Dual-Task vs. Single-Task Gait Training to Improve Spatiotemporal Gait Parameters in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Study Selection
2.4. Data Extraction and Management
2.5. Risk of Bias Assessment
2.6. Data Synthesis and Analysis
2.7. Assessment of Heterogeneity and Subgroup Analysis
2.8. Quality of the Evidence
3. Results
3.1. Flow of the Screening Process
3.2. Characteristics of Included Studies
3.3. Methodological Quality Assessment
3.4. Participants
3.5. Intervention
3.6. Outcomes
3.7. Efficacy of Dual-Task Training vs. Single-Task Gait Training
3.7.1. Speed
3.7.2. Stride Length
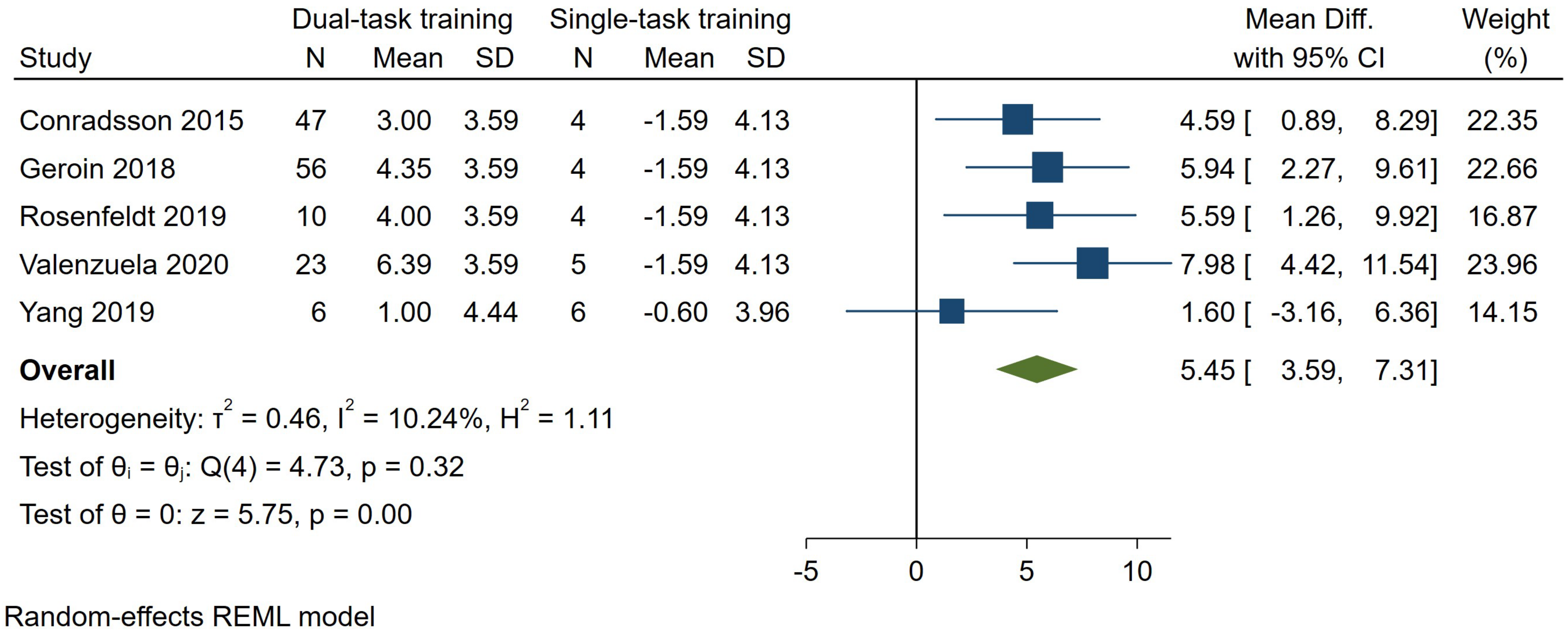
3.7.3. Cadence
| Certainty Assessment | № of Patients | Effect | Certainty | Comments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Dual-Task Gait Training | Single-Task Gait Training | Relative (95% CI) | Absolute (95% CI) | ||
| Dual-task speed (assessed with: m/s) | ||||||||||||
| 11 | randomized trials | not serious | not serious | very serious (a) | not serious | none | 358 | 86 | - | 0.48 standard deviations higher (0.20 to 0.77 higher) | ⨁⨁◯◯ Low | Moderate effect favoring dual-task gait training relative to single-task gait training in improving dual-task speed in people with PD |
| Dual-task stride length (assessed with: m) | ||||||||||||
| 4 | randomized trials | serious (b) | not serious | very serious (c) | not serious | none | 109 | 23 | - | 0.09 m higher (0.04 to 0.14 higher) | ⨁◯◯◯ Very Low | Dual-task relative to single-task gait training leads to higher dual-task stride length improvement (0.09 m) in people with PD |
| Dual-task cadence (assessed with: steps/min) | ||||||||||||
| 5 | randomized trials | serious (d) | not serious | very serious (c) | not serious | none | 142 | 23 | - | 5.45 steps/min higher (3.59 to 7.31 higher) | ⨁◯◯◯ Very Low | Dual-task relative to single-task gait training leads to higher dual-task cadence improvement (5.62 steps/min) in people with PD |
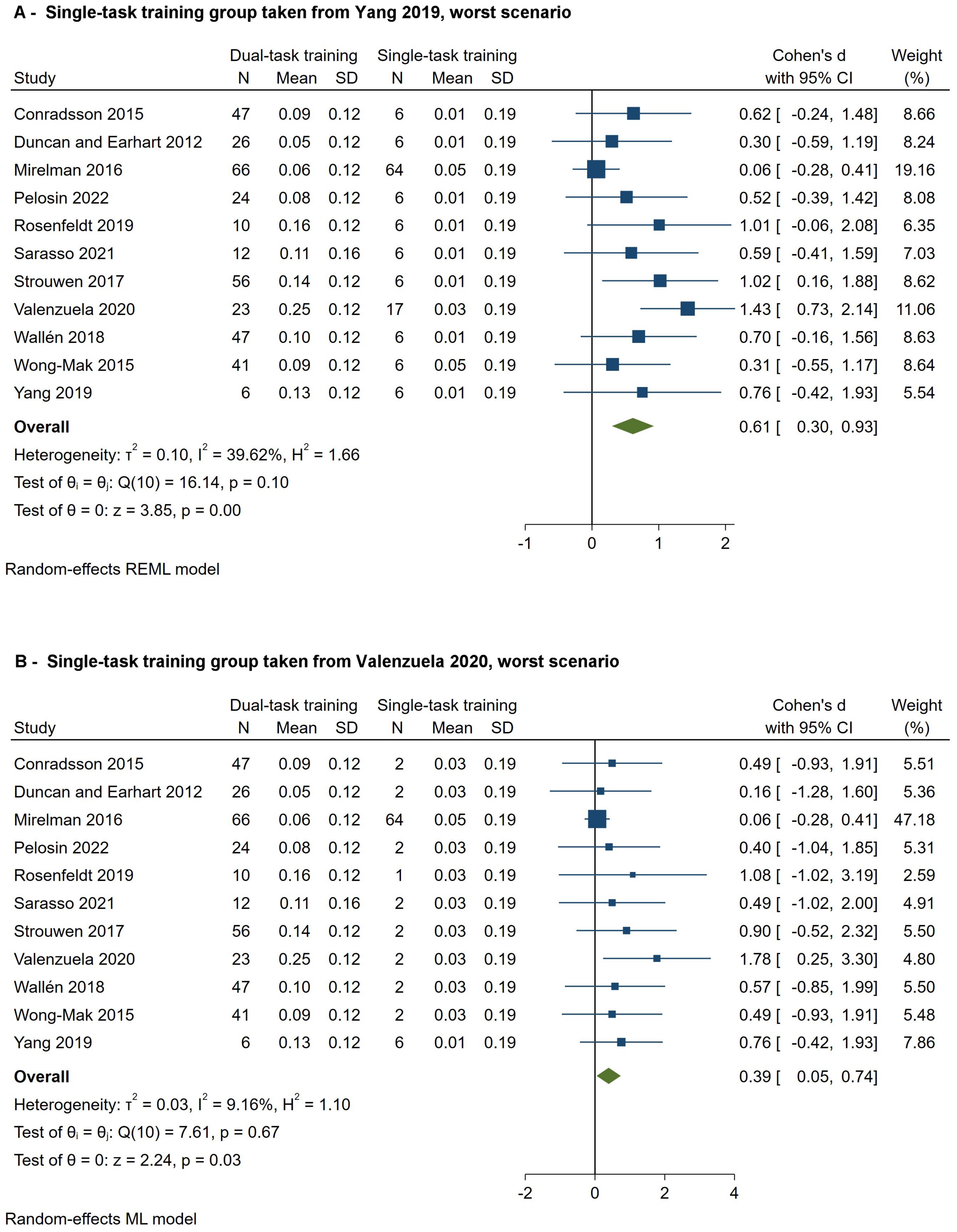
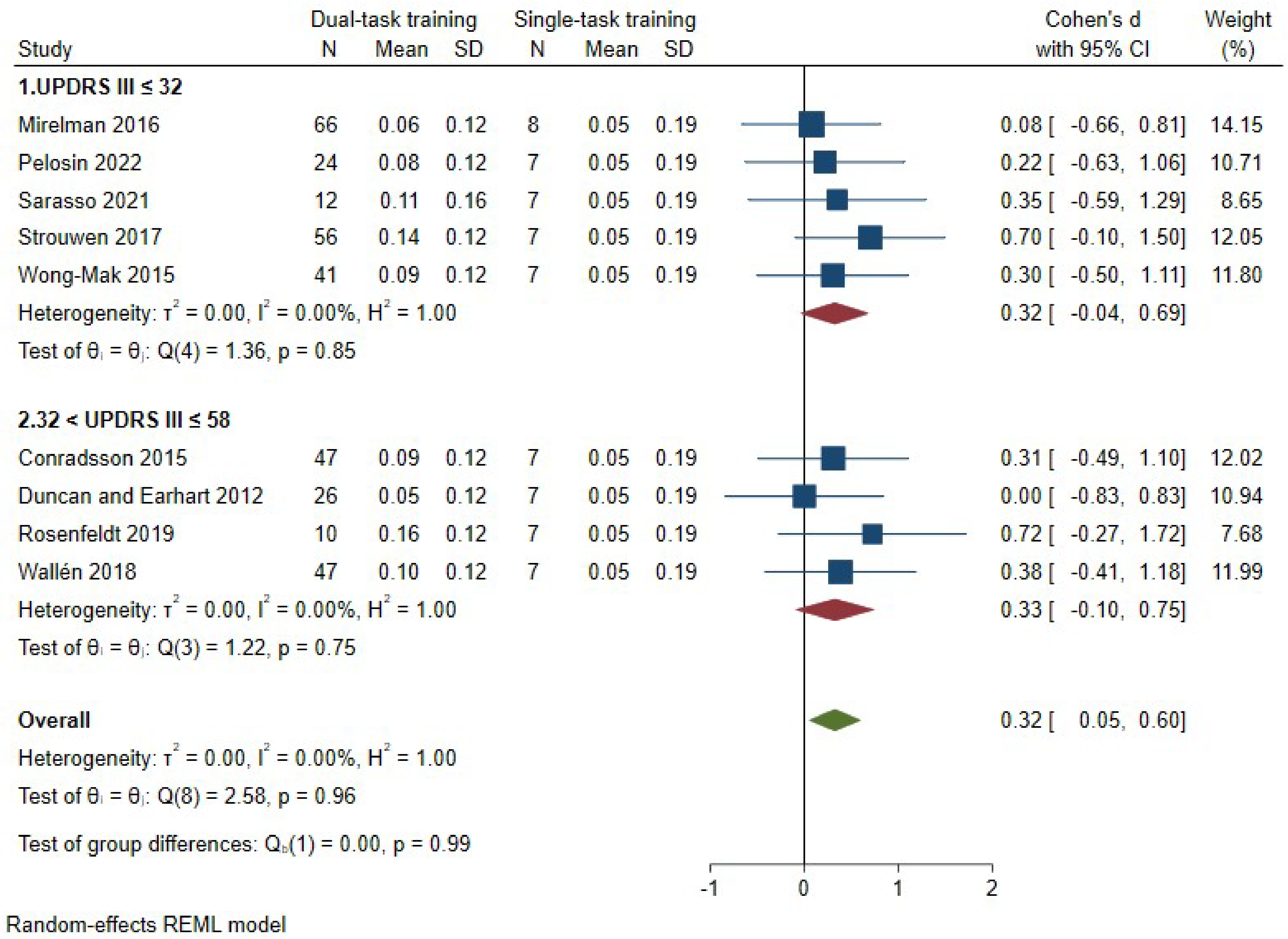
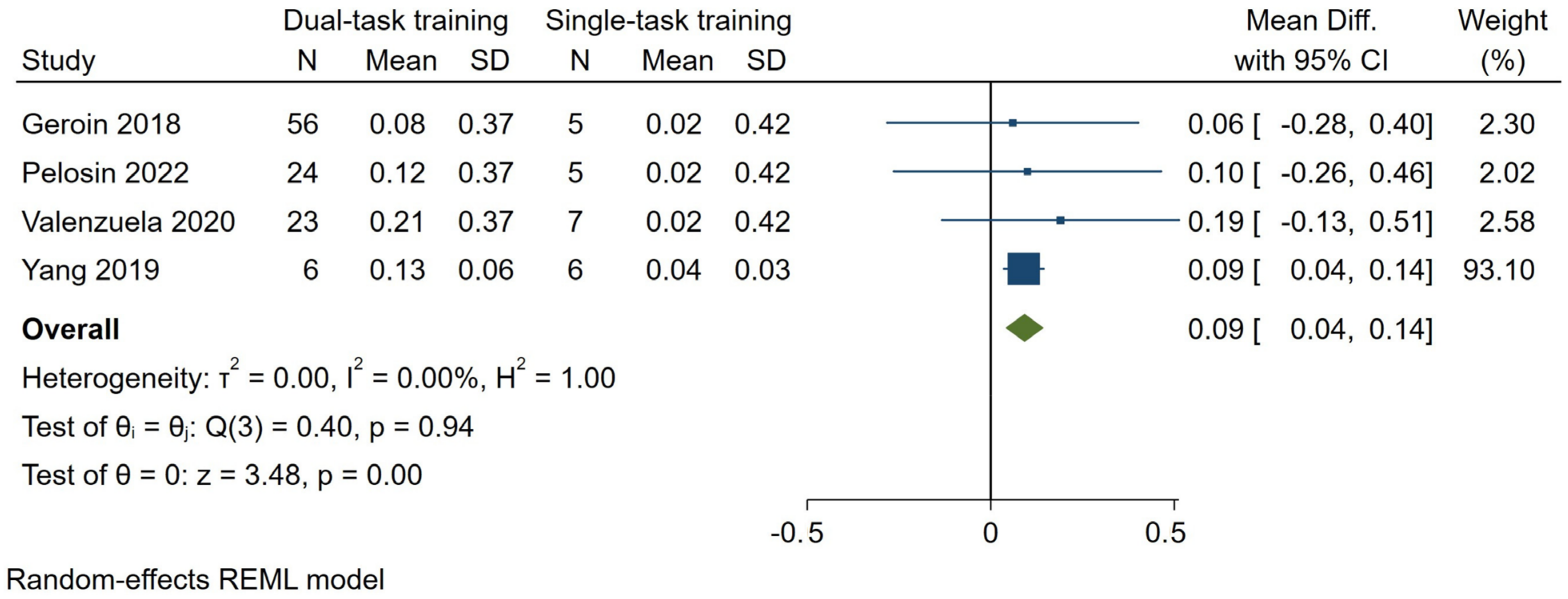
3.7.4. Secondary Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson Disease |
| pwPD | people with Parkinson Disease |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| CENTRAL | Cochrane Central Register of Controlled Trials |
| RCTs | Randomized controlled trials |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| H&Y | Hoehn and Yahr |
| RoB 2 | Version 2 of the Cochrane risk-of-bias tool for randomized trials |
| SDs | Standard Deviations |
| MD | Mean Difference |
| SMD | Standardized Mean Difference |
| CI | Confidence Interval |
| GRADE | Grading of Recommendation Assessment, Development and Evaluation |
| FES-I | Falls Efficacy Scale International |
| ABC | Activity-specific Balance Confidence Scale |
| PDQ-39 | Parkinson’s Disease Questionnaire |
| SF-36 | Short Form Health survey |
References
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Penko, A.L.; Streicher, M.C.; Koop, M.M.; Dey, T.; Rosenfeldt, A.B.; Bazyk, A.S.; Alberts, J.L. Dual-task Interference Disrupts Parkinson’s Gait Across Multiple Cognitive Domains. Neuroscience 2018, 379, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kelly, V.E.; AEusterbrock, J.; Shumway-Cook, A. A review of dual-task walking deficits in people with Parkinson’s disease: Motor and cognitive contributions, mechanisms, and clinical implications. Park. Dis. 2012, 2012, 918719. [Google Scholar] [CrossRef] [PubMed]
- Rochester, L.; Hetherington, V.; Jones, D.; Nieuwboer, A.; Willems, A.-M.; Kwakkel, G.; Van Wegen, E. Attending to the task: Interference effects of functional tasks on walking in Parkinson’s disease and the roles of cognition, depression, fatigue, and balance. Arch. Phys. Med. Rehabil. 2004, 85, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Penko, A.L.; Streicher, M.C.; Dey, T.; Rosenfeldt, A.B.; Alberts, J.L. Parkinson’s gait kinematics deteriorates across multiple cognitive domains under dual-task paradigms. Clin. Neurol. Neurosurg. 2020, 197, 106083. [Google Scholar] [CrossRef] [PubMed]
- Strouwen, C.; Molenaar, E.A.L.M.; Münks, L.; Keus, S.H.J.; Bloem, B.R.; Rochester, L.; Nieuwboer, A. Dual tasking in Parkinson’s disease: Should we train hazardous behavior? Expert Rev. Neurother. 2015, 15, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, E.; Gardoni, A.; Piramide, N.; Volontè, M.A.; Canu, E.; Tettamanti, A.; Filippi, M.; Agosta, F. Dual-task clinical and functional MRI correlates in Parkinson’s disease with postural instability and gait disorders. Park. Relat. Disord. 2021, 91, 88–95. [Google Scholar] [CrossRef]
- Sarasso, E.; Agosta, F.; Piramide, N.; Gardoni, A.; Canu, E.; Leocadi, M.; Castelnovo, V.; Basaia, S.; Tettamanti, A.; Volontè, M.A.; et al. Action Observation and Motor Imagery Improve Dual Task in Parkinson’s Disease: A Clinical/fMRI Study. Mov. Disord. 2021, 36, 2569–2582. [Google Scholar] [CrossRef] [PubMed]
- Cameron, I.G.M.; Watanabe, M.; Pari, G.; Munoz, D.P. Executive impairment in Parkinson’s disease: Response automaticity and task switching. Neuropsychologia 2010, 48, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, T.B.; Leite, P.H.W.; Doná, F.; Pompeu, J.E.; Swarowsky, A.; Torriani-Pasin, C. The effects of dual task gait and balance training in Parkinson’s disease: A systematic review. Physiother. Theory Pract. 2020, 36, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Â.; Rocha, N.; Santos, R.; Tavares, J.M.R.S. Effects of dual-task training on balance and executive functions in Parkinson’s disease: A pilot study. Somat. Mot. Res. 2015, 32, 122–127. [Google Scholar] [CrossRef]
- Li, Z.; Wang, T.; Liu, H.; Jiang, Y.; Wang, Z.; Zhuang, J. Dual-task training on gait, motor symptoms, and balance in patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2020, 34, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.-L.; Cheng, S.-J.; Yang, Y.-R.; Wang, R.-Y. Effects of Dual Task Training on Dual Task Gait Performance and Cognitive Function in Individuals With Parkinson Disease: A Meta-analysis and Meta-regression. Arch. Phys. Med. Rehabil. 2023, 104, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Folkerts, A.-K.; Hammarström, I.; Kalbe, E.; Leavy, B. Effects of motor-cognitive training on dual-task performance in people with Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. 2023, 270, 2890–2907. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions. Available online: www.training.cochrane.org/handbook (accessed on 15 May 2024).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Stata Statistical Software, Release 16; StataCorp LLC: College Station, TX, USA, 2019.
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration, 2011; Available online: www.handbook.cochrane.org (accessed on 15 May 2024).
- Martínez-Martín, P.; Rodríguez-Blázquez, C.; Mario, A.; Arakaki, T.; Arillo, V.C.; Chaná, P.; Fernández, W.; Garretto, N.; Martínez-Castrillo, J.C.; Rodríguez-Violante, M.; et al. Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Park. Relat. Disord. 2015, 21, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Conradsson, D.; Löfgren, N.; Nero, H.; Hagströmer, M.; Ståhle, A.; Lökk, J.; Franzén, E. The Effects of Highly Challenging Balance Training in Elderly With Parkinson’s Disease: A Randomized Controlled Trial. Neurorehabil. Neural Repair. 2015, 29, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.P.; Earhart, G.M. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil. Neural Repair. 2012, 26, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Geroin, C.; Nonnekes, J.; de Vries, N.M.; Strouwen, C.; Smania, N.; Tinazzi, M.; Nieuwboer, A.; Bloem, B.R. Does dual-task training improve spatiotemporal gait parameters in Parkinson’s disease? Park. Relat. Disord. 2018, 55, 86–91. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; Mancini, M.; Smulders, K.; Harker, G.; Lapidus, J.A.; Ramsey, K.; Carlson-Kuhta, P.; Fling, B.W.; Nutt, J.G.; Peterson, D.S.; et al. Cognitively Challenging Agility Boot Camp Program for Freezing of Gait in Parkinson Disease. Neurorehabil. Neural Repair. 2020, 34, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Rochester, L.; Maidan, I.; Del Din, S.; Alcock, L.; Nieuwhof, F.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): A randomised controlled trial. Lancet 2016, 388, 1170–1182. [Google Scholar] [CrossRef]
- Pelosin, E.; Ponte, C.; Putzolu, M.; Lagravinese, G.; Hausdorff, J.M.; Nieuwboer, A.; Ginis, P.; Rochester, L.; Alcock, L.; Bloem, B.R.; et al. Motor-Cognitive Treadmill Training With Virtual Reality in Parkinson’s Disease: The Effect of Training Duration. Front. Aging Neurosci. 2021, 13, 753381. [Google Scholar] [CrossRef] [PubMed]
- Penko, A.L.; Barkley, J.E.; Rosenfeldt, A.B.; Alberts, J.L. Multimodal Training Reduces Fall Frequency as Physical Activity Increases in Individuals With Parkinson’s Disease. J. Phys. Act. Health 2019, 16, 1085–1091. [Google Scholar] [CrossRef]
- Rosenfeldt, A.B.; Penko, A.L.; Streicher, M.C.; Zimmerman, N.M.; Koop, M.M.; Alberts, J.L. Improvements in temporal and postural aspects of gait vary following single- and multi-modal training in individuals with Parkinson’s disease. Park. Relat. Disord. 2019, 64, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Strouwen, C.; Molenaar, E.A.L.M.; Münks, L.; Keus, S.H.J.; Zijlmans, J.C.M.; Vandenberghe, W.; Bloem, B.R.; Nieuwboer, A. Training dual tasks together or apart in Parkinson’s disease: Results from the DUALITY trial. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- San Martín Valenzuela, C.; Moscardó, L.D.; López-Pascual, J.; Serra-Añó, P.; Tomás, J.M. Effects of Dual-Task Group Training on Gait, Cognitive Executive Function, and Quality of Life in People With Parkinson Disease: Results of Randomized Controlled DUALGAIT Trial. Arch. Phys. Med. Rehabil. 2020, 101, 1849–1856. [Google Scholar] [CrossRef]
- Wallén, M.B.; Hagströmer, M.; Conradsson, D.; Sorjonen, K.; Franzén, E. Long-term effects of highly challenging balance training in Parkinson’s disease-a randomized controlled trial. Clin. Rehabil. 2018, 32, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Wong-Yu, I.S.K.; Mak, M.K.Y. Multi-dimensional balance training programme improves balance and gait performance in people with Parkinson’s disease: A pragmatic randomized controlled trial with 12-month follow-up. Park. Relat. Disord. 2015, 21, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-R.; Cheng, S.-J.; Lee, Y.-J.; Liu, Y.-C.; Wang, R.-Y. Cognitive and motor dual task gait training exerted specific training effects on dual task gait performance in individuals with Parkinson’s disease: A randomized controlled pilot study. PLoS ONE 2019, 14, e0218180. [Google Scholar] [CrossRef] [PubMed]
- Dubost, V.; Kressig, R.W.; Gonthier, R.; Herrmann, F.R.; Aminian, K.; Najafi, B.; Beauchet, O. Relationships between dual-task related changes in stride velocity and stride time variability in healthy older adults. Hum. Mov. Sci. 2006, 25, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Mirek, E.; Kubica, J.L.; Szymura, J.; Pasiut, S.; Rudzińska, M.; Chwała, W. Assessment of Gait Therapy Effectiveness in Patients with Parkinson’s Disease on the Basis of Three-Dimensional Movement Analysis. Front. Neurol. 2016, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Gómez, D.; Pedder, H.; Welton, N.J.; Dwan, K.; Dias, S. Variability in Meta-Analysis Estimates of Continuous Outcomes Using Different Standardization and Scale-Specific Re-Expression Methods. J. Clin. Epidemiol. 2024, 165, 111213. [Google Scholar] [CrossRef] [PubMed]
- Abellan van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Buracchio, T.; Dodge, H.H.; Howieson, D.; Wasserman, D.; Kaye, J. The trajectory of gait speed preceding mild cognitive impairment. Arch. Neurol. 2010, 67, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking speed: The functional vital sign. J. Aging Phys. Act. 2015, 23, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Kyrdalen, I.L.; Thingstad, P.; Sandvik, L.; Ormstad, H. Associations between gait speed and well-known fall risk factors among community-dwelling older adults. Physiother. Res. Int. 2019, 24, e1743. [Google Scholar] [CrossRef] [PubMed]
- van der Marck, M.A.; Klok, M.P.C.; Okun, M.S.; Giladi, N.; Munneke, M.; Bloem, B.R.; NPF Falls Task Force. Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson’s disease. Park. Relat. Disord. 2014, 20, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Peterson, D.S.; Earhart, G.M. Gait coordination in Parkinson disease: Effects of step length and cadence manipulations. Gait Posture 2013, 38, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Martini, D.N.; Smulders, K.; Kelly, V.E.; Zabetian, C.P.; Poston, K.; Hiller, A.; Chung, K.A.; Yang, L.; Hu, S.-C.; et al. Cognitive Associations with Comprehensive Gait and Static Balance Measures in Parkinson’s disease. Park. Relat. Disord. 2019, 69, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kang, Y.-J.; Horak, F.B. What Is Wrong with Balance in Parkinson’s Disease? J. Mov. Disord. 2015, 8, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, H.; de Los Angeles Castillo-Pintor, M.; Castro-Sanchez, A.M.; Lara-Palomo, I.C.; Obrero-Gaitan, E.; Cortes-Perez, I. Efficacy of Dual-Task Training in Patients with Parkinson’s Disease: A Systematic Review with Meta-Analysis. Mov. Disord. Clin. Pract. 2023, 10, 1268–1284. [Google Scholar] [CrossRef] [PubMed]
- Gassner, H.; Trutt, E.; Seifferth, S.; Friedrich, J.; Zucker, D.; Salhani, Z.; Adler, W.; Winkler, J.; Jost, W.H. Treadmill training and physiotherapy similarly improve dual task gait performance: A randomized-controlled trial in Parkinson’s disease. J Neural Transm. 2022, 129, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Group, C. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Maidan, I.; Herman, T.; Deutsch, J.; Giladi, N.; Hausdorff, J. Virtual reality for gait training: Can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson‘s disease? J. Gerontol. Ser. A 2011, 66, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Munks, L.; Strouwen, C.; Molenaars, E.; Munneke, M.; Keus, S.; Rochester, L. Dual tasking in Parkinson’s disease increases walking speed: The influence of repeated measures. Mov. Disord. 2012, 27, S280. [Google Scholar]
- Li, Z.; Wang, T.; Shen, M.; Song, T.; He, J.; Guo, W.; Wang, Z.; Zhuang, J. Comparison of Wuqinxi Qigong with Stretching on Single-and Dual-Task Gait, Motor Symptoms and Quality of Life in Parkinson’s Disease: A Preliminary Randomized Control Study. Int. J. Environ. Res. Public Health 2022, 19, 8042. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.; Chivers-Seymour, K.; Summers, R.; Lamb, S.; Goodwin, V.; Rochester, L.; Nieuwboer, A.; Rowsell, A.; Ewing, S.; Ashburn, A. ‘PDSAFE’—A multi-dimensional model of falls-rehabilitation for people with Parkinson‘s. A mixed methods analysis of therapists’ delivery and experience. Physiotherapy 2021, 110, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Capato, T.; De Vries, N.; IntHout, J.; Ramjith, J.; Barbosa, E.; Nonnekes, J.; Bloem, B. A randomized clinical trial of multimodal balance training with rhythmical cues: Effects on freezing of gait in Parkinson’s disease. Mov. Disord. 2019, 34, S32. [Google Scholar]
- Beck, E.N.; Intzandt, B.N.; Almeida, Q.J. Can Dual Task Walking Improve in Parkinson’s Disease After External Focus of Attention Exercise? A Single Blind Randomized Controlled Trial. Neurorehabil. Neural Repair. 2018, 32, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Diaz, G.; Osypiuk, K.; Hausdorff, J.M.; Bonato, P.; Gow, B.J.; Miranda, J.G.; Sudarsky, L.R.; Tarsy, D.; Fox, M.D.; Gardiner, P.; et al. Tai chi for reducing dual-task gait variability, a potential mediator of fall risk in parkinson’s disease: A pilot randomized controlled trial. Global Adv. Health Med. 2018, 7, 2164956118775385. [Google Scholar] [CrossRef] [PubMed]
- Chomiak, T.; Watts, A.; Meyer, N.; Pereira, F.V.; Hu, B. A training approach to improve stepping automaticity while dual-tasking in Parkinson’s disease. Medicine 2017, 96, e5934. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; de Bruin, N.; Doan, J.; Suchowersky, O.; Hu, B. Novel challenges to gait in Parkinson‘s disease: The effect of concurrent music in single- and dual-task contexts. Arch. Phys. Med. Rehabil. 2009, 90, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Wressle, E.; Lundin, F.; Enthoven, P.; Dizdar, N. Group-based music intervention in Parkinson’s disease—Findings from a mixed-methods study. Clin. Rehabil. 2020, 34, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.V.C.; Simão, C.R.; De Melo Santiago, L.M.; Spaniol, A.P.; Oliveira, D.; Lindquist, R.R. Effects of treadmill training on dual-task gait in people with parkinson’s disease. Arch. Phys. Med. Rehabil. 2013, 94, e14–e15. [Google Scholar] [CrossRef]
- Vieira-Yano, B.; Martini, D.N.; Horak, F.B.; de Lima-Pardini, A.; Almeida, F.; Santana, V.P.; Lima, D.; Batista, A.X.; Marquesini, R.; Lira, J.; et al. The Adapted Resistance Training with Instability Randomized Controlled Trial for Gait Automaticity. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.D.N.; Afonso, S.V.; Felipe, L.R.; Oliveira, R.A.; Patrizzi Martins, L.J.; Pascucci Sande de Souza, L.A. Dual-task intervention based on trail making test: Effects on Parkinson’s disease. J. Bodywork Mov. Ther. 2021, 27, 628–633. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, E.; Guidon, M. Fear of falling and dual-task performance in people with Parkinson’s disease. Europ. J. Physiother. 2016, 18, 167–172. [Google Scholar] [CrossRef]
- Sousa, A.V.C.; Santiago, L.M.M.; Silva, R.E.O.; Spaniol, A.P.; Oliveira, D.A.; Galvão, É.R.V.P.; Ribeiro, T.S.; Lindquist, A.R.R. Can treadmill training facilitate the dual-task gait in Parkinson’s disease? Mov. Disord. 2016, 31, S649. [Google Scholar]
- Pompeu, S.; Okamoto, E.; Piemonte, M.E. Dual-task performance assessment: Motor performance of gait, balance, posture and manual skill in dual-tasks. Physiotherapy 2011, 97, eS1014. [Google Scholar]
- Piemonte, M.; Pikel, M.; Mendes, F.; Maciel, L.; Lopes, A. Improvement of the gait performance under dual-task condition after mental practice in patients with Parkinson‘s disease: A single-blind, randomised clinical trial. Mov. Disord. 2015, 30, S111. [Google Scholar]
- Pimentel Piemonte, M.; Mendes, F.; Pompeu, J.; Lobo, A.; Silva, K.; Oliveira, T.; Petersson, A. Improvement of gait, functional and cognitive performance in patients with parkinson‘s disease after motor and cognitive training. Physiotherapy 2011, 97, eS1002. [Google Scholar]
- Criminger, C.; Swank, C. Influence of tDCS on Dual-Task Mobility Elements in Individuals with Parkinson‘s Disease Using 2D Kinematic Analysis Technology. Arch. Phys. Med. Rehabil. 2020, 101, e143. [Google Scholar] [CrossRef]
- Hu, B.; De Bruin, N.; Doan, J.; Turnbull, G.; Suchowersky, O.; Bonfield, S.; Brown, L. Walking with music is a safe and viable tool for gait training in parkinson‘s disease: The effect of a 13-week feasibility study on single and dual task walking. Park. Dis. 2010, 2010, 483530. [Google Scholar]
- Bedeschi Ferrari, C.; Rodrigues, L.; Bauer, D.; Manfredi, A.; Pimentel Piemonte, M. Gait training associated with executive functions tasks in subjects with Parkinson‘s disease: Improvement of performance and effects in motor learning. Mov. Disord. 2012, 27, S12. [Google Scholar]
- Bedeschi Ferrari, C.; Rodrigues, L.; Bauer, D.; Piemonte, M. Improvement of gait, functional and cognitive performance in patients with parkinson‘s disease after gait training associated with executive function tasks. Physiotherapy 2011, 97, eS998–eS999. [Google Scholar]
- Silva, A.Z.D.; Israel, V.L. Effects of dual-task aquatic exercises on functional mobility, balance and gait of individuals with Parkinson‘s disease: A randomized clinical trial with a 3-month follow-up. Complement. Ther. Med. 2019, 42, 119–124. [Google Scholar] [CrossRef] [PubMed]
- King, L.; Smulders, K.; Mancini, M.; Lapidus, J.; Carlson-Kuhta, P.; Fling, B.; Nutt, J.; Horak, F. A combined cognitive and motor exercise program for people with Parkinson’s disease and Freezing of gait: A pilot study. Mov. Disord. 2017, 32, 873. [Google Scholar]
- Lofgren, N.; Conradsson, D.; Rennie, L.; Moe-Nilssen, R.; Franzén, E. Highly challenging gait and balance training can improve cognitive processing during dual-task conditions in elderly with Parkinson’s disease. J. Park. Dis. 2016, 6, 212–213. [Google Scholar]
- Kim, H.; Kim, E.; Yun, S.J.; Kang, M.-G.; Shin, H.I.; Oh, B.-M.; Seo, H.G. Robot-assisted gait training with auditory and visual cues in Parkinson’s disease: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2022, 65, 101620. [Google Scholar] [CrossRef] [PubMed]
- Jabre, M.G.; Elias, N.; Karam, R.; Haddad, I.; Habib, K.; Bejjani, B.P. Efficacy of double-task training on gait performance in Parkinson’s disease: A randomized, controlled, double-blind study. Mov. Disord. 2012, 27, S305. [Google Scholar]
- Rios Romenets, S.; Anang, J.; Fereshtehnejad, S.; Pelletier, A.; Postuma, R. Tango for treatment of motor and non-motor manifestations in Parkinson‘s disease: A randomized control study. Complement. Ther. Med. 2015, 23, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Vitorio, R.; Teixeira-Arroyo, C.; Lirani-Silva, E.; Barbieri, F.A.; Caetano, M.J.D.; Gobbi, S.; Stella, F.; Teresa Bucken Gobbi, L. Effects of 6-month, Multimodal Exercise Program on Clinical and Gait Parameters of Patients with Idiopathic Parkinson’s Disease: A Pilot Study. ISRN Neurol. 2011, 2011, 714947. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarasso, E.; Parente, M.P.; Agosta, F.; Filippi, M.; Corbetta, D. Dual-Task vs. Single-Task Gait Training to Improve Spatiotemporal Gait Parameters in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Brain Sci. 2024, 14, 517. https://doi.org/10.3390/brainsci14050517
Sarasso E, Parente MP, Agosta F, Filippi M, Corbetta D. Dual-Task vs. Single-Task Gait Training to Improve Spatiotemporal Gait Parameters in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Brain Sciences. 2024; 14(5):517. https://doi.org/10.3390/brainsci14050517
Chicago/Turabian StyleSarasso, Elisabetta, Marco Pietro Parente, Federica Agosta, Massimo Filippi, and Davide Corbetta. 2024. "Dual-Task vs. Single-Task Gait Training to Improve Spatiotemporal Gait Parameters in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis" Brain Sciences 14, no. 5: 517. https://doi.org/10.3390/brainsci14050517
APA StyleSarasso, E., Parente, M. P., Agosta, F., Filippi, M., & Corbetta, D. (2024). Dual-Task vs. Single-Task Gait Training to Improve Spatiotemporal Gait Parameters in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Brain Sciences, 14(5), 517. https://doi.org/10.3390/brainsci14050517