Neuroinflammation and Dyskinesia: A Possible Causative Relationship?
Abstract
1. Introduction
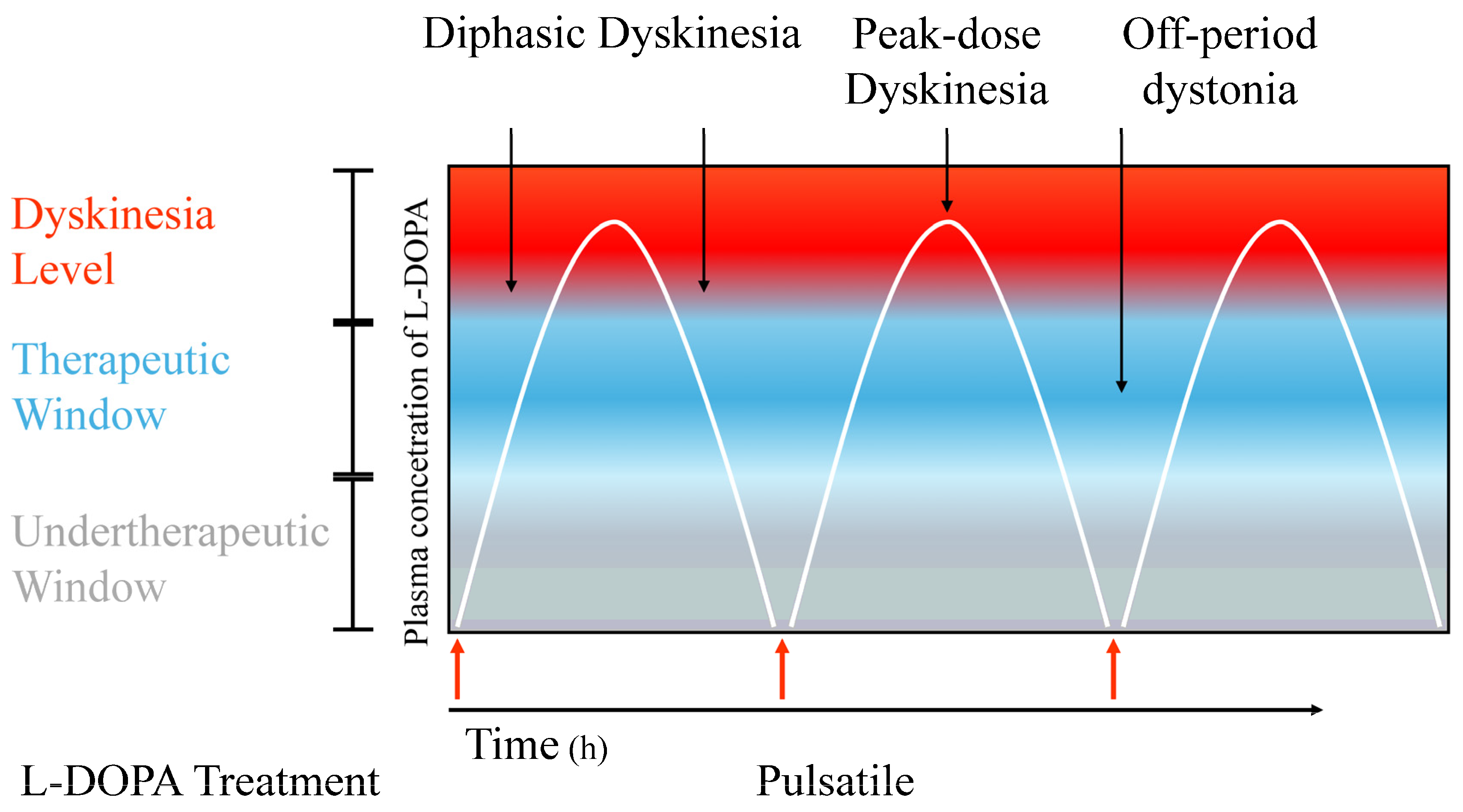
2. Pathophysiology of LIDs
2.1. General Features
2.2. Principal Mechanisms Involved
3. Neuroinflammation and Its Role in LID Development
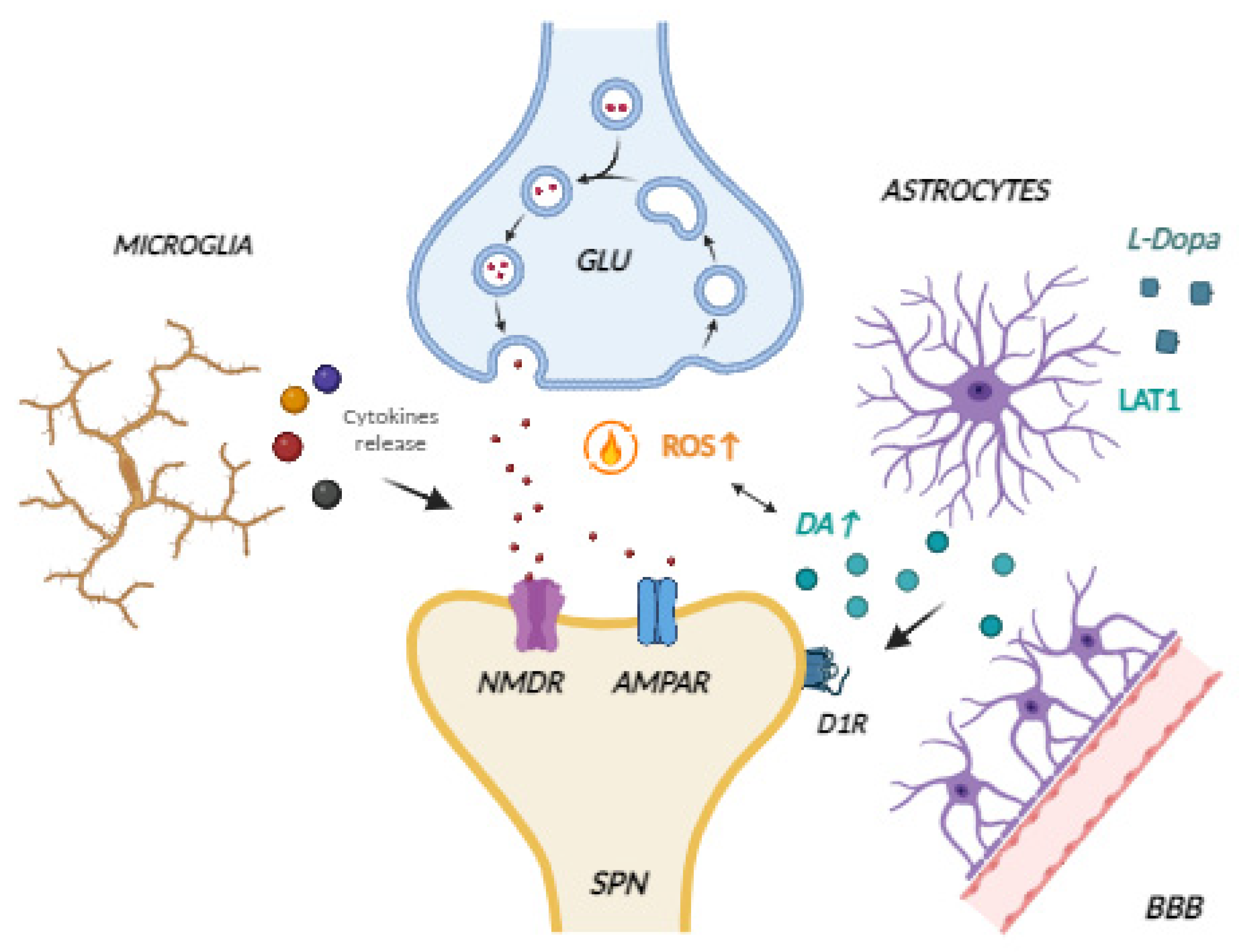
4. Therapeutic Interventions
5. Conclusions
6. Limitations of the Studies
- -
- Could have serious adverse reactions;
- -
- Could reduce the effectiveness of L-dopa;
- -
- Have excellent results in pre-clinical practice but no evidence in medical use due to the lack of clinical trials.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AADC | Aromatic L-Amino Acid Decarboxylase |
| AIMs | Abnormal Involuntary Movements |
| BBB | Blood–Brain Barrier |
| cAMP | Cyclic Adenosine Monophosphate |
| CD68 | Cluster of Differentiation 68 |
| CSF | Cerebrospinal Fluid |
| COMT | Catechol-O-Methyltransferase |
| DA | Dopamine |
| DARPP-32 | Dopamine and Camp-Regulated Protein of 32 kDa |
| DAT | Dopamine Transporter |
| DID | Dyskinesia-Improvement Dyskinesia |
| ERK | Extracellular Signal-Regulated Kinases |
| IBA1 | Ionized Calcium Binding Adaptor Molecule 1 |
| IDI | Improvement-Dyskinesia-Improvement |
| GFAP | Glial Fibrillary Acidic Protein |
| GPi | Internal Globus Pallidus |
| Il-1β | Interleukin-1 beta |
| iNOS | Inducible NO Synthase |
| IFN-γ | Interferon Gamma |
| LAT1 | L-Type Amino Acid Transporter 1 |
| LID | L-DOPA-Induced Dyskinesia |
| L-DOPA | l-3,4-Dihydroxyphenylalanine Levodopa |
| LTD | Long-Term Depression |
| LTP | Long-Term Potentiation |
| MAO-B | Monoamine Oxidase |
| MPEP | Metabotropic Glutamate Receptor 5 Antagonist |
| MPTP | 1-Metil 4-Fenil 1,2,3,6-Tetraidro-Piridina |
| mTOR | Mammalian Target of Rapamycin |
| SPNs | Striatal Projection Neurons |
| PDE10 | Phosphodiesterase 10 |
| PKA | cAMP-dependent Protein Kinase A |
| PD | Parkinson’s Disease |
| TGFβ1 | Transforming Growth Factor beta type 1 |
| TNF-α | Tumor Necrosis Factor |
| TNFR2 | TNF Receptor 2 |
| TNR1 | TNF Receptor 1 |
References
- Cardinale, A.; Calabrese, V. The intricate debate on neurodegeneration and neuroinflammation in Parkinson’s disease: Which came first? Neural Regen. Res. 2023, 18, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, A.; Calabrese, V.; de Iure, A.; Picconi, B. Alpha-Synuclein as a Prominent Actor in the Inflammatory Synaptopathy of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 6517. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived with Disability of Parkinson’s Disease in 204 Countries/Territories From 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef]
- Group, G.B.D.N.D.C. Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. (Vienna) 2017, 124, 901–905. [Google Scholar] [CrossRef]
- AlShimemeri, S.; Fox, S.H.; Visanji, N.P. Emerging drugs for the treatment of L-DOPA-induced dyskinesia: An update. Expert Opin. Emerg. Drugs 2020, 25, 131–144. [Google Scholar] [CrossRef]
- Kwon, D.K.; Kwatra, M.; Wang, J.; Ko, H.S. Levodopa-Induced Dyskinesia in Parkinson’s Disease: Pathogenesis and Emerging Treatment Strategies. Cells 2022, 11, 3736. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Vo, T.N.N.; Frei, K.; Truong, D.D. Levodopa-induced dyskinesia: Clinical features, incidence, and risk factors. J. Neural Transm. (Vienna) 2018, 125, 1109–1117. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Tuo, J.; Zhang, L.; Zhang, J.; Yu, C.; Xu, Z. Levodopa-induced dyskinesia: Interplay between the N-methyl-D-aspartic acid receptor and neuroinflammation. Front. Immunol. 2023, 14, 1253273. [Google Scholar] [CrossRef]
- Cesaroni, V.; Blandini, F.; Cerri, S. Dyskinesia and Parkinson’s disease: Animal model, drug targets, and agents in preclinical testing. Expert Opin. Ther. Targets 2022, 26, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Cenci, M.A.; Lundblad, M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson’s disease in rats and mice. Curr. Protoc. Neurosci. 2007, 41, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E.; Muenter, M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001, 16, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Rascol, O.; Korczyn, A.D.; Jon Stoessl, A.; Watts, R.L.; Poewe, W.; De Deyn, P.P.; Lang, A.E. Ten-year follow-up of Parkinson’s disease patients randomized to initial therapy with ropinirole or levodopa. Mov. Disord. 2007, 22, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.A.; Morris, J.G.; Reid, W.G.; Trafficante, R. Sydney Multicenter Study of Parkinson’s disease: Non-L-dopa-responsive problems dominate at 15 years. Mov. Disord 2005, 20, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.C.; Ruiz, P.J.; Del Pozo, S.V.; Bernardos, V.S. Motor complications in Parkinson’s disease: Ten year follow-up study. Mov. Disord. 2010, 25, 2735–2739. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, P.; Egan, T.; Kuriakose, A.; Stayte, S.; Vissel, B. The ratio of M1 to M2 microglia in the striatum determines the severity of L-Dopa-induced dyskinesias. J. Neurochem. 2023, 167, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Picconi, B.; Centonze, D.; Hakansson, K.; Bernardi, G.; Greengard, P.; Fisone, G.; Cenci, M.A.; Calabresi, P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat. Neurosci. 2003, 6, 501–506. [Google Scholar] [CrossRef]
- Zhang, Y.; Meredith, G.E.; Mendoza-Elias, N.; Rademacher, D.J.; Tseng, K.Y.; Steece-Collier, K. Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: Involvement of corticostriatal but not thalamostriatal synapses. J. Neurosci. 2013, 33, 11655–11667. [Google Scholar] [CrossRef]
- Carta, A.R.; Mulas, G.; Bortolanza, M.; Duarte, T.; Pillai, E.; Fisone, G.; Vozari, R.R.; Del-Bel, E. l-DOPA-induced dyskinesia and neuroinflammation: Do microglia and astrocytes play a role? Eur. J. Neurosci. 2017, 45, 73–91. [Google Scholar] [CrossRef]
- Zesiewicz, T.A.; Sullivan, K.L.; Hauser, R.A. Levodopa-induced dyskinesia in Parkinson’s disease: Epidemiology, etiology, and treatment. Curr. Neurol. Neurosci. Rep. 2007, 7, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Srivanitchapoom, P. Levodopa-induced Dyskinesia: Clinical Features, Pathophysiology, and Medical Management. Ann. Indian Acad. Neurol. 2017, 20, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Picconi, B.; Tozzi, A.; Ghiglieri, V.; Di Filippo, M. Direct and indirect pathways of basal ganglia: A critical reappraisal. Nat. Neurosci. 2014, 17, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E. Experimental reappraisal of continuous dopaminergic stimulation against L-dopa-induced dyskinesia. Mov. Disord. 2013, 28, 1021–1022. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Yousaf, T.; Politis, M. PET Molecular Imaging Research of Levodopa-Induced Dyskinesias in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2017, 17, 90. [Google Scholar] [CrossRef]
- Lindgren, H.S.; Andersson, D.R.; Lagerkvist, S.; Nissbrandt, H.; Cenci, M.A. L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: Temporal and quantitative relationship to the expression of dyskinesia. J. Neurochem. 2010, 112, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Contin, M.; Martinelli, P. Pharmacokinetics of levodopa. J. Neurol. 2010, 257 (Suppl. S2), S253–S261. [Google Scholar] [CrossRef]
- Olanow, C.W.; Obeso, J.A.; Stocchi, F. Continuous dopamine-receptor treatment of Parkinson’s disease: Scientific rationale and clinical implications. Lancet Neurol. 2006, 5, 677–687. [Google Scholar] [CrossRef]
- Nutt, J.G.; Obeso, J.A.; Stocchi, F. Continuous dopamine-receptor stimulation in advanced Parkinson’s disease. Trends Neurosci. 2000, 23 (Suppl. S10), S109–S115. [Google Scholar] [CrossRef]
- Corsi, S.; Stancampiano, R.; Carta, M. Serotonin/dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesia: An update. Prog. Brain Res. 2021, 261, 287–302. [Google Scholar] [PubMed]
- Navailles, S.; De Deurwaerdere, P. Imbalanced Dopaminergic Transmission Mediated by Serotonergic Neurons in L-DOPA-Induced Dyskinesia. Parkinsons Dis. 2012, 2012, 323686. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, R.; Mishra, N.; Rana, R.; Kaur, G.; Ghoneim, M.M.; Alshehri, S.; Mustafa, G.; Ahmad, J.; Alhakamy, N.A.; Mishra, A. Molecular Mechanisms and Therapeutic Strategies for Levodopa-Induced Dyskinesia in Parkinson’s Disease: A Perspective Through Preclinical and Clinical Evidence. Front. Pharmacol. 2022, 13, 805388. [Google Scholar] [CrossRef] [PubMed]
- Campanelli, F.; Marino, G.; Barsotti, N.; Natale, G.; Calabrese, V.; Cardinale, A.; Ghiglieri, V.; Maddaloni, G.; Usiello, A.; Calabresi, P.; et al. Serotonin drives striatal synaptic plasticity in a sex-related manner. Neurobiol. Dis. 2021, 158, 105448. [Google Scholar] [CrossRef] [PubMed]
- Politis, M.; Oertel, W.H.; Wu, K.; Quinn, N.P.; Pogarell, O.; Brooks, D.J.; Bjorklund, A.; Lindvall, O.; Piccini, P. Graft-induced dyskinesias in Parkinson’s disease: High striatal serotonin/dopamine transporter ratio. Mov. Disord. 2011, 26, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.; Bezard, E. Contribution of pre-synaptic mechanisms to L-DOPA-induced dyskinesia. Neuroscience 2011, 198, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Politis, M.; Wu, K.; Loane, C.; Quinn, N.P.; Brooks, D.J.; Rehncrona, S.; Bjorklund, A.; Lindvall, O.; Piccini, P. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci. Transl. Med. 2010, 2, 38ra46. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.; Carlsson, T.; Kirik, D.; Bjorklund, A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 2007, 130 Pt 7, 1819–1833. [Google Scholar] [CrossRef] [PubMed]
- Kempadoo, K.A.; Mosharov, E.V.; Choi, S.J.; Sulzer, D.; Kandel, E.R. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. USA 2016, 113, 14835–14840. [Google Scholar] [CrossRef]
- Bezard, E.; Brotchie, J.M.; Gross, C.E. Pathophysiology of levodopa-induced dyskinesia: Potential for new therapies. Nat. Rev. Neurosci. 2001, 2, 577–588. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Keefe, K.A.; Gauda, E.B. D1 and D2 dopamine receptor function in the striatum: Coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J. Neurosci. 1995, 15, 8167–8176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calabrese, V.; Di Maio, A.; Marino, G.; Cardinale, A.; Natale, G.; De Rosa, A.; Campanelli, F.; Mancini, M.; Napolitano, F.; Avallone, L.; et al. Rapamycin, by Inhibiting mTORC1 Signaling, Prevents the Loss of Striatal Bidirectional Synaptic Plasticity in a Rat Model of L-DOPA-Induced Dyskinesia. Front. Aging Neurosci. 2020, 12, 230. [Google Scholar] [CrossRef]
- Santini, E.; Valjent, E.; Usiello, A.; Carta, M.; Borgkvist, A.; Girault, J.A.; Herve, D.; Greengard, P.; Fisone, G. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J. Neurosci. 2007, 27, 6995–7005. [Google Scholar] [CrossRef]
- Santini, E.; Heiman, M.; Greengard, P.; Valjent, E.; Fisone, G. Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci. Signal 2009, 2, ra36. [Google Scholar] [CrossRef]
- Cardinale, A.; Fusco, F.R. Inhibition of phosphodiesterases as a strategy to achieve neuroprotection in Huntington’s disease. CNS Neurosci. Ther. 2018, 24, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Niccolini, F.; Foltynie, T.; Reis Marques, T.; Muhlert, N.; Tziortzi, A.C.; Searle, G.E.; Natesan, S.; Kapur, S.; Rabiner, E.A.; Gunn, R.N.; et al. Loss of phosphodiesterase 10A expression is associated with progression and severity in Parkinson’s disease. Brain 2015, 138 Pt 10, 3003–3015. [Google Scholar] [CrossRef] [PubMed]
- Sgambato-Faure, V.; Cenci, M.A. Glutamatergic mechanisms in the dyskinesias induced by pharmacological dopamine replacement and deep brain stimulation for the treatment of Parkinson’s disease. Prog. Neurobiol. 2012, 96, 69–86. [Google Scholar] [CrossRef]
- Ahmed, I.; Bose, S.K.; Pavese, N.; Ramlackhansingh, A.; Turkheimer, F.; Hotton, G.; Hammers, A.; Brooks, D.J. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain 2011, 134 Pt 4, 979–986. [Google Scholar] [CrossRef]
- Gardoni, F.; Picconi, B.; Ghiglieri, V.; Polli, F.; Bagetta, V.; Bernardi, G.; Cattabeni, F.; Di Luca, M.; Calabresi, P. A critical interaction between NR2B and MAGUK in L-DOPA induced dyskinesia. J. Neurosci. 2006, 26, 2914–2922. [Google Scholar] [CrossRef]
- Nash, J.E.; Brotchie, J.M. Characterisation of striatal NMDA receptors involved in the generation of parkinsonian symptoms: Intrastriatal microinjection studies in the 6-OHDA-lesioned rat. Mov. Disord. 2002, 17, 455–466. [Google Scholar] [CrossRef]
- Calabresi, P.; Centonze, D.; Gubellini, P.; Marfia, G.A.; Pisani, A.; Sancesario, G.; Bernardi, G. Synaptic transmission in the striatum: From plasticity to neurodegeneration. Prog. Neurobiol. 2000, 61, 231–265. [Google Scholar] [CrossRef] [PubMed]
- Menegoz, M.; Lau, L.F.; Herve, D.; Huganir, R.L.; Girault, J.A. Tyrosine phosphorylation of NMDA receptor in rat striatum: Effects of 6-OH-dopamine lesions. Neuroreport 1995, 7, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, C.; Yeom, H.D.; Nguyen, K.V.A.; Eom, S.; Lee, S.; Jung, J.H.; Lee, J.H.; Kim, S.H.; Kim, I.K.; et al. Subunit-specific effects of poricoic acid A on NMDA receptors. Pharmacol. Rep. 2020, 72, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, C.M.; Shuhama, R.; Fachim, H.A.; Menezes, P.R.; Del-Ben, C.M.; Louzada-Junior, P. Low plasma concentrations of N-methyl-d-aspartate receptor subunits as a possible biomarker for psychosis. Schizophr. Res. 2018, 202, 55–63. [Google Scholar] [CrossRef]
- Pokkula, S.; Thakur, S.R. Icariin ameliorates partial sciatic nerve ligation induced neuropathic pain in rats: An evidence of in silico and in vivo studies. J. Pharm. Pharmacol. 2021, 73, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.J.; Bartlett, M.J.; Root, B.K.; Parent, K.L.; Heien, M.L.; Porreca, F.; Polt, R.; Sherman, S.J.; Falk, T. The combination of the opioid glycopeptide MMP-2200 and a NMDA receptor antagonist reduced l-DOPA-induced dyskinesia and MMP-2200 by itself reduced dopamine receptor 2-like agonist-induced dyskinesia. Neuropharmacology 2018, 141, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Prescott, I.A.; Dostrovsky, J.O.; Moro, E.; Hodaie, M.; Lozano, A.M.; Hutchison, W.D. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson’s disease patients. Brain 2009, 132 Pt 2, 309–318. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W. Maladaptive Synaptic Plasticity in L-DOPA-Induced Dyskinesia. Front. Neural. Circuits 2016, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.; Dwivedi, N.; Kumar, A.; Singh, V.K.; Pathak, A.; Chaurasia, R.N.; Mishra, V.N.; Mohanty, S.; Joshi, D. Association of Catechol-O-Methyltransferase Gene rs4680 Polymorphism and Levodopa Induced Dyskinesia in Parkinson’s Disease: A Meta-Analysis and Systematic Review. J. Geriatr. Psychiatry Neurol. 2023, 36, 98–106. [Google Scholar] [CrossRef]
- Lohle, M.; Mangone, G.; Hermann, W.; Hausbrand, D.; Wolz, M.; Mende, J.; Reichmann, H.; Hermann, A.; Corvol, J.C.; Storch, A. Functional MAOB Gene Intron 13 Polymorphism Predicts Dyskinesia in Parkinson’s Disease. Parkinsons Dis. 2022, 2022, 5597503. [Google Scholar] [CrossRef]
- Yoon, W.; Min, S.; Ryu, H.S.; Chung, S.J.; Chung, J. Discovery of levodopa-induced dyskinesia-associated genes using genomic studies in patients and Drosophila behavioral analyses. Commun. Biol. 2022, 5, 872. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Rojo, A.I.; Manda, G.; Bosca, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Preeti, K.; Fernandes, V.; Khatri, D.K.; Singh, S.B. Glia: A major player in glutamate-GABA dysregulation-mediated neurodegeneration. J. Neurosci. Res. 2021, 99, 3148–3189. [Google Scholar] [CrossRef] [PubMed]
- Iovino, M.; Messana, T.; De Pergola, G.; Iovino, E.; Guastamacchia, E.; Licchelli, B.; Vanacore, A.; Giagulli, V.A.; Triggiani, V. Brain Angiotensinergic Regulation of the Immune System: Implications for Cardiovascular and Neuroendocrine Responses. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, A.; Boi, L.; Mulas, G.; Spiga, S.; Fenu, S.; Carta, A.R. Neuroinflammation in L-DOPA-induced dyskinesia: Beyond the immune function. J. Neural Transm. (Vienna) 2018, 125, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.P.; Frey, B.N. Disruption in the Blood-Brain Barrier: The Missing Link between Brain and Body Inflammation in Bipolar Disorder? Neural Plast. 2015, 2015, 708306. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.E.; Kooij, G.; Frenkel, D.; Georgopoulos, S.; Monsonego, A.; Janigro, D. Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: Implications for clinical disease. Epilepsia 2012, 53 (Suppl. S6), 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kuter, K.Z.; Cenci, M.A.; Carta, A.R. The role of glia in Parkinson’s disease: Emerging concepts and therapeutic applications. Prog. Brain Res. 2020, 252, 131–168. [Google Scholar]
- De Chiara, V.; Motta, C.; Rossi, S.; Studer, V.; Barbieri, F.; Lauro, D.; Bernardi, G.; Centonze, D. Interleukin-1beta alters the sensitivity of cannabinoid CB1 receptors controlling glutamate transmission in the striatum. Neuroscience 2013, 250, 232–239. [Google Scholar] [CrossRef]
- Centonze, D.; Muzio, L.; Rossi, S.; Cavasinni, F.; De Chiara, V.; Bergami, A.; Musella, A.; D’Amelio, M.; Cavallucci, V.; Martorana, A.; et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J. Neurosci. 2009, 29, 3442–3452. [Google Scholar] [CrossRef]
- Harry, G.J. Microglia in Neurodegenerative Events-An Initiator or a Significant Other? Int. J. Mol. Sci. 2021, 22, 5818. [Google Scholar] [CrossRef] [PubMed]
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019, 18, 573–586. [Google Scholar] [CrossRef]
- Bortolanza, M.; Cavalcanti-Kiwiatkoski, R.; Padovan-Neto, F.E.; da-Silva, C.A.; Mitkovski, M.; Raisman-Vozari, R.; Del-Bel, E. Glial activation is associated with l-DOPA induced dyskinesia and blocked by a nitric oxide synthase inhibitor in a rat model of Parkinson’s disease. Neurobiol. Dis. 2015, 73, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Teema, A.M.; Zaitone, S.A.; Moustafa, Y.M. Ibuprofen or piroxicam protects nigral neurons and delays the development of l-dopa induced dyskinesia in rats with experimental Parkinsonism: Influence on angiogenesis. Neuropharmacology 2016, 107, 432–450. [Google Scholar] [CrossRef]
- Barnum, C.J.; Eskow, K.L.; Dupre, K.; Blandino, P., Jr.; Deak, T.; Bishop, C. Exogenous corticosterone reduces L-DOPA-induced dyskinesia in the hemi-parkinsonian rat: Role for interleukin-1beta. Neuroscience 2008, 156, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Bortolanza, M.; Padovan-Neto, F.E.; Cavalcanti-Kiwiatkoski, R.; Dos Santos-Pereira, M.; Mitkovski, M.; Raisman-Vozari, R.; Del-Bel, E. Are cyclooxygenase-2 and nitric oxide involved in the dyskinesia of Parkinson’s disease induced by L-DOPA? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140190. [Google Scholar] [CrossRef]
- Martinez, A.A.; Morgese, M.G.; Pisanu, A.; Macheda, T.; Paquette, M.A.; Seillier, A.; Cassano, T.; Carta, A.R.; Giuffrida, A. Activation of PPAR gamma receptors reduces levodopa-induced dyskinesias in 6-OHDA-lesioned rats. Neurobiol. Dis. 2015, 74, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Paudel, Y.N.; Julian, T.; Shaikh, M.F.; Piperi, C. Pivotal Role of Fyn Kinase in Parkinson’s Disease and Levodopa-Induced Dyskinesia: A Novel Therapeutic Target? Mol. Neurobiol. 2021, 58, 1372–1391. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Purves-Tyson, T.; Geddes, A.E.; Huang, X.F.; Newell, K.A.; Weickert, C.S. N-Methyl-d-Aspartate receptor and inflammation in dorsolateral prefrontal cortex in schizophrenia. Schizophr. Res. 2022, 240, 61–70. [Google Scholar] [CrossRef]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Munoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef]
- Inyushin, M.Y.; Huertas, A.; Kucheryavykh, Y.V.; Kucheryavykh, L.Y.; Tsydzik, V.; Sanabria, P.; Eaton, M.J.; Skatchkov, S.N.; Rojas, L.V.; Wessinger, W.D. L-DOPA Uptake in Astrocytic Endfeet Enwrapping Blood Vessels in Rat Brain. Parkinsons Dis. 2012, 2012, 321406. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Zorec, R. Astroglial signalling in health and disease. Neurosci. Lett. 2019, 689, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, M.; Miyazaki, I.; Murakami, S.; Diaz-Corrales, F.J.; Ogawa, N. Striatal astrocytes act as a reservoir for L-DOPA. PLoS ONE 2014, 9, e106362. [Google Scholar] [CrossRef] [PubMed]
- Cenci, M.A. Presynaptic Mechanisms of l-DOPA-Induced Dyskinesia: The Findings, the Debate, and the Therapeutic Implications. Front. Neurol. 2014, 5, 118136. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, H.; Tomiyama, M. What Mechanisms Are Responsible for the Reuptake of Levodopa-Derived Dopamine in Parkinsonian Striatum? Front. Neurosci. 2016, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C. Neuroinflammation: Fanning the fire of l-dopa-induced dyskinesia. Mov. Disord. 2019, 34, 1758–1760. [Google Scholar] [CrossRef] [PubMed]
- Mulas, G.; Espa, E.; Fenu, S.; Spiga, S.; Cossu, G.; Pillai, E.; Carboni, E.; Simbula, G.; Jadzic, D.; Angius, F.; et al. Differential induction of dyskinesia and neuroinflammation by pulsatile versus continuous l-DOPA delivery in the 6-OHDA model of Parkinson’s disease. Exp. Neurol. 2016, 286, 83–92. [Google Scholar] [CrossRef]
- Morissette, M.; Bourque, M.; Tremblay, M.E.; Di Paolo, T. Prevention of L-Dopa-Induced Dyskinesias by MPEP Blockade of Metabotropic Glutamate Receptor 5 Is Associated with Reduced Inflammation in the Brain of Parkinsonian Monkeys. Cells 2022, 11, 691. [Google Scholar] [CrossRef]
- Lanza, K.; Perkins, A.E.; Deak, T.; Bishop, C. Late aging-associated increases in L-DOPA-induced dyskinesia are accompanied by heightened neuroinflammation in the hemi-parkinsonian rat. Neurobiol. Aging 2019, 81, 190–199. [Google Scholar] [CrossRef]
- Warren Olanow, C.; Kieburtz, K.; Rascol, O.; Poewe, W.; Schapira, A.H.; Emre, M.; Nissinen, H.; Leinonen, M.; Stocchi, F.; Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease (STRIDE-PD) Investigators. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov. Disord. 2013, 28, 1064–1071. [Google Scholar] [CrossRef]
- Kumar, N.; Van Gerpen, J.A.; Bower, J.H.; Ahlskog, J.E. Levodopa-dyskinesia incidence by age of Parkinson’s disease onset. Mov. Disord. 2005, 20, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Bez, F.; Francardo, V.; Cenci, M.A. Dramatic differences in susceptibility to l-DOPA-induced dyskinesia between mice that are aged before or after a nigrostriatal dopamine lesion. Neurobiol. Dis. 2016, 94, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Dyavar, S.R.; Potts, L.F.; Beck, G.; Dyavar Shetty, B.L.; Lawson, B.; Podany, A.T.; Fletcher, C.V.; Amara, R.R.; Papa, S.M. Transcriptomic approach predicts a major role for transforming growth factor beta type 1 pathway in L-Dopa-induced dyskinesia in parkinsonian rats. Genes Brain Behav. 2020, 19, e12690. [Google Scholar] [CrossRef]
- De Lella Ezcurra, A.L.; Chertoff, M.; Ferrari, C.; Graciarena, M.; Pitossi, F. Chronic expression of low levels of tumor necrosis factor-alpha in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol. Dis. 2010, 37, 630–640. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.K.; Tansey, M.G. TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J. Neuroinflamm. 2008, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Beattie, E.C.; Stellwagen, D.; Morishita, W.; Bresnahan, J.C.; Ha, B.K.; Von Zastrow, M.; Beattie, M.S.; Malenka, R.C. Control of synaptic strength by glial TNFalpha. Science 2002, 295, 2282–2285. [Google Scholar] [CrossRef] [PubMed]
- Calon, F.; Rajput, A.H.; Hornykiewicz, O.; Bedard, P.J.; Di Paolo, T. Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson’s disease. Neurobiol. Dis. 2003, 14, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Konitsiotis, S.; Blanchet, P.J.; Verhagen, L.; Lamers, E.; Chase, T.N. AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeys. Neurology 2000, 54, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, B.; Hoyer, D.; Gregoire, L.; Morissette, M.; Gasparini, F.; Gomez-Mancilla, B.; Di Paolo, T. Changes of AMPA receptors in MPTP monkeys with levodopa-induced dyskinesias. Neuroscience 2010, 167, 1160–1167. [Google Scholar] [CrossRef]
- Lewitus, G.M.; Pribiag, H.; Duseja, R.; St-Hilaire, M.; Stellwagen, D. An adaptive role of TNFalpha in the regulation of striatal synapses. J. Neurosci. 2014, 34, 6146–6155. [Google Scholar] [CrossRef]
- Balosso, S.; Ravizza, T.; Pierucci, M.; Calcagno, E.; Invernizzi, R.; Di Giovanni, G.; Esposito, E.; Vezzani, A. Molecular and functional interactions between tumor necrosis factor-alpha receptors and the glutamatergic system in the mouse hippocampus: Implications for seizure susceptibility. Neuroscience 2009, 161, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Boi, L.; Pisanu, A.; Greig, N.H.; Scerba, M.T.; Tweedie, D.; Mulas, G.; Fenu, S.; Carboni, E.; Spiga, S.; Carta, A.R. Immunomodulatory drugs alleviate l-dopa-induced dyskinesia in a rat model of Parkinson’s disease. Mov. Disord. 2019, 34, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, A.; Adriani, G.; Catalano, A.; Carocci, A.; Rao, L.; Lentini, G.; Cavalluzzi, M.M.; Franchini, C.; Vacca, A.; Corbo, F. A Mini-Review on Thalidomide: Chemistry, Mechanisms of Action, Therapeutic Potential and Anti-Angiogenic Properties in Multiple Myeloma. Curr. Med. Chem. 2017, 24, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, J.; Zhang, J.; Huang, F. Thalidomide reduces recurrence of ankylosing spondylitis in patients following discontinuation of etanercept. Rheumatol. Int. 2013, 33, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Caracciolo, L.; Tweedie, D.; Choi, S.H.; Greig, N.H.; Barlati, S.; Bosetti, F. 3,6’-Dithiothalidomide, a new TNF-alpha synthesis inhibitor, attenuates the effect of Abeta1-42 intracerebroventricular injection on hippocampal neurogenesis and memory deficit. J. Neurochem. 2012, 122, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Tweedie, D.; Ferguson, R.A.; Fishman, K.; Frankola, K.A.; Van Praag, H.; Holloway, H.W.; Luo, W.; Li, Y.; Caracciolo, L.; Russo, I.; et al. Tumor necrosis factor-alpha synthesis inhibitor 3,6’-dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer’s disease. J. Neuroinflamm. 2012, 9, 106. [Google Scholar] [CrossRef]
- Tweedie, D.; Frankola, K.A.; Luo, W.; Li, Y.; Greig, N.H. Thalidomide Analogues Suppress Lipopolysaccharide-Induced Synthesis of TNF-alpha and Nitrite, an Intermediate of Nitric Oxide, in a Cellular Model of Inflammation. Open Biochem. J. 2011, 5, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.; Sampaio, E.P.; Zmuidzinas, A.; Frindt, P.; Smith, K.A.; Kaplan, G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J. Exp. Med. 1993, 177, 1675–1680. [Google Scholar] [CrossRef]
- Sampaio, E.P.; Sarno, E.N.; Galilly, R.; Cohn, Z.A.; Kaplan, G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J. Exp. Med. 1991, 173, 699–703. [Google Scholar] [CrossRef]
- Blanchette, J.; Jaramillo, M.; Olivier, M. Signalling events involved in interferon-gamma-inducible macrophage nitric oxide generation. Immunology 2003, 108, 513–522. [Google Scholar] [CrossRef]
- Ferrari, D.P.; Bortolanza, M.; Del Bel, E.A. Interferon-gamma Involvement in the Neuroinflammation Associated with Parkinson’s Disease and L-DOPA-Induced Dyskinesia. Neurotox Res. 2021, 39, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Roy, A.; Choudhury, S.; Banerjee, R.; Dey, S.; Kumar, H. Levodopa-induced Dyskinesia in Parkinson’s Disease: Plausible Inflammatory and Oxidative Stress Biomarkers. Can. J. Neurol. Sci. 2024, 51, 104–109. [Google Scholar] [CrossRef]
- Zhang, S.F.; Xie, C.L.; Lin, J.Y.; Wang, M.H.; Wang, X.J.; Liu, Z.G. Lipoic acid alleviates L-DOPA-induced dyskinesia in 6-OHDA parkinsonian rats via anti-oxidative stress. Mol. Med. Rep. 2018, 17, 1118–1124. [Google Scholar] [CrossRef]
- Bido, S.; Marti, M.; Morari, M. Amantadine attenuates levodopa-induced dyskinesia in mice and rats preventing the accompanying rise in nigral GABA levels. J. Neurochem. 2011, 118, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.H.; Lacoste, A.M.B.; Visanji, N.P.; Lang, A.E.; Fox, S.H.; Brotchie, J.M. Repurposing drugs to treat l-DOPA-induced dyskinesia in Parkinson’s disease. Neuropharmacology 2019, 147, 11–27. [Google Scholar] [CrossRef]
- Daneault, J.F.; Carignan, B.; Sadikot, A.F.; Panisset, M.; Duval, C. Drug-induced dyskinesia in Parkinson’s disease. Should success in clinical management be a function of improvement of motor repertoire rather than amplitude of dyskinesia? BMC Med. 2013, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Rudzinska, M.; Bukowczan, S.; Stozek, J.; Zajdel, K.; Mirek, E.; Chwala, W.; Wojcik-Pedziwiatr, M.; Banaszkiewicz, K.; Szczudlik, A. Causes and consequences of falls in Parkinson disease patients in a prospective study. Neurol. Neurochir. Pol. 2013, 47, 423–430. [Google Scholar] [CrossRef]
- Bachmann, C.G.; Trenkwalder, C. Body weight in patients with Parkinson’s disease. Mov. Disord. 2006, 21, 1824–1830. [Google Scholar] [CrossRef]
- Ashburn, A.; Stack, E.; Pickering, R.M.; Ward, C.D. A community-dwelling sample of people with Parkinson’s disease: Characteristics of fallers and non-fallers. Age Ageing 2001, 30, 47–52. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Barton, B.; de Bie, R.M.A.; Seppi, K.; Coelho, M.; Sampaio, C.; Movement Disorder Society Evidence‐Based Medicine Committee. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Ravina, B.; Seppi, K.; Coelho, M.; Poewe, W.; Rascol, O.; Goetz, C.G.; Sampaio, C. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2011, 26 (Suppl. S3), S2–S41. [Google Scholar] [CrossRef] [PubMed]
- Hubsher, G.; Haider, M.; Okun, M.S. Amantadine: The journey from fighting flu to treating Parkinson disease. Neurology 2012, 78, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, H.W.; Hwang, J.; Kim, J.; Lee, M.J.; Han, H.S.; Lee, W.H.; Suk, K. Microglia-inhibiting activity of Parkinson’s disease drug amantadine. Neurobiol. Aging 2012, 33, 2145–2159. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Pereira, M.; do Nascimento, G.C.; Bortolanza, M.; Michel, P.P.; Raisman-Vozari, R.; Del Bel, E. Doxycycline attenuates l-DOPA-induced dyskinesia through an anti-inflammatory effect in a hemiparkinsonian mouse model. Front. Pharmacol. 2022, 13, 1045465. [Google Scholar] [CrossRef] [PubMed]
- Bariotto-Dos-Santos, K.; Padovan-Neto, F.E.; Bortolanza, M.; Dos-Santos-Pereira, M.; Raisman-Vozari, R.; Tumas, V.; Del Bel, E. Repurposing an established drug: An emerging role for methylene blue in L-DOPA-induced dyskinesia. Eur. J. Neurosci. 2019, 49, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 2008, 27, 2783–2802. [Google Scholar] [CrossRef] [PubMed]
- Murad, F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006, 355, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, J.; Wang, Z.; Feng, M.; Shen, Y.; Cao, S.; Li, T.; Peng, Y.; Fan, L.; Chen, J.; et al. Methylene blue attenuates neuroinflammation after subarachnoid hemorrhage in rats through the Akt/GSK-3beta/MEF2D signaling pathway. Brain Behav. Immun. 2017, 65, 125–139. [Google Scholar] [CrossRef]
- Fenn, A.M.; Skendelas, J.P.; Moussa, D.N.; Muccigrosso, M.M.; Popovich, P.G.; Lifshitz, J.; Eiferman, D.S.; Godbout, J.P. Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. J. Neurotrauma 2015, 32, 127–138. [Google Scholar] [CrossRef]
- Dibaj, P.; Zschuntzsch, J.; Steffens, H.; Scheffel, J.; Goricke, B.; Weishaupt, J.H.; Le Meur, K.; Kirchhoff, F.; Hanisch, U.K.; Schomburg, E.D.; et al. Influence of methylene blue on microglia-induced inflammation and motor neuron degeneration in the SOD1(G93A) model for ALS. PLoS ONE 2012, 7, e43963. [Google Scholar] [CrossRef]
- Zheng, C.Q.; Fan, H.X.; Li, X.X.; Li, J.J.; Sheng, S.; Zhang, F. Resveratrol Alleviates Levodopa-Induced Dyskinesia in Rats. Front. Immunol. 2021, 12, 683577. [Google Scholar] [CrossRef] [PubMed]
- Bayo-Olugbami, A.; Nafiu, A.B.; Amin, A.; Ogundele, O.M.; Lee, C.C.; Owoyele, B.V. Cholecalciferol (VD3) Attenuates L-DOPA-Induced Dyskinesia in Parkinsonian Mice Via Modulation of Microglia and Oxido-Inflammatory Mechanisms. Niger. J. Physiol. Sci. 2022, 37, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Golan, D.; Staun-Ram, E.; Glass-Marmor, L.; Lavi, I.; Rozenberg, O.; Dishon, S.; Barak, M.; Ish-Shalom, S.; Miller, A. The influence of vitamin D supplementation on melatonin status in patients with multiple sclerosis. Brain Behav. Immun. 2013, 32, 180–185. [Google Scholar] [CrossRef]
- Buttner, S.; Faes, L.; Reichelt, W.N.; Broeskamp, F.; Habernig, L.; Benke, S.; Kourtis, N.; Ruli, D.; Carmona-Gutierrez, D.; Eisenberg, T.; et al. The Ca2+/Mn2+ ion-pump PMR1 links elevation of cytosolic Ca2+ levels to alpha-synuclein toxicity in Parkinson’s disease models. Cell Death Differ. 2013, 20, 465–477. [Google Scholar] [CrossRef] [PubMed]
| Drug Name | Characteristics | References |
|---|---|---|
| Amantadine | As a mild glutamate receptor antagonist, it is used to treat Parkinson’s disease (PD), boosting dopamine and preventing its reuptake | [7,11,115,120] |
| Corticosterone | Hormone with potent immunomodulatory properties | [75] |
| Ibuprofen | A non-selective COX inhibitor | [74] |
| MPEP | Metabotropic glutamate receptor 5 antagonist | [88] |
| Doxycycline | A semisynthetic tetracycline antibiotic | [124] |
| Methylene blue (MB) | A non-selective inhibitor of the soluble enzyme guanylyl cyclase (sGC) | [98,125,126,127,128,129,130] |
| Resveratrol (trans-3, 4, 5-trihydroxystilbene, RES) | Class of plant micronutrients called polyphenols | [131] |
| Cholecalciferol (VD3) | Vitamin | [132,133,134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinale, A.; de Iure, A.; Picconi, B. Neuroinflammation and Dyskinesia: A Possible Causative Relationship? Brain Sci. 2024, 14, 514. https://doi.org/10.3390/brainsci14050514
Cardinale A, de Iure A, Picconi B. Neuroinflammation and Dyskinesia: A Possible Causative Relationship? Brain Sciences. 2024; 14(5):514. https://doi.org/10.3390/brainsci14050514
Chicago/Turabian StyleCardinale, Antonella, Antonio de Iure, and Barbara Picconi. 2024. "Neuroinflammation and Dyskinesia: A Possible Causative Relationship?" Brain Sciences 14, no. 5: 514. https://doi.org/10.3390/brainsci14050514
APA StyleCardinale, A., de Iure, A., & Picconi, B. (2024). Neuroinflammation and Dyskinesia: A Possible Causative Relationship? Brain Sciences, 14(5), 514. https://doi.org/10.3390/brainsci14050514








