. A non-invasive biomechanical model of mild TBI in larval zebrafish. PLoS ONE 2022, 17, e0268901. [Google Scholar] [CrossRef]
- Beppi, C.; Penner, M.; Straumann, D.; Bögli, S.Y. Biomechanical induction of mild brain trauma in larval zebrafish: Effects on visual startle reflex habituation. Brain Commun. 2023, 5, fcad062. [Google Scholar] [CrossRef]
- Drake, M.E.; Weate, S.J.; Newell, S.A. Auditory evoked potentials in postconcussive syndrome. Electromyogr. Clin. Neurophysiol. 1996, 36, 457–462. [Google Scholar]
- Ottaviani, F.; Almadori, G.; Calderazzo, A.B.; Frenguelli, A.; Paludetti, G. Auditory brain-stem (ABss) and middle latency auditory responses (MLRs) in the prognosis of severely head-injured patients. Electroencephalogr. Clin. Neurophysiol/Evoked Potentials Sect. 1986, 65, 196–202. [Google Scholar] [CrossRef]
- Staab, J.P.; Eckhardt-Henn, A.; Horii, A.; Jacob, R.; Strupp, M.; Brandt, T.; Bronstein, A. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): Consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J. Vestib. Res. 2017, 27, 191–208. [Google Scholar] [CrossRef]
- Murofushi, T.; Goto, F.; Ushio, M. Habituation disorders in auditory middle latency response (AMLR) of persistent postural-perceptual dizziness (PPPD) patients. Front. Neurol. 2024, 15, 1366420. [Google Scholar] [CrossRef]
- Adler, L.E.; Olincy, A.; Waldo, M.; Harris, J.G.; Griffith, J.; Stevens, K.; Flach, K.; Nagamoto, H.; Bickford, P.; Leonard, S.; et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr. Bull. 1998, 24, 189–202. [Google Scholar] [CrossRef]
- Budnick, B.; Braff, D.L. Sensory gating deficits in schizophrenia: New results. Am. J. Psychiatry 1992, 149, 489–491. [Google Scholar]
- Boutros, N.N.; Overall, J.; Zouridakis, G. Test-retest reliability of the P50 mid-latency auditory evoked response. Psychiatry Res. 1991, 39, 181–192. [Google Scholar] [CrossRef]
- Van Gils, A.; Stone, J.; Welch, K.; Davidson, L.R.; Kerslake, D.; Caesar, D.; McWhirter, L.; Carson, A. Management of mild traumatic brain injury. Pract. Neurol. 2020, 20, 213–221. [Google Scholar] [CrossRef]
- Terry, D.P.; Reddi, P.J.; Cook, N.E.; Seifert, T.; Maxwell, B.A.; Zafonte, R.; Berkner, P.D.; Iverson, G.L. Acute effects of concussion in youth with pre-existing migraines. Clin. J. Sport Med. 2021, 31, 430–437. [Google Scholar] [CrossRef]
- Covassin, T.; Crutcher, B.; Belanger, S. Preinjury history of migraine headache: Effects on neurocognitive performance and symptoms in athletes with concussion. Athl. Train. Sports Health Care. 2014, 6, 220–227. [Google Scholar] [CrossRef]
- Jo, J.; Berkner, P.D.; Stephenson, K.; Maxwell, B.A.; Iverson, G.L.; Zuckerman, S.L.; Terry, D.P. Examining acute symptoms after sport-related concussion in collegiate athletes with preinjury migraines. Clin. J. Sport Med. 2022, 32, 10–1097. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Chou, T.L.; Lai, W.S.; Hsieh, M.H.; Liu, C.C.; Liu, C.M.; Hwu, H.G. P50, N100, and P200 auditory sensory gating deficits in schizophrenia patients. Front. Psychiatry 2020, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Cancelli, I.; Cadore, I.P.; Merlino, G.; Valentinis, L.; Moratti, U.; Bergonzi, P.; Gigli, G.L.; Valente, M. Sensory gating deficit assessed by P50/Pb middle latency event-related potential in Alzheimer’s disease. J. Clin. Neurophysiol. 2006, 23, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas, D.; Olincy, A.; Topkoff, J.; McRae, K.; Cawthra, E.; Filley, C.M.; Reite, M.; Adler, L.E. Impaired auditory gating and P50 nonsuppression following traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 77–85. [Google Scholar] [CrossRef]
- Soustiel, J.F.; Hafner, H.; Chistyakov, A.V.; Barzilai, A.; Feinsod, M. Trigeminal and auditory evoked responses in minor head injuries and post-concussion syndrome. Brain Inj. 1995, 9, 805–813. [Google Scholar] [CrossRef]
- Munjal, S.K.; Panda, N.K.; Pathak, A. Relationship between severity of traumatic brain injury (TBI) and extent of auditory dysfunction. Brain Inj. 2010, 24, 525–532. [Google Scholar] [CrossRef]
- Gaetz, M.; Weinberg, H. Electrophysiological indices of persistent post-concussion symptoms. Brain Inj. 2000, 14, 815–832. [Google Scholar]
- Musiek, F.E.; Baran, J.A.; Shinn, J. Assessment and remediation of an auditory processing disorder associated with head trauma. J. Am. Acad. Audiol. 2004, 15, 117–132. [Google Scholar] [CrossRef]
- Davis, G.A.; Patricios, J.; Schneider, K.J.; Iverson, G.L.; Silverberg, N.D. Definition of sport-related concussion: The 6th International Conference on Concussion in Sport. Br. J. Sports Med. 2023, 57, 617–618. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Iverson, G.L.; Group, A.B.I.S.; Cogan, A.; Dams-O-Connor, K.; Delmonico, R.; Zemek, R. The American Congress of Rehabilitation Medicine diagnostic criteria for mild traumatic brain injury. Arch. Phys. Med. Rehabil. 2023, 104, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Carl, A.C.; Hohman, M.H.; Cornejo, J. Audiology pure tone evaluation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Frizzo, A.C. Auditory evoked potential: A proposal for further evaluation in children with learning disabilities. Front. Psychol. 2015, 6, 788. [Google Scholar] [CrossRef] [PubMed]
- Neves, I.F.; Gonçalves, I.C.; Leite, R.A.; Magliaro, F.C.; Matas, C.G. Middle latency response study of auditory evoked potentials’ amplitudes and latencies in audiologically normal individuals. Rev. Bras. Otorrinolaringol. 2007, 73, 75–80. [Google Scholar] [CrossRef]
- Frizzo, A.C.; Funayama, C.A.; Isaac, M.L.; Colafêmina, J.F. Auditory middle latency responses: A study of healthy children. Rev. Bras. Otorrinolaringol. 2007, 73, 398–403. [Google Scholar] [CrossRef]
- Biagianti, B.; Stocchetti, N.; Brambilla, P.; Van Vleet, T. Brain dysfunction underlying prolonged post-concussive syndrome: A systematic review. J. Affect. Disord. 2020, 262, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Gumus, M.; Santos, A.; Tartaglia, M.C. Diffusion and functional MRI findings and their relationship to behavior in postconcussion syndrome: A scoping review. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1259–1270. [Google Scholar] [CrossRef]
- Hampshire, A.; MacDonald, A.; Owen, A.M. Hypoconnectivity and hyperfrontality in retired American football players. Sci. Rep. 2013, 3, 2972. [Google Scholar] [CrossRef]
- Chen, J.K.; Johnston, K.M.; Petrides, M.; Ptito, A. Recovery from mild head injury in sports: Evidence from serial functional magnetic resonance imaging studies in male athletes. Clin. J. Sport Med. 2008, 18, 241–247. [Google Scholar] [CrossRef]
- Chang, W.T.; Jääskeläinen, I.P.; Belliveau, J.W.; Huang, S.; Hung, A.Y.; Rossi, S.; Ahveninen, J. Combined MEG and EEG show reliable patterns of electromagnetic brain activity during natural viewing. Neuroimage 2015, 114, 49–56. [Google Scholar] [CrossRef]
- Rytter, H.M.; Graff, H.J.; Henriksen, H.K.; Aaen, N.; Hartvigsen, J.; Hoegh, M.; Nisted, I.; Næss-Schmidt, E.T.; Pedersen, L.L.; Schytz, H.W.; et al. Nonpharmacological treatment of persistent postconcussion symptoms in adults: A systematic review and meta-analysis and guideline recommendation. JAMA Netw. Open 2021, 4, e2132221. [Google Scholar] [CrossRef]
- Moser, N.; Gargoum, S.; Popovic, M.R.; Kalsi-Ryan, S. Effectiveness of non-pharmacological therapy on physical symptoms in patients with persistent concussion symptoms: A systematic review. J. Neurotrauma 2024, 41, 1473–1493. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S. Posttraumatic headache: Clinical characterization and management. Curr. Pain Headache Rep. 2015, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Sengul, Y.; Bal, N.; Louis, E.D. Evidence of central involvement in essential tremor: A detailed study of auditory pathway physiology. J. Neural Transm. 2020, 127, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Matas, C.G.; Matas, S.L.; Oliveira, C.R.; Gonçalves, I.C. Auditory evoked potentials and multiple sclerosis. Arq. Neuropsiquiatr. 2010, 68, 528–534. [Google Scholar] [CrossRef]
- Romero, A.C.; Funayama, C.A.; Capellini, S.A.; Frizzo, A.C. Auditory middle latency response and phonological awareness in students with learning disabilities. Int. Arch. Otorhinolaryngol. 2015, 19, 325–330. [Google Scholar]
- Eggers, S.D.Z.; Staab, J.P. Vestibular migraine and persistent postural perceptual dizziness. Handb. Clin. Neurol. 2024, 199, 389–411. [Google Scholar]
- Ponsford, J.; Cameron, P.; Fitzgerald, M.; Grant, M.; Mikocka-Walus, A. Long-term outcomes after uncomplicated mild traumatic brain injury: A comparison with trauma controls. J. Neurotrauma 2011, 28, 937–946. [Google Scholar] [CrossRef]
- Kelman, L.; Tanis, D. The relationship between migraine pain and other associated symptoms. Cephalalgia 2006, 26, 548–553. [Google Scholar] [CrossRef]
- Ashina, M.; Terwindt, G.M.; Al-Karagholi, M.A.M.; De Boer, I.; Lee, M.J.; Hay, D.L.; Schulte, L.H.; Hadjikhani, N.; Sinclair, A.J.; Ashina, H.; et al. Migraine: Disease characterisation, biomarkers, and precision medicine. Lancet 2021, 397, 1496–1504. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Suleiman, A.; Lithgow, B.; Dastgheib, Z.; Mansouri, B.; Moussavi, Z. Quantitative measurement of post-concussion syndrome using electrovestibulography. Sci. Rep. 2017, 7, 16371. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, M.; Drisdelle, B.L.; Lefebvre, C.; Gagnon, J.F.; De Beaumont, L.; Jolicoeur, P. Interhemispheric differences in P1 and N1 amplitude in EEG and MEG differ across older individuals with a concussion compared with age-matched controls. Psychophysiology 2021, 58, e13751. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, D.; Wennberg, R.; Gütling, E.; Hess, K. Whiplash and concussion: Similar acute changes in middle-latency SEPs. Can. J. Neurol. Sci. 2006, 33, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Mizota, A.; Tormene, A.P.; International Society for Clinical Electrophysiology of Vision. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Wang, D.H.; Trojian, T.; Leddy, J.J. Post–COVID-19 neurological syndrome and concussion. Clin. J. Sport Med. 2022, 32, 555–557. [Google Scholar] [CrossRef]
- Daniel, M.J.; Koffinas, L.; Hughes, K.A. Habituation underpins preference for mates with novel phenotypes in the guppy. Proc. Biol. Sci. 2019, 286, 20190435. [Google Scholar] [CrossRef]
- Rankin, C.H.; Abrams, T.; Barry, R.J.; Bhatnagar, S.; Clayton, D.F.; Colombo, J.; Coppola, G.; Geyer, M.A.; Glanzman, D.L.; Marsland, S.; et al. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 2009, 92, 135–138. [Google Scholar] [CrossRef]
- McBurney, D.H.; Balaban, C.D. A heuristic model of sensory adaptation. Atten. Percept. Psychophys. 2009, 71, 1941–1961. [Google Scholar] [CrossRef]
- Kuenen, L.P.; Baker, T.C. Habituation versus sensory adaptation as the cause of reduced attraction following pulsed and constant sex pheromone pre-exposure in Trichoplusiani. J. Insect Physiol. 1981, 27, 721–726. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beppi, C.; Agostino, D.; Palla, A.; Feddermann-Demont, N.; Dlugaiczyk, J.; Straumann, D. Regression-Based Classification of the Middle-Latency Auditory-Evoked Potentials in Vestibular Migraine and Concussion Patients with Dizziness. Brain Sci. 2025, 15, 1. https://doi.org/10.3390/brainsci15010001
Beppi C, Agostino D, Palla A, Feddermann-Demont N, Dlugaiczyk J, Straumann D. Regression-Based Classification of the Middle-Latency Auditory-Evoked Potentials in Vestibular Migraine and Concussion Patients with Dizziness. Brain Sciences. 2025; 15(1):1. https://doi.org/10.3390/brainsci15010001
Chicago/Turabian StyleBeppi, Carolina, Daniel Agostino, Antonella Palla, Nina Feddermann-Demont, Julia Dlugaiczyk, and Dominik Straumann. 2025. "Regression-Based Classification of the Middle-Latency Auditory-Evoked Potentials in Vestibular Migraine and Concussion Patients with Dizziness" Brain Sciences 15, no. 1: 1. https://doi.org/10.3390/brainsci15010001
APA StyleBeppi, C., Agostino, D., Palla, A., Feddermann-Demont, N., Dlugaiczyk, J., & Straumann, D. (2025). Regression-Based Classification of the Middle-Latency Auditory-Evoked Potentials in Vestibular Migraine and Concussion Patients with Dizziness. Brain Sciences, 15(1), 1. https://doi.org/10.3390/brainsci15010001





