Hyperactivity in ADHD: Friend or Foe?
Abstract
1. Introduction
- How does movement during an EF task impact performance in children with and without ADHD?
- How does movement during an EF task impact left DLPFC activity in children with and without ADHD?
- How does movement during an EF task impact self-efficacy in children with and without ADHD?
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Demographic Questionnaire
2.2.2. Vanderbilt ADHD Diagnostic Parent Rating Scale
2.2.3. Executive Functioning: Stroop Task
2.2.4. Self-Efficacy
2.2.5. Heart Rate
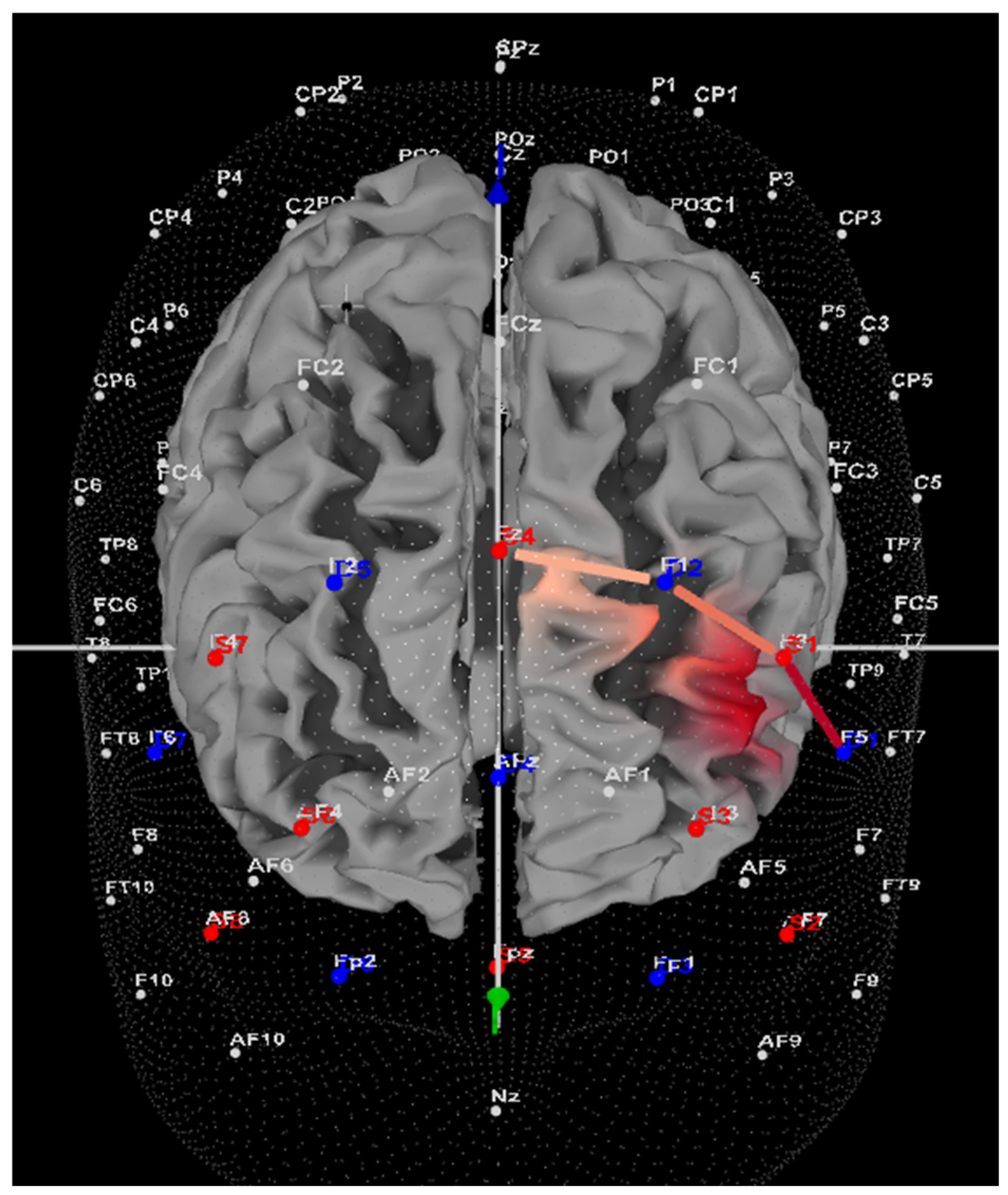
2.2.6. Left DLPFC Activity Using Functional Near-Infrared Spectroscopy (fNIRS)
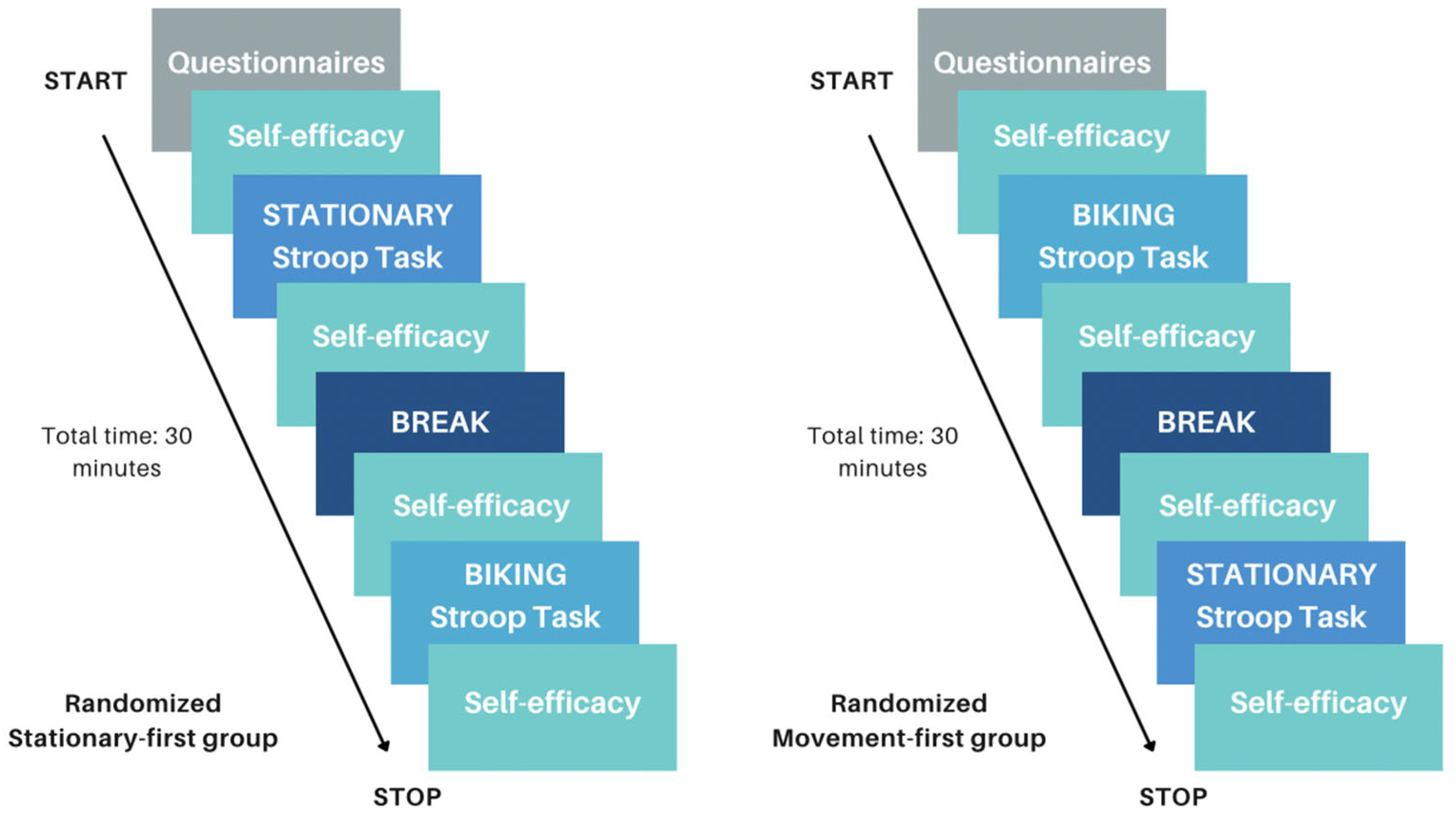
2.2.7. Design
Movement Condition
Stationary Condition
Procedure
2.3. Statistical Analysis
2.3.1. Executive Functioning and Self-Efficacy
2.3.2. fNIRS and Left DLPFC Activity
3. Results
3.1. Preliminary Analyses
3.2. Executive Functioning: Stroop
3.3. fNIRS: Left DLPFC Oxygenation
3.4. Self-Efficacy
3.5. Missing Cases
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polanczyk, G.V.; Willcutt, E.G.; Salum, G.A.; Kieling, C.; Rohde, L.A. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014, 43, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, M.; Görlich, E.; Loyens, S.; Wong, J.; Paas, F. Effects of Desk-Bike Cycling on Phonological Working Memory Performance in Adolescents with Attention Deficit Hyperactivity Disorder. Front. Educ. 2022, 7, 841576. [Google Scholar] [CrossRef]
- Van Riper, S.M.; Tempest, G.D.; Piccirilli, A.; Ma, Q.; Reiss, A.L. Aerobic Exercise, Cognitive Performance, and Brain Activity in Adolescents with Attention-Deficit/Hyperactivity Disorder. Med. Sci. Sports Exerc. 2023, 55, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Antshel, K.M.; Hier, B.O.; Barkley, R.A. Executive Functioning Theory and ADHD. In Handbook of Executive Functioning; Springer: New York, NY, USA, 2014; pp. 107–120. [Google Scholar] [CrossRef]
- Agnew-Blais, J.C.; Polanczyk, G.V.; Danese, A.; Wertz, J.; Moffitt, T.E.; Arseneault, L. Evaluation of the Persistence, Remission, and Emergence of Attention-Deficit/Hyperactivity Disorder in Young Adulthood. JAMA Psychiatry 2016, 73, 713. [Google Scholar] [CrossRef]
- Zang, Y.-F.; Jin, Z.; Weng, X.-C.; Zhang, L.; Zeng, Y.-W.; Yang, L.; Wang, Y.-F.; Seidman, L.J.; Faraone, S.V. Functional MRI in attention-deficit hyperactivity disorder: Evidence for hypofrontality. Brain Dev. 2005, 27, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Rapport, M.D.; Bolden, J.; Kofler, M.J.; Sarver, D.E.; Raiker, J.S.; Alderson, R.M. Hyperactivity in Boys with Attention-Deficit/Hyperactivity Disorder (ADHD): A Ubiquitous Core Symptom or Manifestation of Working Memory Deficits? J. Abnorm. Child Psychol. 2009, 37, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Ota, T.; Iida, J.; Yamamuro, K.; Yoshino, H.; Kishimoto, N.; Kishimoto, T. Reduced prefrontal hemodynamic response in adult attention-deficit hyperactivity disorder as measured by near-infrared spectroscopy. Psychiatry Clin. Neurosci. 2018, 72, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Gossé, L.K.; Bell, S.W.; Hosseini, S.M.H. Functional near-infrared spectroscopy in developmental psychiatry: A review of attention deficit hyperactivity disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, K.; Dan, I.; Suwabe, K.; Kyutoku, Y.; Yamada, Y.; Akahori, M.; Byun, K.; Kato, M.; Soya, H. Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol. Aging 2012, 33, 2621–2632. [Google Scholar] [CrossRef]
- Negoro, H.; Sawada, M.; Iida, J.; Ota, T.; Tanaka, S.; Kishimoto, T. Prefrontal Dysfunction in Attention-Deficit/Hyperactivity Disorder as Measured by Near-Infrared Spectroscopy. Child Psychiatry Hum. Dev. 2010, 41, 193–203. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Dan, I.; Tsuzuki, D.; Kato, M.; Okamoto, M.; Kyutoku, Y.; Soya, H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage 2010, 50, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Sarver, D.E.; Rapport, M.D.; Kofler, M.J.; Raiker, J.S.; Friedman, L.M. Hyperactivity in Attention-Deficit/Hyperactivity Disorder (ADHD): Impairing Deficit or Compensatory Behavior? J. Abnorm. Child Psychol. 2015, 43, 1219–1232. [Google Scholar] [CrossRef]
- Hartanto, T.A.; Krafft, C.E.; Iosif, A.M.; Schweitzer, J.B. A trial-by-trial analysis reveals more intense physical activity is associated with better cognitive control performance in attention-deficit/hyperactivity disorder. Child. Neuropsychol. 2016, 22, 618–626. [Google Scholar] [CrossRef]
- French, M.; Fregni, F.; Chen, E. Aerobic Exercise Effects on Cognition: A Functional Near Infrared Spectroscopy Systematic Review. Front. Hum. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Tsujii, T.; Komatsu, K.; Sakatani, K. Acute Effects of Physical Exercise on Prefrontal Cortex Activity in Older Adults: A Functional Near-Infrared Spectroscopy Study. In Oxygen Transport to Tissue XXXIV; Springer: New York, NY, USA, 2013; pp. 293–298. [Google Scholar] [CrossRef]
- Endo, K.; Matsukawa, K.; Liang, N.; Nakatsuka, C.; Tsuchimochi, H.; Okamura, H.; Hamaoka, T. Dynamic exercise improves cognitive function in association with increased prefrontal oxygenation. J. Physiol. Sci. 2013, 63, 287–298. [Google Scholar] [CrossRef]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Müller, N. Applications of Functional Near-Infrared Spectroscopy (fNIRS) Neuroimaging in Exercise–Cognition Science: A Systematic, Methodology-Focused Review. J. Clin. Med. 2018, 7, 466. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; Saliba, B.J.; Raine, L.B.; Picchietti, D.L.; Hillman, C.H. Exercise Improves Behavioral, Neurocognitive, and Scholastic Performance in Children with Attention-Deficit/Hyperactivity Disorder. J. Pediatr. 2013, 162, 543–551. [Google Scholar] [CrossRef]
- Benzing, V.; Chang, Y.-K.; Schmidt, M. Acute Physical Activity Enhances Executive Functions in Children with ADHD. Sci. Rep. 2018, 8, 12382. [Google Scholar] [CrossRef]
- Neudecker, C.; Mewes, N.; Reimers, A.K.; Woll, A. Exercise Interventions in Children and Adolescents with ADHD: A Systematic Review. J. Atten. Disord. 2019, 23, 307–324. [Google Scholar] [CrossRef]
- Bigelow, H.; Gottlieb, M.D.; Ogrodnik, M.; Graham, J.D.; Fenesi, B. The Differential Impact of Acute Exercise and Mindfulness Meditation on Executive Functioning and Psycho-Emotional Well-Being in Children and Youth with ADHD. Front. Psychol. 2021, 12, 660845. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Gu, Q.; Deng, Z.; Tsai, C.; Xue, Y.; Zhang, J.; Zou, L.; Chen, Z.; Wang, K. Executive Function Performance in Young Adults When Cycling at an Active Workstation: An fNIRS Study. Int. J. Environ. Res. Public Health 2019, 16, 1119. [Google Scholar] [CrossRef]
- Xiang, M.; Li, G.; Ye, J.; Wu, M.; Xu, R.; Hu, M. Effects of combined physical and cognitive training on executive function of adolescent shooting athletes: A functional near-infrared spectroscopy study. Sports Med. Health Sci. 2023, 5, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T.; Li, B.-M. Neurobiology of Executive Functions: Catecholamine Influences on Prefrontal Cortical Functions. Biol. Psychiatry 2005, 57, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Heisz, J.J.; Clark, I.B.; Bonin, K.; Paolucci, E.M.; Michalski, B.; Becker, S.; Fahnestock, M. The Effects of Physical Exercise and Cognitive Training on Memory and Neurotrophic Factors. J. Cogn. Neurosci. 2017, 29, 1895–1907. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Ha, C.H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Self-Efficacy: The Exercise of Control; W.H. Freeman: New York, NY, USA; Times Books: New York, NY, USA; Henry Holt & Co.: New York, NY, USA, 1997. [Google Scholar]
- Komarraju, M.; Nadler, D. Self-efficacy and academic achievement: Why do implicit beliefs, goals, and effort regulation matter? Learn. Individ. Differ. 2013, 25, 67–72. [Google Scholar] [CrossRef]
- Major, A.; Martinussen, R.; Wiener, J. Self-efficacy for self-regulated learning in adolescents with and without attention deficit hyperactivity disorder (ADHD). Learn. Individ. Differ. 2013, 27, 149–156. [Google Scholar] [CrossRef]
- Newark, P.E.; Elsässer, M.; Stieglitz, R.-D. Self-Esteem, Self-Efficacy, and Resources in Adults with ADHD. J. Atten. Disord. 2016, 20, 279–290. [Google Scholar] [CrossRef]
- Zhang, G.; Feng, W.; Zhao, L.; Zhao, X.; Li, T. The association between physical activity, self-efficacy, stress self-management and mental health among adolescents. Sci. Rep. 2024, 14, 5488. [Google Scholar] [CrossRef]
- Perchtold-Stefan, C.M.; Fink, A.; Rominger, C.; Weiss, E.M.; Papousek, I. More habitual physical activity is linked to the use of specific, more adaptive cognitive reappraisal strategies in dealing with stressful events. Stress Health 2020, 36, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Qiu, H.; Wang, P.; Sit, C.H.P. The impacts of a combined exercise on executive function in children with ADHD: A randomized controlled trial. Scand. J. Med. Sci. Sports 2022, 32, 1297–1312. [Google Scholar] [CrossRef] [PubMed]
- Bard, D.E.; Wolraich, M.L.; Neas, B.; Doffing, M.; Beck, L. The Psychometric Properties of the Vanderbilt Attention-Deficit Hyperactivity Disorder Diagnostic Parent Rating Scale in a Community Population. J. Dev. Behav. Pediatr. 2013, 34, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.P.; Langberg, J.M.; Vaughn, A.J.; Epstein, J.N. Clinical Utility of the Vanderbilt ADHD Diagnostic Parent Rating Scale Comorbidity Screening Scales. J. Dev. Behav. Pediatr. 2012, 33, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Assef, E.C.D.S.; Capovilla, A.G.S.; Capovilla, F.C. Computerized Stroop Test to Assess Selective Attention in Children with Attention Deficit Hyperactivity Disorder. Span. J. Psychol. 2007, 10, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, A.; Kokubo, N.; Yamamoto, H.; Yasumura, Y.; Nakagawa, E.; Kaga, M.; Hiraki, K.; Inagaki, M. Neurobehavioral and hemodynamic evaluation of Stroop and reverse Stroop interference in children with attention-deficit/hyperactivity disorder. Brain Dev. 2014, 36, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A.; Wessels, S. Self-Efficacy; W.H. Freeman & Company: New York, NY, USA, 1997. [Google Scholar]
- Monitor Your Heart Rate with Apple Watch. Available online: https://support.apple.com/en-us/120277 (accessed on 9 July 2024).
- Dooley, E.E.; Golaszewski, N.M.; Bartholomew, J.B. Estimating Accuracy at Exercise Intensities: A Comparative Study of Self-Monitoring Heart Rate and Physical Activity Wearable Devices. JMIR Mhealth Uhealth 2017, 5, e34. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, F.L.; Monteiro, E.; Ankrah, E.; Beltran, J.A.; Tavakoulnia, A.; Schuck, S.E.B.; Hayes, G.R.; Lakes, K.D. Parents’ perspectives on a smartwatch intervention for children with ADHD: Rapid deployment and feasibility evaluation of a pilot intervention to support distance learning during COVID-19. PLoS ONE 2021, 16, e0258959. [Google Scholar] [CrossRef] [PubMed]
- Almajidy, R.K.; Mankodiya, K.; Abtahi, M.; Hofmann, U.G. A Newcomer’s Guide to Functional Near Infrared Spectroscopy Experiments. IEEE Rev. Biomed. Eng. 2020, 13, 292–308. [Google Scholar] [CrossRef]
- Rahman, M.A.; Siddik, A.B.; Ghosh, T.K.; Khanam, F.; Ahmad, M. A Narrative Review on Clinical Applications of fNIRS. J. Digit. Imaging 2020, 33, 1167–1184. [Google Scholar] [CrossRef]
- Thomas, A.G.; Dennis, A.; Bandettini, P.A.; Johansen-Berg, H. The Effects of Aerobic Activity on Brain Structure. Front. Psychol. 2012, 3, 86. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, D.; Dan, I. Spatial registration for functional near-infrared spectroscopy: From channel position on the scalp to cortical location in individual and group analyses. NeuroImage 2014, 85, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Fishburn, F.A.; Ludlum, R.S.; Vaidya, C.J.; Medvedev, A.V. Temporal Derivative Distribution Repair (TDDR): A motion correction method for fNIRS. Neuroimage 2019, 184, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Tak, S.; Ye, J.C. Statistical analysis of fNIRS data: A comprehensive review. NeuroImage 2014, 85, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Corkum, P.; Tannock, R.; Moldofsky, H. Sleep Disturbances in Children with Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child. Adolesc. Psychiatry 1998, 37, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, S.G.; Bannon, K.; Castellanos, F.X.; Milham, M.P. The neural correlates of attention deficit hyperactivity disorder: An ALE meta-analysis. J. Child Psychol. Psychiatry 2006, 47, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E.; Hodgkins, P.; Kahle, J.; Madhoo, M.; Kewley, G. Long-Term Outcomes of ADHD: Academic Achievement and Performance. J. Atten. Disord. 2020, 24, 73–85. [Google Scholar] [CrossRef]
- Graham, J.D.; Bremer, E.; Fenesi, B.; Cairney, J. Examining the Acute Effects of Classroom-Based Physical Activity Breaks on Executive Functioning in 11- to 14-Year-Old Children: Single and Additive Moderation Effects of Physical Fitness. Front. Pediatr. 2021, 9, 688251. [Google Scholar] [CrossRef] [PubMed]
- Bantoft, C.; Summers, M.J.; Tranent, P.J.; Palmer, M.A.; Cooley, P.D.; Pedersen, S.J. Effect of Standing or Walking at a Workstation on Cognitive Function. Hum. Factors J. Hum. Factors Ergon. Soc. 2016, 58, 140–149. [Google Scholar] [CrossRef]
- Torbeyns, T.; de Gues, B.; Bailey, S.; Decroix, L.; Van Cutsem, J.; De Pauw, K.; Meeusen, R. Bike Desks in the Classroom: Energy Expenditure, Physical Health, Cognitive Performance, Brain Functioning, and Academic Performance. J. Phys. Act. Health 2017, 14, 429–439. [Google Scholar] [CrossRef]
- Ohlinger, C.M.; Horn, T.S.; Berg, W.P.; Cox, R.H. The Effect of Active Workstation Use on Measures of Cognition, Attention, and Motor Skill. J. Phys. Act. Health 2011, 8, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kujach, S.; Byun, K.; Hyodo, K.; Suwabe, K.; Fukuie, T.; Laskowski, R.; Dan, I.; Soya, H. A transferable high-intensity intermittent exercise improves executive performance in association with dorsolateral prefrontal activation in young adults. Neuroimage 2018, 169, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Falck, R.S.; Davis, J.C.; Liu-Ambrose, T. What is the association between sedentary behaviour and cognitive function? A systematic review. Br. J. Sports Med. 2017, 51, 800–811. [Google Scholar] [CrossRef]
- Martin, R.; Murtagh, E.M. Effect of Active Lessons on Physical Activity, Academic, and Health Outcomes: A Systematic Review. Res. Q. Exerc. Sport 2017, 88, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Mullender-Wijnsma, M.J.; Hartman, E.; de Greeff, J.W.; Doolaard, S.; Bosker, R.J.; Visscher, C. Physically Active Math and Language Lessons Improve Academic Achievement: A Cluster Randomized Controlled Trial. Pediatrics 2016, 137, e20152743. [Google Scholar] [CrossRef] [PubMed]
- Poliakova, E.; Conrad, A.L.; Schieltz, K.M.; O’Brien, M.J. Using fNIRS to evaluate ADHD medication effects on neuronal activity: A systematic literature review. Front. Neuroimaging 2023, 2, 1083036. [Google Scholar] [CrossRef]
- Silverstein, M.; Hironaka, L.K.; Feinberg, E.; Sandler, J.; Pellicer, M.; Chen, N.; Cabral, H. Using clinical data to predict accurate ADHD diagnoses among urban children. Clin. Pediatr. 2015, 55, 326–332. [Google Scholar] [CrossRef]

| Characteristic | ADHD Value (%) | Control Value (%) |
|---|---|---|
| Age of Child (years), mean (SD) | 9.8 (1.7) | 10.3 (1.2) |
| Age of Guardian (years), mean (SD) | 38.8 (3.8) | 43.4 (4.9) |
| Sex of Participant | ||
| Male | 7 (78) | 10 (67) |
| Female | 2 (22) | 5 (33) |
| Sex of Guardian | ||
| Male | 1 (11) | 2 (13) |
| Female | 8 (89) | 13 (87) |
| Guardian’s Education Level (n) | ||
| Some high school, no diploma | 0 (0) | 0 (0) |
| High school graduate, diploma or the equivalent | 0 (0) | 0 (0) |
| Some college credit, no degree | 0 (0) | 2 (13) |
| Trade/technical/vocational training | 0 (0) | 1 (7) |
| Associate degree | 1 (11) | 1 (7) |
| Bachelor’s degree | 4 (44) | 5 (33) |
| Master’s degree | 2 (22) | 4 (27) |
| Professional degree | 1 (11) | 2 (13) |
| Guardian’s Employment (n) | ||
| Employed for wages | 3 (33) | 6 (40) |
| Self-employed | 3 (33) | 6 (40) |
| Out of work | 1 (11) | 1 (7) |
| Homemaker | 1 (11) | 2 (13) |
| No response | 1 (11) | - |
| Household Income | ||
| Prefer not to say | 2 (22) | 3 (20) |
| <CAD 30,000 | 0 (0) | 1 (7) |
| CAD 30,000–CAD 40,000 | 0 (0) | 1 (7) |
| CAD 40,000–CAD 50,000 | 0 (0) | 0 (0) |
| CAD 50,000–CAD 60,000 | 1 (11) | 4 (27) |
| CAD 60,000–CAD 70,000 | 1 (11) | 2 (13) |
| CAD 70,000–CAD 80,000 | 0 (0) | 1 (7) |
| CAD 80,000–CAD 90,000 | 2 (22) | 1 (7) |
| CAD 90,000–CAD 100,000 | 1 (11) | 2 (13) |
| >CAD 100,000 | 2 (22) | 0 (0) |
| Age of ADHD diagnosis (n) | - | |
| Unsure | 1 (11) | - |
| 3 | 3 (33) | - |
| 7 | 2 (22) | - |
| 8 | 1 (11) | - |
| 9 | 1 (11) | - |
| 10 | 1 (11) | - |
| ADHD Subtype (n) | - | |
| Predominantly inattentive | 2 (22) | - |
| Predominantly hyperactive | 0 (0) | - |
| Combined subtype | 4 (44) | - |
| Unsure/no diagnosis given | 3 (33) | - |
| Currently Taking ADHD Medication | - | |
| No response | 1 (11) | - |
| Yes | 6 (67) | - |
| No | 2 (22) | - |
| Other Diagnosis Present | - | |
| Yes | 3 (33) | - |
| No | 6 (67) | - |
| Medicated for Another Diagnosis | - | |
| Yes | 3 (33) | - |
| No | 5 (56) | - |
| No Response | 1 (11) | - |
| ADHD N (%) | Control N (%) | |
|---|---|---|
| Predominantly Inattentive subtype | ||
| Clinically significant | 2 (22) | 0 (0) |
| Not clinically significant | 7 (78) | 15 (100) |
| Predominantly Hyperactive/Impulsive subtype | ||
| Clinically significant | 1 (11) | 0 (0) |
| Not clinically significant | 8 (89) | 15 (100) |
| ADHD Combined Inattention/Hyperactivity | ||
| Clinically significant | 2 (22) | 0 (0) |
| Not clinically significant | 7 (78) | 15 (100) |
| Oppositional-defiant disorder | ||
| Clinically significant | 3 (33) | 0 (0) |
| Not clinically significant | 6 (67) | 15 (100) |
| Conduct disorder | ||
| Clinically significant | 1 (11) | 0 (0) |
| Not clinically significant | 8 (89) | 15 (100) |
| Anxiety | ||
| Clinically significant | 2 (22) | 0 (0) |
| Not clinically significant | 7 (78) | 15 (100) |
| Impairment | ||
| Clinically significant | 6 (67) | 1 (7) |
| Not clinically significant | 3 (33) | 14 (93) |
| Outcome Variables | ADHD (N = 9) | Control (N = 15) | ||
|---|---|---|---|---|
| Stationary Mean (SD) | Movement Mean (SD) | Stationary Mean (SD) | Movement Mean (SD) | |
| Stroop RT (ms) | 1268.40 (439.70) | 935.11 (170.07) | 1141.62 (459.97) | 964.75 (181.22) |
| Stroop Proportion Correct | 0.89 (0.14) | 0.89 (0.16) | 0.90 (0.12) | 0.90 (0.14) |
| Condition | ADHD (N = 9) | Control (N = 15) | ||
|---|---|---|---|---|
| Self-Efficacy Pre-Stroop Mean (SD) | Self-Efficacy Post-Stroop Mean (SD) | Self-Efficacy Pre-Stroop Mean (SD) | Self-Efficacy Post-Stroop Mean (SD) | |
| Stationary | 7.22 (2.54) | 7.44 (3.25) | 6.87 (2.9) | 7.67 (2.02) |
| Movement | 7.11 (3.62) | 8.33 (2.06) | 7.43 (2.31) | 7.43 (2.41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoy, B.-A.; Bi, M.; Lam, M.; Krishnasamy, G.; Abdalmalak, A.; Fenesi, B. Hyperactivity in ADHD: Friend or Foe? Brain Sci. 2024, 14, 719. https://doi.org/10.3390/brainsci14070719
Hoy B-A, Bi M, Lam M, Krishnasamy G, Abdalmalak A, Fenesi B. Hyperactivity in ADHD: Friend or Foe? Brain Sciences. 2024; 14(7):719. https://doi.org/10.3390/brainsci14070719
Chicago/Turabian StyleHoy, Beverly-Ann, Michelle Bi, Matthew Lam, Gayuni Krishnasamy, Androu Abdalmalak, and Barbara Fenesi. 2024. "Hyperactivity in ADHD: Friend or Foe?" Brain Sciences 14, no. 7: 719. https://doi.org/10.3390/brainsci14070719
APA StyleHoy, B.-A., Bi, M., Lam, M., Krishnasamy, G., Abdalmalak, A., & Fenesi, B. (2024). Hyperactivity in ADHD: Friend or Foe? Brain Sciences, 14(7), 719. https://doi.org/10.3390/brainsci14070719







