Nicotine’s Effects on Schizophrenia-like Symptoms in a Mice Model: Time Matters
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Protocol
2.2.1. Behavioral Sensitization
- Habituation
- Experiment 1 (Exp. 1)
- Experiment 2 (Exp. 2)
- Behavioral Assessment
2.2.2. Sensorimotor Gating
2.3. Statistical Analysis
3. Results
3.1. Body Mass
3.2. Open Field: Locomotor Activity
3.2.1. Habituation (Tables S3 and S4)
3.2.2. Acquisition Phase 1 (Figure 2)

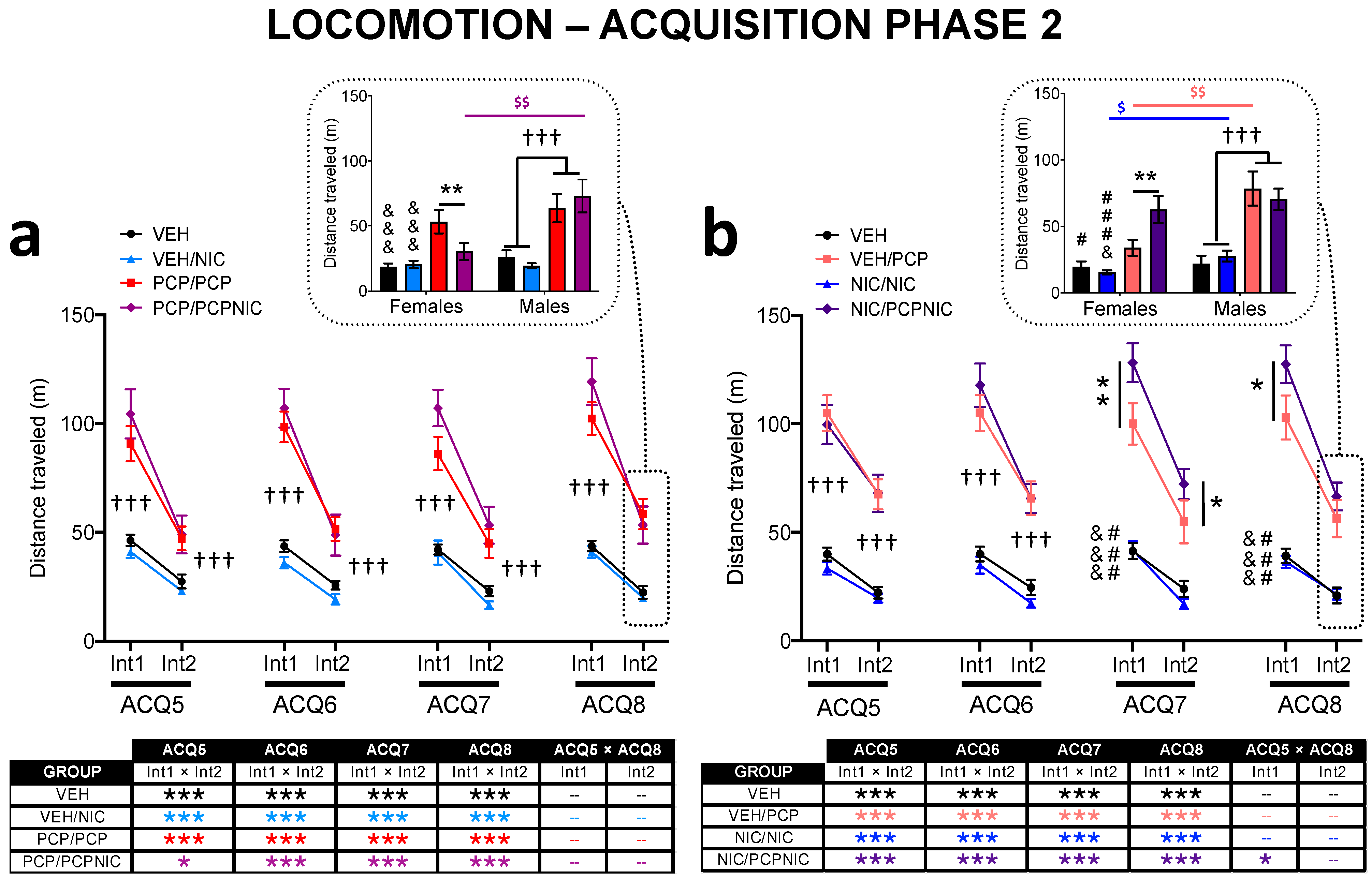
3.2.3. Acquisition Phase 2 (Table 1, Figure 3)
| Experiment 1: Effect or Interaction | Fd.f., p Value |
| Phencyclidine | F1,83 = 76.0, p < 0.001 |
| Phencyclidine × Sex | F1,83 = 4.5, p = 0.036 |
| Day × Phencyclidine | F3,249 = 2.1, p = 0.097 |
| Interval × Phencyclidine | F1,83 = 340.8, p < 0.001 |
| Interval × Nicotine | F1,83 = 4.0, p = 0.051 |
| Interval × Phencyclidine × Nicotine | F1,83 = 3.1, p = 0.082 |
| Experiment 2: Effect or Interaction | Fd.f., p Value |
| Phencyclidine | F1,73 = 232.6, p < 0.001 |
| Nicotine × Phencyclidine | F1,73 = 3.7, p = 0.058 |
| Phencyclidine × Sex | F1,73 = 7.8, p = 0.006 |
| Day × Interval × Phencyclidine | F3,219 = 2.7, p = 0.05 |
| Day × Interval × Phencyclidine × Sex | F3,219 = 3.3, p = 0.021 |
| Day × Interval × Nicotine × Phencyclidine × Sex | F3,219 = 2.8, p = 0.042 |
| Interval × Phencyclidine | F1,73 = 49.1, p < 0.001 |
3.3. Open Field: Rearing
3.3.1. Acquisition Phase 1 (Figure 4a,b)
3.3.2. Acquisition Phase 2 (Table 2, Figure 4c,d)
| Experiment 1: Effect or Interaction | Fd.f., p Value |
| Phencyclidine | F1,74 = 9.0, p = 0.004 |
| Nicotine | F1,74 = 86.3, p < 0.001 |
| Phencyclidine × Nicotine | F1,74 = 23.4, p < 0.001 |
| Day × Interval × Phencyclidine | F1,74 = 3.3, p = 0.072 |
| Interval × Phencyclidine | F1,74 = 74.9, p < 0.001 |
| Interval × Nicotine | F1,74 = 214.9, p < 0.001 |
| Interval × Phencyclidine × Nicotine | F1,74 = 73.6, p < 0.001 |
| Experiment 2: Effect or Interaction | Fd.f., p Value |
| Nicotine | F1,73 = 158.4, p < 0.001 |
| Phencyclidine | F1,73 = 160.9, p < 0.001 |
| Nicotine × Phencyclidine | F1,73 = 112.2, p < 0.001 |
| Day × Nicotine | F1,73 = 3.0, p = 0.087 |
| Interval × Nicotine | F1,73 = 191.8, p < 0.001 |
| Interval × Phencyclidine | F1,73 = 164.5, p < 0.001 |
| Interval × Nicotine × Phencyclidine | F1,73 = 211.1, p < 0.001 |

3.4. Prepulse Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- American Psychiatric Association. Manual Diagnóstico e Estatístico de Transtornos Mentais: DSM-5, 5th ed.; Artmed: Porto Alegre, Brasil, 2014. [Google Scholar]
- Kumari, V.; Postma, P. Nicotine Use in Schizophrenia: The Self Medication Hypotheses. Neurosci. Biobehav. Rev. 2005, 29, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Ohi, K.; Shimada, T.; Kuwata, A.; Kataoka, Y.; Okubo, H.; Kimura, K.; Yasuyama, T.; Uehara, T.; Kawasaki, Y. Smoking Rates and Number of Cigarettes Smoked per Day in Schizophrenia: A Large Cohort Meta-Analysis in a Japanese Population. Int. J. Neuropsychopharmacol. 2019, 22, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hartz, S.M.; Pato, C.N.; Medeiros, H.; Cavazos-Rehg, P.; Sobell, J.L.; Knowles, J.A.; Bierut, L.J.; Pato, M.T.; Genomic Psychiatry Cohort Consortium. Comorbidity of Severe Psychotic Disorders with Measures of Substance Use. JAMA Psychiatry 2014, 71, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.L.; Wayne, G.F.; Kafali, E.N.; Liu, Z.; Shu, C.; Flores, M. Trends in Smoking Among Adults with Mental Illness and Association between Mental Health Treatment and Smoking Cessation. JAMA 2014, 311, 172. [Google Scholar] [CrossRef]
- Han, B.; Volkow, N.D.; Blanco, C.; Tipperman, D.; Einstein, E.B.; Compton, W.M. Trends in Prevalence of Cigarette Smoking Among US Adults with Major Depression or Substance Use Disorders, 2006–2019. JAMA 2022, 327, 1566–1576. [Google Scholar] [CrossRef]
- Szatkowski, L.; McNeill, A. Diverging Trends in Smoking Behaviors According to Mental Health Status. Nicotine Tob. Res. 2015, 17, 356–360. [Google Scholar] [CrossRef]
- Spring, B.; Pingitore, R.; McChargue, D.E. Reward Value of Cigarette Smoking for Comparably Heavy Smoking Schizophrenic, Depressed, and Nonpatient Smokers. Am. J. Psychiatry 2003, 160, 316–322. [Google Scholar] [CrossRef]
- Ziedonis, D.; Hitsman, B.; Beckham, J.C.; Zvolensky, M.; Adler, L.E.; Audrain-McGovern, J.; Breslau, N.; Brown, R.A.; George, T.P.; Williams, J.; et al. Tobacco Use and Cessation in Psychiatric Disorders: National Institute of Mental Health Report. Nicotine Tob. Res. 2008, 10, 1691–1715. [Google Scholar] [CrossRef]
- Lanza, S.T.; Vasilenko, S.A. New Methods Shed Light on Age of Onset as a Risk Factor for Nicotine Dependence. Addict. Behav. 2015, 50, 161–164. [Google Scholar] [CrossRef]
- Cullen, K.A.; Liu, S.T.; Bernat, J.K.; Slavit, W.I.; Tynan, M.A.; King, B.A.; Neff, L.J. Flavored Tobacco Product Use among Middle and High School Students—United States, 2014–2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 839–844. [Google Scholar] [CrossRef]
- Miech, R.; Johnston, L.; O’Malley, P.M.; Bachman, J.G.; Patrick, M.E. Adolescent Vaping and Nicotine Use in 2017–2018—U.S. National Estimates. N. Engl. J. Med. 2019, 380, 192–193. [Google Scholar] [CrossRef]
- Häfner, H. From Onset and Prodromal Stage to a Life-Long Course of Schizophrenia and Its Symptom Dimensions: How Sex, Age, and Other Risk Factors Influence Incidence and Course of Illness. Psychiatry J. 2019, 2019, 9804836. [Google Scholar] [CrossRef]
- Tandon, R.; Nasrallah, H.A.; Keshavan, M.S. Schizophrenia, “Just the Facts” 4. Clinical Features and Conceptualization. Schizophr. Res. 2009, 110, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gourzis, P.; Katrivanou, A.; Beratis, S. Symptomatology of the Initial Prodromal Phase in Schizophrenia. Schizophr. Bull. 2002, 28, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Lally, J.; Spaducci, G.; Gardner-Sood, P.; Atakan, Z.; Greenwood, K.; Di Forti, M.; Ismail, K.; Murphy, K.C.; Smith, S.; McNeill, A.; et al. Tobacco Smoking and Nicotine Dependence in First Episode and Established Psychosis. Asian J. Psychiatry 2019, 43, 125–131. [Google Scholar] [CrossRef]
- Myles, N.; Newall, H.D.; Curtis, J.; Nielssen, O.; Shiers, D.; Large, M. Tobacco Use before, at, and after First-Episode Psychosis: A Systematic Meta-Analysis. J. Clin. Psychiatry 2012, 73, 468–475. [Google Scholar] [CrossRef]
- Raballo, A.; Poletti, M. Advances in Early Identification of Children and Adolescents at Risk for Psychiatric Illness. Curr. Opin. Psychiatry 2020, 33, 611–617. [Google Scholar] [CrossRef]
- Hamasaki, Y.; Nakayama, T.; Hikida, T.; Murai, T. Combined Pattern of Childhood Psycho-Behavioral Characteristics in Patients with Schizophrenia: A Retrospective Study in Japan. BMC Psychiatry 2021, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, T.; He, Y.; Meng, F.; Zhang, K.; Jin, X.; Cui, X.; Luo, X. Value of P300 Amplitude in the Diagnosis of Untreated First-Episode Schizophrenia and Psychosis Risk Syndrome in Children and Adolescents. BMC Psychiatry 2023, 23, 743. [Google Scholar] [CrossRef]
- Green, M.J.; O’Hare, K.; Laurens, K.R.; Tzoumakis, S.; Dean, K.; Badcock, J.C.; Harris, F.; Linscott, R.J.; Carr, V.J. Developmental Profiles of Schizotypy in the General Population: A Record Linkage Study of Australian Children Aged 11–12 Years. Br. J. Clin. Psychol. 2022, 61, 836–858. [Google Scholar] [CrossRef]
- Collin, G.; Bauer, C.C.C.; Anteraper, S.A.; Gabrieli, J.D.E.; Molokotos, E.; Mesholam-Gately, R.; Thermenos, H.W.; Seidman, L.J.; Keshavan, M.S.; Shenton, M.E.; et al. Hyperactivation of Posterior Default Mode Network During Self-Referential Processing in Children at Familial High-Risk for Psychosis. Front. Psychiatry 2021, 12, 613142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Liang, J.; Chen, D.C.; Xiu, M.H.; He, J.; Cheng, W.; Wu, Z.; De Yang, F.; Haile, C.N.; Sun, H.; et al. Cigarette Smoking in Male Patients with Chronic Schizophrenia in a Chinese Population: Prevalence and Relationship to Clinical Phenotypes. PLoS ONE 2012, 7, e30937. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.; Kutlu, M.G.; Gould, T.J. NAChR Dysfunction as a Common Substrate for Schizophrenia and Comorbid Nicotine Addiction: Current Trends and Perspectives. Schizophr. Res. 2016, 171, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, R.E.; Siegel, S.J. The Role of Nicotine in Schizophrenia. Int. Rev. Neurobiol. 2015, 124, 23–78. [Google Scholar]
- Riala, K.; Hakko, H.; Isohanni, M.; Pouta, A.; Räsänen, P. Is Initiation of Smoking Associated with the Prodromal Phase of Schizophrenia? J. Psychiatry Neurosci. 2005, 30, 26–32. [Google Scholar] [PubMed]
- Weeks, J.J.; Grace, A.A.; Sved, A.F. Nicotine Administration Normalizes Behavioral and Neurophysiological Perturbations in the MAM Rodent Model of Schizophrenia. Int. J. Neuropsychopharmacol. 2021, 24, 979–987. [Google Scholar] [CrossRef]
- Tizabi, Y.; Copeland, R.L., Jr.; Brus, R.; Kostrzewa, R.M. Nicotine Blocks Quinpirole-Induced Behavior in Rats: Psychiatric Implications. Psychopharmacology 1999, 145, 433–441. [Google Scholar] [CrossRef]
- Waterhouse, U.; Roper, V.E.; Brennan, K.A.; Ellenbroek, B.A. Nicotine Ameliorates Schizophrenia-like Cognitive Deficits Induced by Maternal LPS Exposure: A Study in Rats. Dis. Model. Mech. 2016, 9, 1159–1167. [Google Scholar] [CrossRef]
- Richtand, N.M.; Woods, S.C.; Berger, S.P.; Strakowski, S.M. D3 Dopamine Receptor, Behavioral Sensitization, and Psychosis. Neurosci. Biobehav. Rev. 2001, 25, 427–443. [Google Scholar] [CrossRef]
- Berg, S.A.; Sentir, A.M.; Cooley, B.S.; Engleman, E.A.; Chambers, R.A. Nicotine Is More Addictive, Not More Cognitively Therapeutic in a Neurodevelopmental Model of Schizophrenia Produced by Neonatal Ventral Hippocampal Lesions. Addict. Biol. 2014, 19, 1020–1031. [Google Scholar] [CrossRef]
- Peeters, L.D.; Wills, L.J.; Cuozzo, A.M.; Ivanich, K.L.; Turney, S.E.; Bullock, L.P.; Price, R.M.; Gass, J.T.; Brown, R.W. Modulation of MGlu5 Reduces Rewarding Associative Properties of Nicotine via Changes in Mesolimbic Plasticity: Relevance to Comorbid Cigarette Smoking in Psychosis. Pharmacol. Biochem. Behav. 2024, 239, 173752. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.A.; Perry, D.C.; O’Neil, J.; Manaye, K.F.; Tizabi, Y. Effects of Nicotine on Sensorimotor Gating Impairment Induced by Long-Term Treatment with Neurotoxic NMDA Antagonism. Neurotox. Res. 2008, 13, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Soeda, K.; Uchida, M.; Goto, S.; Ito, T.; Kitagaki, S.; Mamiya, T.; Yoshimi, A.; Ozaki, N.; Mouri, A. Multiple Nicotinic Acetylcholine Receptor Subtypes Regulate Social or Cognitive Behaviors in Mice Repeatedly Administered Phencyclidine. Behav. Brain Res. 2021, 408, 113284. [Google Scholar] [CrossRef] [PubMed]
- Yavas, E.; Young, A.M.J. Repeated Phencyclidine Disrupts Nicotinic Acetylcholine Regulation of Dopamine Release in Nucleus Accumbens: Implications for Models of Schizophrenia. Neurochem. Int. 2020, 140, 104836. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.A.; Chambers, R.A. Accentuated Behavioral Sensitization to Nicotine in the Neonatal Ventral Hippocampal Lesion Model of Schizophrenia. Neuropharmacology 2008, 54, 1201–1207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, R.W.; Kirby, S.L.; Denton, A.R.; Dose, J.M.; Cummins, E.D.; Drew Gill, W.; Burgess, K.C. An Analysis of the Rewarding and Aversive Associative Properties of Nicotine in the Neonatal Quinpirole Model: Effects on Glial Cell Line-Derived Neurotrophic Factor (GDNF). Schizophr. Res. 2018, 194, 107–114. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Sun, Y.; Hu, X.; Geng, P.; Shu, H.; Wang, X.; Wang, H.; Zhang, J.; Cheng, H.; et al. Nicotine Pretreatment Alleviates MK-801-Induced Behavioral and Cognitive Deficits in Mice by Regulating Pdlim5/CRTC1 in the PFC. Acta Pharmacol. Sin. 2023, 44, 780–790. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Sumikawa, K. Nicotine-Induced Neuroplasticity Counteracts the Effect of Schizophrenia-Linked Neuregulin 1 Signaling on NMDAR Function in the Rat Hippocampus. Neuropharmacology 2017, 113, 386–395. [Google Scholar] [CrossRef]
- Schmidt, S.J.; Schultze-Lutter, F.; Schimmelmann, B.G.; Maric, N.P.; Salokangas, R.K.R.; Riecher-Rössler, A.; van der Gaag, M.; Meneghelli, A.; Nordentoft, M.; Marshall, M.; et al. EPA Guidance on the Early Intervention in Clinical High Risk States of Psychoses. Eur. Psychiatry 2015, 30, 388–404. [Google Scholar] [CrossRef]
- Cannon, T.D. How Schizophrenia Develops: Cognitive and Brain Mechanisms Underlying Onset of Psychosis. Trends Cogn. Sci. 2015, 19, 744–756. [Google Scholar] [CrossRef]
- Dutra-Tavares, A.C.; Manhães, A.C.; Semeão, K.A.; Maia, J.G.; Couto, L.A.; Filgueiras, C.C.; Ribeiro-Carvalho, A.; Abreu-Villaça, Y. Does Nicotine Exposure during Adolescence Modify the Course of Schizophrenia-like Symptoms? Behavioral Analysis in a Phencyclidine-Induced Mice Model. PLoS ONE 2021, 16, e0257986. [Google Scholar] [CrossRef] [PubMed]
- Nespor, A.A.; Tizabi, Y. Effects of Nicotine on Quinpirole- and Dizocilpine (MK-801)-Induced Sensorimotor Gating Impairments in Rats. Psychopharmacology 2008, 200, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Taylor, J.R. Chronic Nicotine Attenuates Phencyclidine-Induced Impulsivity in a Mouse Serial Reaction Time Task. Behav. Brain Res. 2014, 259, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vega, A.; Dutra-Tavares, A.C.; Souza, T.P.; Semeão, K.A.; Filgueiras, C.C.; Ribeiro-Carvalho, A.; Manhães, A.C.; Abreu-Villaça, Y. Nicotine Exposure in a Phencyclidine-Induced Mice Model of Schizophrenia: Sex-Selective Medial Prefrontal Cortex Protein Markers of the Combined Insults in Adolescent Mice. Int. J. Mol. Sci. 2023, 24, 14634. [Google Scholar] [CrossRef]
- Dutra-Tavares, A.C.; Bandeira-Martins, A.; Silva, J.O.; Couto, L.A.; Filgueiras, C.C.; Ribeiro-Carvalho, A.; Manhães, A.C.; Abreu-Villaça, Y. Adolescent Nicotine Potentiates the Inhibitory Effect of Raclopride, a D2R Antagonist, on Phencyclidine-Sensitized Psychotic-like Behavior in Mice. Toxicol. Appl. Pharmacol. 2022, 456, 116282. [Google Scholar] [CrossRef]
- Noda, Y.; Uchida, M.; Mouri, A.; Yamada, S.; Goto, S.; Kitagaki, S.; Mamiya, T.; Kushima, I.; Arioka, Y.; Ozaki, N.; et al. Involvement of Nicotinic Acetylcholine Receptors in Behavioral Abnormalities and Psychological Dependence in Schizophrenia-like Model Mice. Eur. Neuropsychopharmacol. 2020, 41, 92–105. [Google Scholar] [CrossRef]
- Winship, I.R.; Dursun, S.M.; Baker, G.B.; Balista, P.A.; Kandratavicius, L.; Maia-de-Oliveira, J.P.; Hallak, J.; Howland, J.G. An Overview of Animal Models Related to Schizophrenia. Can. J. Psychiatry 2018, 64, 5–17. [Google Scholar] [CrossRef]
- Cadinu, D.; Grayson, B.; Podda, G.; Harte, M.K.; Doostdar, N.; Neill, J.C. NMDA Receptor Antagonist Rodent Models for Cognition in Schizophrenia and Identification of Novel Drug Treatments, an Update. Neuropharmacology 2018, 142, 41–62. [Google Scholar] [CrossRef]
- Allen, P.; Moore, H.; Corcoran, C.M.; Gilleen, J.; Kozhuharova, P.; Reichenberg, A.; Malaspina, D. Emerging Temporal Lobe Dysfunction in People at Clinical High Risk for Psychosis. Front. Psychiatry 2019, 10, 298. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Manhães, A.C.; Krahe, T.E.; Filgueiras, C.C.; Ribeiro-Carvalho, A. Tobacco and Alcohol Use during Adolescence: Interactive Mechanisms in Animal Models. Biochem. Pharmacol. 2017, 144, 1–17. [Google Scholar] [CrossRef]
- Leslie, F.M. Unique, Long-Term Effects of Nicotine on Adolescent Brain. Pharmacol. Biochem. Behav. 2020, 197, 173010. [Google Scholar] [CrossRef]
- Pistillo, F.; Clementi, F.; Zoli, M.; Gotti, C. Nicotinic, Glutamatergic and Dopaminergic Synaptic Transmission and Plasticity in the Mesocorticolimbic System: Focus on Nicotine Effects. Prog. Neurobiol. 2015, 124, 1–27. [Google Scholar] [CrossRef]
- Laviolette, S.R. Molecular and Neuronal Mechanisms Underlying the Effects of Adolescent Nicotine Exposure on Anxiety and Mood Disorders. Neuropharmacology 2021, 184, 108411. [Google Scholar] [CrossRef] [PubMed]
- Ziauddeen, H.; Murray, G.K. The Relevance of Reward Pathways for Schizophrenia. Curr. Opin. Psychiatry 2010, 23, 91–96. [Google Scholar] [CrossRef]
- Sams-Dodd, F. Effects of Continuous D-Amphetamine and Phencyclidine Administration on Social Behaviour, Stereotyped Behaviour, and Locomotor Activity in Rats. Neuropsychopharmacology 1998, 19, 18–25. [Google Scholar] [CrossRef]
- Braff, D.L. Prepulse Inhibition of the Startle Reflex: A Window on the Brain in Schizophrenia. Behav. Neurobiol. Schizophr. Its Treat. 2010, 4, 349–371. [Google Scholar]
- Swerdlow, N.R.; Weber, M.; Qu, Y.; Light, G.A.; Braff, D.L. Realistic Expectations of Prepulse Inhibition in Translational Models for Schizophrenia Research. Psychopharmacology 2008, 199, 331–388. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. Policy: NIH to Balance Sex in Cell and Animal Studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Selvendra, A.; Stewart, A.; Castle, D. Risk Factors in Early and Late Onset Schizophrenia. Compr. Psychiatry 2018, 80, 155–162. [Google Scholar] [CrossRef]
- Edwards, K.; Manoharan, A.; Asfar, T.; Kareff, S.; Lopes, G.; Rodriguez, E.; Olazagasti, C. Disparities in Electronic Cigarette Use: A Narrative Review. Crit. Rev. Oncog. 2024, 29, 91–98. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. E-Cigarette Use among Youth and Young Adults: A Report of the Surgeon General; US Department of Health and Human Services: Atlanta, GA, USA, 2016. [Google Scholar]
- Xu, X.; Domino, E.F. Asymmetric Cross-Sensitization to the Locomotor Stimulant Effects of Phencyclidine and MK-801. Neurochem. Int. 1994, 25, 155–159. [Google Scholar] [CrossRef][Green Version]
- Beraki, S.; Diaz-Heijtz, R.; Tai, F.; Ögren, S.O. Effects of Repeated Treatment of Phencyclidine on Cognition and Gene Expression in C57BL/6 Mice. Int. J. Neuropsychopharmacol. 2009, 12, 243. [Google Scholar] [CrossRef]
- Engel, M.; Snikeris, P.; Jenner, A.; Karl, T.; Huang, X.-F.; Frank, E. Neuregulin 1 Prevents Phencyclidine-Induced Behavioral Impairments and Disruptions to GABAergic Signaling in Mice. Int. J. Neuropsychopharmacol. 2015, 18, pyu114. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Alkhlaif, Y.; Papke, R.L.; Brunzell, D.H.; Damaj, M.I. Impact of Modulation of the A7 Nicotinic Acetylcholine Receptor on Nicotine Reward in the Mouse Conditioned Place Preference Test. Psychopharmacology 2019, 236, 3593–3599. [Google Scholar] [CrossRef] [PubMed]
- Dutra-Tavares, A.C.; Ribeiro-Carvalho, A.; Nunes, F.; Araújo, U.C.; Bruno, V.; Marcourakis, T.; Filgueiras, C.C.; Manhães, A.C.; Abreu-Villaça, Y. Lifetime Caffeine and Adolescent Nicotine Exposure in Mice: Effects on Anxiety-like Behavior and Reward. J. Dev. Orig. Health Dis. 2023, 14, 362–370. [Google Scholar] [CrossRef]
- Dutra-Tavares, A.C.; Souza, T.P.; Silva, J.O.; Semeão, K.A.; Mello, F.F.; Filgueiras, C.C.; Ribeiro-Carvalho, A.; Manhães, A.C.; Abreu-Villaça, Y. Neonatal Phencyclidine as a Model of Sex-Biased Schizophrenia Symptomatology in Adolescent Mice. Psychopharmacology 2023, 240, 2111–2129. [Google Scholar] [CrossRef] [PubMed]
- Braff, D.L.; Geyer, M.A. Sensorimotor Gating and Schizophrenia: Human and Animal Model Studies. Arch. Gen. Psychiatry 1990, 47, 181–188. [Google Scholar] [CrossRef]
- Spielewoy, C.; Markou, A. Strain-Specificity in Nicotine Attenuation of Phencyclidine-Induced Disruption of Prepulse Inhibition in Mice: Relevance to Smoking in Schizophrenia Patients. Behav. Genet. 2004, 34, 343–354. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; University Press: Ames, IA, USA, 1967. [Google Scholar]
- Qiao, J.; Gao, J.; Shu, Q.; Zhang, Q.; Hu, G.; Li, M. Long-Lasting Sensitization Induced by Repeated Risperidone Treatment in Adolescent Sprague-Dawley Rats: A Possible D2 Receptor Mediated Phenomenon? Psychopharmacology 2014, 231, 1649–1659. [Google Scholar] [CrossRef]
- Svensson, T.H. Dysfunctional Brain Dopamine Systems Induced by Psychotomimetic NMDA-Receptor Antagonists and the Effects of Antipsychotic Drugs. Brain Res. Rev. 2000, 31, 320–329. [Google Scholar] [CrossRef]
- Goff, D.C.; Coyle, J.T. The Emerging Role of Glutamate in the Pathophysiology and Treatment of Schizophrenia. Am. J. Psychiatry 2001, 158, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C.; Zukin, S.R.; Heresco-Levy, U.; Umbricht, D. Has an Angel Shown the Way? Etiological and Therapeutic Implications of the PCP/NMDA Model of Schizophrenia. Schizophr. Bull. 2012, 38, 958–966. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lane, H.-Y.; Tsai, G.E. Glutamate Signaling in the Pathophysiology and Therapy of Schizophrenia. Pharmacol. Biochem. Behav. 2012, 100, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Meyer-Lindenberg, A. Striatal Presynaptic Dopamine in Schizophrenia, Part II: Meta-Analysis of [18F/11C]-DOPA PET Studies. Schizophr. Bull. 2013, 39, 33–42. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Gil, R.; Krystal, J.; Baldwin, R.M.; Seibyl, J.P.; Bowers, M.; van Dyck, C.H.; Charney, D.S.; Innis, R.B.; Laruelle, M. Increased Striatal Dopamine Transmission in Schizophrenia: Confirmation in a Second Cohort. Am. J. Psychiatry 1998, 155, 761–767. [Google Scholar] [CrossRef]
- Lau, C.-I.; Wang, H.-C.; Hsu, J.-L.; Liu, M.-E. Does the Dopamine Hypothesis Explain Schizophrenia? Rev. Neurosci. 2013, 24, 389–400. [Google Scholar] [CrossRef]
- Castañé, A.; Santana, N.; Artigas, F. PCP-Based Mice Models of Schizophrenia: Differential Behavioral, Neurochemical and Cellular Effects of Acute and Subchronic Treatments. Psychopharmacology 2015, 232, 4085–4097. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Hart, N.; Trujillo, K.A. Differences between Adolescents and Adults in the Acute Effects of PCP and Ketamine and in Sensitization following Intermittent Administration. Pharmacol. Biochem. Behav. 2017, 157, 24–34. [Google Scholar] [CrossRef]
- Damaj, M.I.; Martin, B.R. Is the Dopaminergic System Involved in the Central Effects of Nicotine in Mice? Psychopharmacology 1993, 111, 106–108. [Google Scholar] [CrossRef]
- Dwoskin, L.P.; Crooks, P.A.; Teng, L.; Green, T.A.; Bardo, M.T. Acute and Chronic Effects of Nornicotine on Locomotor Activity in Rats: Altered Response to Nicotine. Psychopharmacology 1999, 145, 442–451. [Google Scholar] [CrossRef]
- Brielmaier, J.M.; McDonald, C.G.; Smith, R.F. Immediate and Long-Term Behavioral Effects of a Single Nicotine Injection in Adolescent and Adult Rats. Neurotoxicol. Teratol. 2007, 29, 74–80. [Google Scholar] [CrossRef]
- Damaj, M.I.; Glennon, R.A.; Martin, B.R. Involvement of the Serotonergic System in the Hypoactive and Antinociceptive Effects of Nicotine in Mice. Brain Res. Bull. 1994, 33, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Rodvelt, K.R.; Bumgarner, D.M.; Putnam, W.C.; Miller, D.K. WIN-55,212-2 and SR-141716A Alter Nicotine-Induced Changes in Locomotor Activity, but Do Not Alter Nicotine-Evoked [3H]Dopamine Release. Life Sci. 2007, 80, 337–344. [Google Scholar] [CrossRef]
- Rauhut, A.S.; Zentner, I.J.; Mardekian, S.K.; Tanenbaum, J.B. Wistar Kyoto and Wistar Rats Differ in the Affective and Locomotor Effects of Nicotine. Physiol. Behav. 2008, 93, 177–188. [Google Scholar] [CrossRef]
- Wills, L.; Ables, J.L.; Braunscheidel, K.M.; Caligiuri, S.P.B.; Elayouby, K.S.; Fillinger, C.; Ishikawa, M.; Moen, J.K.; Kenny, P.J. Neurobiological Mechanisms of Nicotine Reward and Aversion. Pharmacol. Rev. 2022, 74, 271–310. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Al-Rejaie, S.S.; AlSharari, S.D.; Sari, Y. Targeting Glutamate Homeostasis for Potential Treatment of Nicotine Dependence. Brain Res. Bull. 2016, 121, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Placzek, A.N.; Zhang, T.A.; Dani, J.A. Age Dependent Nicotinic Influences over Dopamine Neuron Synaptic Plasticity. Biochem. Pharmacol. 2009, 78, 686–692. [Google Scholar] [CrossRef]
- Braff, D.L.; Geyer, M.A.; Swerdlow, N.R. Human Studies of Prepulse Inhibition of Startle: Normal Subjects, Patient Groups, and Pharmacological Studies. Psychopharmacology 2001, 156, 234–258. [Google Scholar] [CrossRef]
- Mouri, A.; Noda, Y.; Enomoto, T.; Nabeshima, T. Phencyclidine Animal Models of Schizophrenia: Approaches from Abnormality of Glutamatergic Neurotransmission and Neurodevelopment. Neurochem. Int. 2007, 51, 173–184. [Google Scholar] [CrossRef]
- Cadenhead, K.S. Startle Reactivity and Prepulse Inhibition in Prodromal and Early Psychosis: Effects of Age, Antipsychotics, Tobacco and Cannabis in a Vulnerable Population. Psychiatry Res. 2011, 188, 208–216. [Google Scholar] [CrossRef][Green Version]
- Song, L.; Chen, X.; Chen, M.; Tang, Y.; Wang, J.; Zhang, M.; Lou, F.; Liang, J.; Chen, C. Differences in P50 and Prepulse Inhibition of the Startle Reflex between Male Smokers and Non-Smokers with First Episode Schizophrenia without Medical Treatment. Chin. Med. J. (Engl). 2014, 127, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Dalmus, M.; De Vry, J. Effects of A4/Β2- and A7-Nicotine Acetylcholine Receptor Agonists on Prepulse Inhibition of the Acoustic Startle Response in Rats and Mice. Psychopharmacology 2002, 159, 248–257. [Google Scholar] [CrossRef]
- Pinnock, F.; Bosch, D.; Brown, T.; Simons, N.; Yeomans, J.R.; DeOliveira, C.; Schmid, S. Nicotine Receptors Mediating Sensorimotor Gating and Its Enhancement by Systemic Nicotine. Front. Behav. Neurosci. 2015, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Mansvelder, H.D.; McGehee, D.S. Cellular and Synaptic Mechanisms of Nicotine Addiction. J. Neurobiol. 2002, 53, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.; Nordberg, A. Neuronal Nicotinic Receptors in the Human Brain. Prog. Neurobiol. 2000, 61, 75–111. [Google Scholar] [CrossRef]
- Barendse, M.E.A.; Lara, G.A.; Guyer, A.E.; Swartz, J.R.; Taylor, S.L.; Shirtcliff, E.A.; Lamb, S.T.; Miller, C.; Ng, J.; Yu, G.; et al. Sex and Pubertal Influences on the Neurodevelopmental Underpinnings of Schizophrenia: A Case for Longitudinal Research on Adolescents. Schizophr. Res. 2023, 252, 231–241. [Google Scholar] [CrossRef]
- Salvadé, A.; Golay, P.; Abrahamyan, L.; Bonnarel, V.; Solida, A.; Alameda, L.; Ramain, J.; Conus, P. Gender Differences in First Episode Psychosis: Some Arguments to Develop Gender Specific Treatment Strategies. Schizophr. Res. 2024, 271, 300–308. [Google Scholar] [CrossRef]
- Lewis, D.A.; González-Burgos, G. Neuroplasticity of Neocortical Circuits in Schizophrenia. Neuropsychopharmacology 2008, 33, 141–165. [Google Scholar] [CrossRef]
- Andia, A.M.; Zisook, S.; Heaton, R.K.; Hesselink, J.; Jernigan, T.; Kuck, J.; Moranville, J.; Braff, D.L. Gender Differences in Schizophrenia. J. Nerv. Ment. Dis. 1995, 183, 522–528. [Google Scholar] [CrossRef]
- Szymanski, S.; Lieberman, J.; Alvir, J.; Mayerhoff, D.; Loebel, A.; Geisler, S.; Chakos, M.; Koreen, A.; Jody, D.; Kane, J. Gender Differences in Onset of Illness, Treatment Response, Course, and Biologic Indexes in First-Episode Schizophrenic Patients. Am. J. Psychiatry 1995, 152, 698–703. [Google Scholar] [CrossRef]
- Wickens, M.M.; Bangasser, D.A.; Briand, L.A. Sex Differences in Psychiatric Disease: A Focus on the Glutamate System. Front. Mol. Neurosci. 2018, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Maher, E.E.; Strzelecki, A.M.; Weafer, J.J.; Gipson, C.D. The Importance of Translationally Evaluating Steroid Hormone Contributions to Substance Use. Front. Neuroendocrinol. 2023, 69, 101059. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P. Neuroactive Steroid Regulation of Neurotransmitter Release in the CNS: Action, Mechanism and Possible Significance. Prog. Neurobiol. 2009, 89, 134–152. [Google Scholar] [CrossRef]
- Kumari, V.; Aasen, I.; Sharma, T. Sex Differences in Prepulse Inhibition Deficits in Chronic Schizophrenia. Schizophr. Res. 2004, 69, 219–235. [Google Scholar] [CrossRef]
- Matsuo, J.; Ota, M.; Hori, H.; Hidese, S.; Teraishi, T.; Ishida, I.; Hiraishi, M.; Kunugi, H. A Large Single Ethnicity Study of Prepulse Inhibition in Schizophrenia: Separate Analysis by Sex Focusing on Effect of Symptoms. J. Psychiatr. Res. 2016, 82, 155–162. [Google Scholar] [CrossRef]
- Swanson, C.J.; Heath, S.; Stratford, T.R.; Kelley, A.E. Differential Behavioral Responses to Dopaminergic Stimulation of Nucleus Accumbens Subregions in the Rat. Pharmacol. Biochem. Behav. 1997, 58, 933–945. [Google Scholar] [CrossRef]
- Tarrés-Gatius, M.; López-Hill, X.; Miquel-Rio, L.; Castarlenas, L.; Fabius, S.; Santana, N.; Vilaró, M.T.; Artigas, F.; Scorza, M.C.; Castañé, A. Discrimination of Motor and Sensorimotor Effects of Phencyclidine and MK-801: Involvement of GluN2C-Containing NMDA Receptors in Psychosis-like Models. Neuropharmacology 2022, 213, 109079. [Google Scholar] [CrossRef]
- Kusljic, S.; van den Buuse, M.; Gogos, A. Reassessment of Amphetamine- and Phencyclidine-Induced Locomotor Hyperactivity as a Model of Psychosis-like Behavior in Rats. J. Integr. Neurosci. 2022, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Hertel, P.; Mathé, J.M.; Nomikos, G.G.; Iurlo, M.; Mathé, A.A.; Svensson, T.H. Effects of D-Amphetamine and Phencyclidine on Behavior and Extracellular Concentrations of Neurotensin and Dopamine in the Ventral Striatum and the Medial Prefrontal Cortex of the Rat. Behav. Brain Res. 1995, 72, 103–114. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Slepak, N.; Hyun, J.; Buell, M.R.; Powell, S.B. The Novel Ketamine Analog Methoxetamine Produces Dissociative-like Behavioral Effects in Rodents. Psychopharmacology 2016, 233, 1215–1225. [Google Scholar] [CrossRef]
- Iwamoto, E.T. An Assessment of the Spontaneous Activity of Rats Administered Morphine, Phencyclidine, or Nicotine Using Automated and Observational Methods. Psychopharmacology 1984, 84, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Gresa, P.; Pérez-Martinez, A.; Redolat, R. Behavioral Effects of Combined Environmental Enrichment and Chronic Nicotine Administration in Male NMRI Mice. Physiol. Behav. 2013, 114–115, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.D.; Arends, M.A.; Kenny, P.J. Subtypes of Nicotinic Acetylcholine Receptors in Nicotine Reward, Dependence, and Withdrawal: Evidence from Genetically Modified Mice. Behav. Pharmacol. 2008, 19, 461–484. [Google Scholar] [CrossRef]
- Bruijnzeel, A.W. Tobacco Addiction and the Dysregulation of Brain Stress Systems. Neurosci. Biobehav. Rev. 2012, 36, 1418–1441. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Filgueiras, C.C.; Guthierrez, M.; de Medeiros, A.H.; Mattos, M.A.; Pereira, M.d.S.; Manhães, A.C.; Kubrusly, R.C.C. Exposure to Tobacco Smoke Containing Either High or Low Levels of Nicotine during Adolescence: Differential Effects on Choline Uptake in the Cerebral Cortex and Hippocampus. Nicotine Tob. Res. 2010, 12, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.E. Nicotine and Nonnicotine Factors in Cigarette Addiction. Psychopharmacology 2006, 184, 274–285. [Google Scholar] [CrossRef]
- Villégier, A.-S.; Gallager, B.; Heston, J.; Belluzzi, J.D.; Leslie, F.M. Age Influences the Effects of Nicotine and Monoamine Oxidase Inhibition on Mood-Related Behaviors in Rats. Psychopharmacology 2010, 208, 593–601. [Google Scholar] [CrossRef]
- Bubeníková-Valešová, V.; Horáček, J.; Vrajová, M.; Höschl, C. Models of Schizophrenia in Humans and Animals Based on Inhibition of NMDA Receptors. Neurosci. Biobehav. Rev. 2008, 32, 1014–1023. [Google Scholar] [CrossRef]
- Yee, B.K.; Chang, D.L.T.; Feldon, J. The Effects of Dizocilpine and Phencyclidine on Prepulse Inhibition of the Acoustic Startle Reflex and on Prepulse-Elicited Reactivity in C57BL6 Mice. Neuropsychopharmacology 2004, 29, 1865–1877. [Google Scholar] [CrossRef]
- Brown, R.W.; Schlitt, M.A.; Owens, A.S.; DePreter, C.C.; Cummins, E.D.; Kirby, S.L.; Gill, W.D.; Burgess, K.C. Effects of Environmental Enrichment on Nicotine Sensitization in Rats Neonatally Treated with Quinpirole: Analyses of Glial Cell Line-Derived Neurotrophic Factor and Implications towards Schizophrenia. Dev. Neurosci. 2018, 40, 64–72. [Google Scholar] [CrossRef]

| Experiment 1: Effect or Interaction | Fd.f., p Value |
| dB Intensity | F2,138 = 40.7, p < 0.001 |
| Phencyclidine | F1,69 = 29.8, p < 0.001 |
| dB Intensity × Phencyclidine | F2,138 = 4.2, p = 0.017 |
| dB Intensity × Phencyclidine × Nicotine × Sex | F2,138 = 2.6, p = 0.079 |
| Experiment 2: Effect or Interaction | Fd.f., p Value |
| dB Intensity | F2,160 = 61.5, p < 0.001 |
| Phencyclidine | F1,80 = 7.6, p = 0.007 |
| dB Intensity × Phencyclidine | F2,160 = 2.7, p = 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutra-Tavares, A.C.; Couto, L.A.; Souza, T.P.; Bandeira-Martins, A.; Silva, J.O.; Filgueiras, C.C.; Ribeiro-Carvalho, A.; Manhães, A.C.; Abreu-Villaça, Y. Nicotine’s Effects on Schizophrenia-like Symptoms in a Mice Model: Time Matters. Brain Sci. 2024, 14, 855. https://doi.org/10.3390/brainsci14090855
Dutra-Tavares AC, Couto LA, Souza TP, Bandeira-Martins A, Silva JO, Filgueiras CC, Ribeiro-Carvalho A, Manhães AC, Abreu-Villaça Y. Nicotine’s Effects on Schizophrenia-like Symptoms in a Mice Model: Time Matters. Brain Sciences. 2024; 14(9):855. https://doi.org/10.3390/brainsci14090855
Chicago/Turabian StyleDutra-Tavares, Ana Carolina, Luciana Araújo Couto, Thainá P. Souza, Anais Bandeira-Martins, Juliana Oliveira Silva, Claudio C. Filgueiras, Anderson Ribeiro-Carvalho, Alex C. Manhães, and Yael Abreu-Villaça. 2024. "Nicotine’s Effects on Schizophrenia-like Symptoms in a Mice Model: Time Matters" Brain Sciences 14, no. 9: 855. https://doi.org/10.3390/brainsci14090855
APA StyleDutra-Tavares, A. C., Couto, L. A., Souza, T. P., Bandeira-Martins, A., Silva, J. O., Filgueiras, C. C., Ribeiro-Carvalho, A., Manhães, A. C., & Abreu-Villaça, Y. (2024). Nicotine’s Effects on Schizophrenia-like Symptoms in a Mice Model: Time Matters. Brain Sciences, 14(9), 855. https://doi.org/10.3390/brainsci14090855







