The Role of Immersive Virtual Reality Interventions in Pediatric Cerebral Palsy: A Systematic Review across Motor and Cognitive Domains
Abstract
1. Introduction
2. Materials and Methods
2.1. PICO Model
2.2. Search Strategy and Eligibility Criteria
2.3. Assess Quality of Included Studies—Risk of Bias
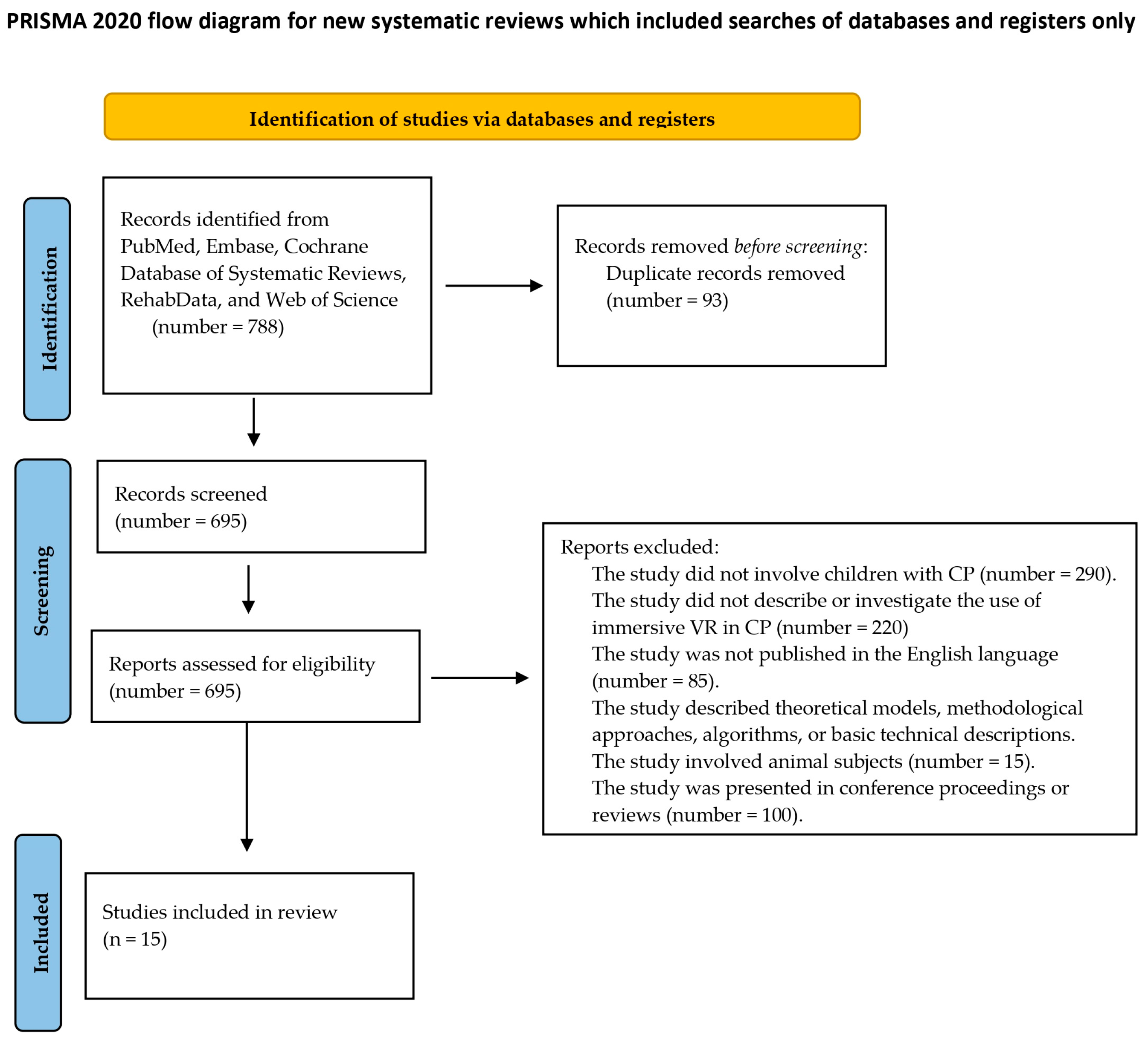
3. Results
4. Key Finding
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dar, H.; Stewart, K.; McIntyre, S.; Paget, S. Multiple motor disorders in cerebral palsy. Dev. Med. Child. Neurol. 2024, 66, 317–325. [Google Scholar] [CrossRef]
- Thomas, S.P.; Novak, I.; Ritterband-Rosenbaum, A.; Lind, K.; Webb, A.; Gross, P.; McNamara, M.; CP Global Clinical Trials Network. The critical need to accelerate cerebral palsy research with consumer engagement, global networks, and adaptive designs. J. Pediatr. Rehabil. Med. 2024, 17, 9–17. [Google Scholar] [CrossRef]
- McIntyre, S.; Goldsmith, S.; Webb, A.; Ehlinger, V.; Hollung, S.J.; McConnell, K.; Arnaud, C.; Smithers-Sheedy, H.; Oskoui, M.; Khandaker, G.; et al. Global prevalence of cerebral palsy: A systematic analysis. Dev. Med. Child. Neurol. 2022, 64, 1494–1506. [Google Scholar] [CrossRef]
- Wimalasundera, N.; Stevenson, V.L. Cerebral palsy. Pract. Neurol. 2016, 16, 184–194. [Google Scholar] [CrossRef]
- Galli, M.; Cimolin, V.; Rigoldi, C.; Tenore, N.; Albertini, G. Gait patterns in hemiplegic children with cerebral palsy: Comparison of right and left hemiplegia. Res. Dev. Disabil. 2010, 31, 1340–1345. [Google Scholar] [CrossRef]
- De Luca, R.; Lauria, P.; Bonanno, M.; Corallo, F.; Rifici, C.; Castorina, M.V.; Trifirò, S.; Gangemi, A.; Lombardo, C.; Quartarone, A.; et al. Neurophysiological and Psychometric Outcomes in Minimal Consciousness State after Advanced Audio-Video Emotional Stimulation: A Retrospective Study. Brain Sci. 2023, 13, 1619. [Google Scholar] [CrossRef]
- Katsma, M.; Liu, H.; Pan, X.; Ryan, K.J.; Roye, D.P.; Chambers, H.G. Management and treatment of musculoskeletal problems in adults with cerebral palsy: Experience gained from two lifespan clinics. J. Pediatr. Rehabil. Med. 2024, 17, 19–33. [Google Scholar] [CrossRef]
- Camara Machado, F.R.; Novak, G.D.S.; Kato, S.K.; Oliveira, A.A. Evaluation of the Usability of a Serious Game in Virtual Reality with a Focus on the Perception and Experience of Health Professionals for Motor Rehabilitation in Children with Cerebral Palsy. Games Health J. 2024. ahead of print. [Google Scholar] [CrossRef]
- De Luca, R.; Portaro, S.; Le Cause, M.; De Domenico, C.; Maggio, M.G.; Ferrera, M.C.; Giuffrè, G.; Bramanti, A.; Calabrò, R.S. Cognitive rehabilitation using immersive virtual reality at young age: A case report on traumatic brain injury. Appl. Neuropsychol. Child. 2020, 9, 282–287. [Google Scholar] [CrossRef]
- Amirthalingam, J.; Paidi, G.; Alshowaikh, K.; Jayarathna, A.I.; Salibindla, D.B.A.M.R.; Karpinska-Leydier, K.; Ergin, H.E. Virtual Reality Intervention to Help Improve Motor Function in Patients Undergoing Rehabilitation for Cerebral Palsy, Parkinson’s Disease, or Stroke: A Systematic Review of Randomized Controlled Trials. Cureus 2021, 13, e16763. [Google Scholar] [CrossRef]
- Meyer-Heim, A.; van Hedel, H.J. Robot-assisted and computer-enhanced therapies for children with cerebral palsy: Current state and clinical implementation. Semin. Pediatr. Neurol. 2013, 20, 139–145. [Google Scholar] [CrossRef]
- Komariah, M.; Amirah, S.; Abdurrahman, M.F.; Handimulya, M.F.S.; Platini, H.; Maulana, S.; Nugrahani, A.D.; Mulyana, A.M.; Qadous, S.G.; Mediani, H.S.; et al. Effectivity of Virtual Reality to Improve Balance, Motor Function, Activities of Daily Living, and Upper Limb Function in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Ther. Clin. Risk Manag. 2024, 20, 95–109. [Google Scholar] [CrossRef]
- Bell, J.; Decker, B.; Eichmann, A.; Palkovich, C.; Reji, C. Effectiveness of Virtual Reality for Upper Extremity Function and Motor Performance of Children With Cerebral Palsy: A Systematic Review. Am. J. Occup. Ther. 2024, 78, 7802180180. [Google Scholar] [CrossRef]
- Wiskerke, E.; Kool, J.; Hilfiker, R.; Sattelmayer, M.; Verheyden, G. Neurorehabilitation including Virtual-Reality-Based Balance Therapy: Factors Associated with Training Response. Brain Sci. 2024, 14, 263. [Google Scholar] [CrossRef]
- Laver, K.E.; George, S.; Thomas, S.; Deutsch, J.E.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2015, 2, CD008349. [Google Scholar] [CrossRef]
- Maggio, M.G.; Naro, A.; Manuli, A.; Maresca, G.; Balletta, T.; Latella, D.; De Luca, R.; Calabrò, R.S. Effects of Robotic Neurorehabilitation on Body Representation in Individuals with Stroke: A Preliminary Study Focusing on an EEG-Based Approach. Brain Topogr. 2021, 34, 348–362. [Google Scholar] [CrossRef]
- De Luca, R.; Maggio, M.G.; Maresca, G.; Latella, D.; Cannavò, A.; Sciarrone, F.; Voi, E.L.; Accorinti, M.; Bramanti, P.; Calabrò, R.S. Improving Cognitive Function after Traumatic Brain Injury: A Clinical Trial on the Potential Use of the Semi-Immersive Virtual Reality. Behav. Neurol. 2019, 2019, 9268179. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Quinn, S.; Suskauer, S.J.; Birch, J.; Busch, T.; Svingos, A.; Crawfis, R.; Yeates, K.O.; Taylor, H.G. Efficacy of a virtual reality-based cognitive interactive training program for children with traumatic brain injuries: Study protocol for a parallel-group randomized controlled trial. Trials 2024, 25, 185. [Google Scholar] [CrossRef]
- Maggio, M.G.; Bonanno, M.; Manuli, A.; Onesta, M.P.; De Luca, R.; Quartarone, A.; Calabrò, R.S. Do Individuals with Spinal Cord Injury Benefit from Semi-Immersive Virtual Reality Cognitive Training? Preliminary Results from an Exploratory Study on an Underestimated Problem. Brain Sci. 2023, 13, 945. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Brown, D. A Review of the PubMed PICO Tool: Using Evidence-Based Practice in Health Education. Health Promot. Pract. 2019, 21, 496–498. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Gagliardi, C.; Turconi, A.C.; Biffi, E.; Maghini, C.; Marelli, A.; Cesareo, A.; Diella, E.; Panzeri, D. Immersive Virtual Reality to Improve Walking Abilities in Cerebral Palsy: A Pilot Study. Ann. Biomed. Eng. 2018, 46, 1376–1384. [Google Scholar] [CrossRef]
- Nossa, R.; Gagliardi, C.; Panzeri, D.; Diella, E.; Maghini, C.; Genova, C.; Turconi, A.C.; Biffi, E. Could Immersive Virtual Reality Training Improve Navigation Skills in Children with Cerebral Palsy? A Pilot Controlled Study. J. Clin. Med. 2022, 11, 6146. [Google Scholar] [CrossRef]
- Biffi, E.; Gagliardi, C.; Maghini, C.; Genova, C.; Panzeri, D.; Redaelli, D.F.; Turconi, A.C. Learning My Way: A Pilot Study of Navigation Skills in Cerebral Palsy in Immersive Virtual Reality. Front. Psychol. 2020, 11, 591296. [Google Scholar] [CrossRef]
- van Gelder, L.; Booth, A.T.C.; van de Port, I.; Buizer, A.I.; Harlaar, J.; van der Krogt, M.M. Real-time feedback to improve gait in children with cerebral palsy. Gait Posture 2017, 52, 76–82. [Google Scholar] [CrossRef]
- van der Krogt, M.M.; Sloot, L.H.; Harlaar, J. Overground versus self-paced treadmill walking in a virtual environment in children with cerebral palsy. Gait Posture 2014, 40, 587–593. [Google Scholar] [CrossRef]
- Barton, G.J.; Hawken, M.B.; Foster, R.J.; Holmes, G.; Butler, P.B. The effects of virtual reality game training on trunk to pelvis coupling in a child with cerebral palsy. J. Neuroeng. Rehabil. 2013, 10, 15. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, Y.; Kang, X.; Shao, M.; Siemelink, L.; Zhang, Y. Gait Characteristics of Children with Spastic Cerebral Palsy during Inclined Treadmill Walking under a Virtual Reality Environment. Appl. Bionics Biomech. 2019, 2019, 8049156. [Google Scholar] [CrossRef]
- Bortone, I.; Barsotti, M.; Leonardis, D.; Crecchi, A.; Tozzini, A.; Bonfiglio, L.; Frisoli, A. Immersive Virtual Environments and Wearable Haptic Devices in rehabilitation of children with neuromotor impairments: A single-blind randomized controlled crossover pilot study. J. Neuroeng. Rehabil. 2020, 17, 144. [Google Scholar] [CrossRef]
- Shum, L.C.; Valdes, B.A.; Hodges, N.J.; Van der Loos, H.F.M. Error Augmentation in Immersive Virtual Reality for Bimanual Upper-Limb Rehabilitation in Individuals With and Without Hemiplegic Cerebral Palsy. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 541–549. [Google Scholar] [CrossRef]
- Jung, Y.G.; Chang, H.J.; Jo, E.S.; Kim, D.H. The Effect of a Horse-Riding Simulator with Virtual Reality on Gross Motor Function and Body Composition of Children with Cerebral Palsy: Preliminary Study. Sensors 2022, 22, 2903. [Google Scholar] [CrossRef]
- Chang, H.J.; Jung, Y.G.; Park, Y.S.; O, S.H.; Kim, D.H.; Kim, C.W. Virtual Reality-Incorporated Horse-Riding Simulator to Improve Motor Function and Balance in Children with Cerebral Palsy: A Pilot Study. Sensors 2021, 21, 6394. [Google Scholar] [CrossRef]
- Sloot, L.H.; Harlaar, J.; van der Krogt, M.M. Self-paced versus fixed speed walking and the effect of virtual reality in children with cerebral palsy. Gait Posture 2015, 42, 498–504. [Google Scholar] [CrossRef]
- Booth, A.T.; Buizer, A.I.; Harlaar, J.; Steenbrink, F.; van der Krogt, M.M. Immediate Effects of Immersive Biofeedback on Gait in Children With Cerebral Palsy. Arch. Phys. Med. Rehabil. 2019, 100, 598–605. [Google Scholar] [CrossRef]
- Saussez, G.; Bailly, R.; Araneda, R.; Paradis, J.; Ebner-Karestinos, D.; Klöcker, A.; Sogbossi, E.S.; Riquelme, I.; Brochard, S.; Bleyenheuft, Y. Efficacy of integrating a semi-immersive virtual device in the HABIT-ILE intervention for children with unilateral cerebral palsy: A non-inferiority randomized controlled trial. J. Neuroeng. Rehabil. 2023, 20, 98. [Google Scholar] [CrossRef]
- Bortone, I.; Leonardis, D.; Mastronicola, N.; Crecchi, A.; Bonfiglio, L.; Procopio, C.; Solazzi, M.; Frisoli, A. Wearable Haptics and Immersive Virtual Reality Rehabilitation Training in Children With Neuromotor Impairments. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1469–1478. [Google Scholar] [CrossRef]
- Choi, J.Y.; Yi, S.H.; Ao, L.; Tang, X.; Xu, X.; Shim, D.; Yoo, B.; Park, E.S.; Rha, D.W. Virtual reality rehabilitation in children with brain injury: A randomized controlled trial. Dev. Med. Child. Neurol. 2021, 63, 480–487. [Google Scholar] [CrossRef]
- Tieri, G.; Morone, G.; Paolucci, S.; Iosa, M. Virtual reality in cognitive and motor rehabilitation: Facts, fiction and fallacies. Expert. Rev. Med. Devices 2018, 15, 107–117. [Google Scholar] [CrossRef]
- Reale, G.; Fusco, A.; Calciano, R.; Vallario, N.; Vagnarelli, G.; Caliandro, P.; Castelli, L.; Moci, M.; Tieri, G.; Iasevoli, L.; et al. The Immediate Effects of Immersive Virtual Reality on Autonomic Nervous System Function in Patients with Disorders of Consciousness after Severe Acquired Brain Injury: A Pilot Study. J. Clin. Med. 2023, 12, 7639. [Google Scholar] [CrossRef]
- Tuena, C.; Serino, S.; Stramba-Badiale, C.; Pedroli, E.; Goulene, K.M.; Stramba-Badiale, M.; Riva, G. Usability of an Embodied CAVE System for Spatial Navigation Training in Mild Cognitive Impairment. J. Clin. Med. 2023, 12, 1949. [Google Scholar] [CrossRef]
- Martino Cinnera, A.; Verna, V.; Marucci, M.; Tavernese, A.; Magnotti, L.; Matano, A.; D’acunto, C.; Paolucci, S.; Morone, G.; Betti, V.; et al. Immersive Virtual Reality for Treatment of Unilateral Spatial Neglect via Eye-Tracking Biofeedback: RCT Protocol and Usability Testing. Brain Sci. 2024, 14, 283. [Google Scholar] [CrossRef]
- Demeco, A.; Zola, L.; Frizziero, A.; Martini, C.; Palumbo, A.; Foresti, R.; Buccino, G.; Costantino, C. Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review. Sensors 2023, 23, 1712. [Google Scholar] [CrossRef]
- Lee, K.; Oh, H.; Lee, G. Fully immersive virtual reality game-based training for an adolescent with spastic diplegic cerebral palsy: A case Report. Children 2022, 9, 1512. [Google Scholar] [CrossRef]
- You, S.H.; Jang, S.H.; Kim, Y.H.; Hallett, M.; Ahn, S.H.; Kwon, Y.H.; Kim, J.H.; Lee, M.Y. Virtual reality–induced cortical reorganization and associated locomotor recovery in chronic stroke: An experimenter-blind randomized study. Stroke 2005, 36, 1166–1171. [Google Scholar] [CrossRef]
- Impellizzeri, F.; Naro, A.; Basile, G.; Bramanti, A.; Gazia, F.; Galletti, F.; Militi, D.; Petralito, F.; Calabrò, R.S.; Milardi, D. Does cybersickness affect virtual reality training using the Computer Assisted Rehabilitation Environment (CAREN)? Preliminary results from a case-control study in Parkinson’s disease. Physiother. Theory Pract. 2022, 38, 2603–2611. [Google Scholar] [CrossRef]
- Chen, Q.C.; Fleming, A.; Lepkowsky, A.; Narouze, S. Virtual reality cybersickness and the headache patient. Pain. Med. 2024, pnae014. [Google Scholar] [CrossRef]
- Cano-De-La-Cuerda, R.; Blázquez-Fernández, A.; Marcos-Antón, S.; Sánchez-Herrera-Baeza, P.; Fernández-González, P.; Collado-Vázquez, S.; Jiménez-Antona, C.; Laguarta-Val, S. Economic Cost of Rehabilitation with Robotic and Virtual Reality Systems in People with Neurological Disorders: A Systematic Review. J. Clin. Med. 2024, 13, 1531. [Google Scholar] [CrossRef]
- Burridge, J.H.; Hughes, A.M. Potential for new technologies in clinical practice. Curr. Opin. Neurol. 2010, 23, 671–677. [Google Scholar] [CrossRef]
| Study | Selection | Comparability | Outcome Assessment | Total Score |
|---|---|---|---|---|
| Gagliardi et al. [23] | 3 | 1 | 2 | 6 |
| Nossa et al. [24] | 3 | 2 | 2 | 7 |
| Biffi et al. [25] | 3 | 1 | 2 | 6 |
| van Gelder et al. [26] | 3 | 1 | 2 | 6 |
| van der Krogt et al. [27] | 3 | 1 | 2 | 6 |
| Barton et al. [28] | 1 | 2 | 1 | 4 |
| Ma et al. [29] | 2 | 1 | 2 | 5 |
| Bortone et al. [30] | 1 | 2 | 1 | 4 |
| Shum et al. [31] | 3 | 1 | 2 | 6 |
| Jung et al. [32] | 3 | 2 | 2 | 7 |
| Chang et al. [33] | 3 | 2 | 2 | 7 |
| Sloot et al. [34] | 3 | 2 | 2 | 7 |
| Booth et al. [35] | 3 | 1 | 2 | 6 |
| Saussez et al. [36] | 3 | 2 | 2 | 7 |
| Bortone et al. [37] | 2 | 1 | 2 | 5 |
| Ref. | Type of Studies | Participant | Intervention | Frequency and Duration | Primary Outcome | Secondary Outcome | Drop Out | Major Findings |
|---|---|---|---|---|---|---|---|---|
| Gagliardi et al. [23] | Pilot Study | 16 children with bilateral CP diplegia SMI level I, II, and III (7–16 years); 10 males and 6 females | IVR using GRAIL system for exercises targeting walking and balance | One daily session lasting 30 min, 5 days a week (18 sessions) | GMFM 88 6MWT FAQ | The Sensewear Armband wearable device was used to measure energy expenditure | No drop-out | Motor skills, including standing, walking, running, and jumping, significantly improved, along with enhanced walking performance indicated by kinematic and kinetic parameters. Progress was observed in hip and ankle functions. |
| Nossa et al. [24] | Pilot Study | 41 children (7–15 years): -14 TD 35 preterm spastic diplegia CP and SMI level I, II, and III. | GRAIL system Regular IVR training The IVR navigation Training | GC and GI underwent 18 daily sessions, each lasting 45 min | Corsi Block Test Subtest Labyrinth of WISC-III “Star-Maze” app | GMFCS MACS | No drop-out | All children with CP showed improved visuospatial abilities after both training courses, indicating the effectiveness of the VR programs. Overall, children with CP demonstrated enhanced performance and motor efficiency. |
| Biffi et al. [25] | Pilot Study | 28 children: 15 with bilateral CP and SMI level I–III, (6–14 years: 11 males and 4 females) 13 TD (5 males and 8 females) | IVR GRAIL system for gait and balance | 21 explorations of the maze, with 16 attempts to freely explore the environment plus five interposed trials | Corsi Block Test Subtest Labyrinth of WISC-III “Star-Maze” app on the GRAIL system | Raven’s progressive matrices | No drop-out | Both groups improved navigation skills in the virtual maze over trials. Typically developing participants quickly mastered maze navigation. Participants with CP navigated similarly once performance stabilized, suggesting minimal impact of motor impairment. |
| van Gelder et al. [26] | Clinical Study | 27 children: 16 spastic CP and SMI level I–III (6–16 years) 11 TD (6–16 years) | IVR GRAIL system And 3D motion capture (Vicon, Oxford, UK) | Self-selected walking speed was assessed for the first 3 min without feedback, followed by feedback on knee extension and hip extension | HBM outputted 3D kinematic data, GPS, and MAP incorporating trunk kinematics | No drop-out | All the children, except one, improved hip and/or knee extension. | |
| van der Krogt et al. [27] | Clinical Study | 20 children: 9 with spastic CP, SMI level I or II (8–14 years) 11 TD (7 males and 4 females between 8 and 15 years old) | 3 treatment: conventional gait lab; GRAIL system; indoor courtyard | Four different 3 min test trials were collected in random order | Various parameters including joint angles, gait velocity, step width, stance motion | GPS MAP The similarity to walking in the street, whether they could walk alone, preferred speed, and fatigue in walking | No drop-out | After training, all children walked independently on the treadmill. Step width and knee/ankle movements varied systematically in PCI; potentially clinically relevant. Walking speed in both labs was slower than natural. |
| Barton et al. [28] | Case Study | 1 child with spastic CP diplegia, SMI level I (10 years old) | IRV Goblin Post Office game CAREN Vicon system. MATLAB’s CONVHULL The exercise takes place on the knees | Treatment of 30 min, 2 times a week, for 6 weeks (13 sessions) | Segmental Assessment of Trunk Control Gait Deviation Index | N/A | No | Both groups exhibited significant improvements, particularly in the least affected hand with the REAtouch intervention and in the most affected hand with the HABIT-ILE group. Additionally, there was no significant disadvantage in virtual reality (RV) treatment |
| Ma et al. [29] | Clinical Study | 20 children: 10 spastic CP and SMI level I–II (6 males and 4 females: age 6–12 years) 10 children TD | IVR CAREN system 3D motion capture system | 1 session | Joint kinematics, walking speed, peak pelvic tilt, ankle dorsiflexion, trunk rotation, stance phase, and ankle angle | Position of the center of pressure The position of the center of mass | No drop-out | During uphill walking, both groups slowed down and shortened steps, with increased pelvic tilt, ankle dorsiflexion, and hip flexion. Children with CP further reduced walking speed and step length, showing altered hip and ankle mechanics compared to TD children. |
| Bortone et al. [30] | Pilot Study Cross-over | 8 children with neuromotor impairments: 3 CP SMI levels I–IV and MACS levels I–III 5 DD | IVR and wearable haptic devices (VERA) using the HMD (Oculus Rift VK2) in the first period + conventional therapy in the second | 8 h (2 sessions per week for 4 weeks) of VERA rehabilitation before receiving conventional therapy | 9-HPT | Zoia’s Protocol for DD Melbourne Assessment of unilateral upper limb function kinesiological assessment | No drop-out | Both conventional and VR-assisted therapies exhibited similar efficacy in improving kinesiological indices for specific tasks, suggesting VR therapy’s potential as a safe alternative or complement to conventional methods. |
| Shum et al. [31] | crossover counterbalanced design | 17 children: 12 TD (13–21 years) 5 hemiplegic CP SMI level I-III | Bimanual treatment of the upper limb using Oculus Rift system, l’Oculus touch controller | The study was a single-session experiment | Symmetry Root-Mean-Squared Error (RMSE) in cm | Rom peak velocity per reach time to peak velocity movement smoothness trunk compensation | No drop-out | There were improvements in the symmetry of bimanual movements in both groups that used increased error feedback during the use of virtual reality. |
| Jung et al. [32] | Clinical Study | 17 children with spastic CP diplegia SMI level I–IV EG: 10 CP (7 males and 3 females) CG: 7 CP (4 males and 3 females) | EG: IVR HRS and conventional Ph CG: home-based aerobic exercise and conventional Ph | Twice a week for a total of 16 sessions for both groups | GMFM | BIA PBS TUG | No drop-out | The study demonstrated that high-resistance strength training with virtual reality yielded positive effects on motor function, balance, mobility, and body composition in children with spastic CP, notably increasing skeletal muscle mass, without significant adverse events. |
| Chang et al. [33] | Pilot Study | 16 children with CP SMI levels I–IV (5–17 years); 6 females and 10 males | IVR HRS | 30 min twice a week over a period of 8 weeks (total of 16 sessions) | PBS GMFM-88 GMFM-66 | N/A | No drop-out | Statistically significant improvements in PBS, GMFM-66, and GMFM-88 scores, particularly in standing and walking, were observed without any reported adverse events. |
| Sloot et al. [34] | Clinical Study | 20 children: 9 with CP SMI level I or II (5 females and 4 males, age 8–14 years) 11 TD (4 females and 7 males aged 8–15 years) | GRAIL system and Three markers of movement to the pelvis, thighs, shanks, and feet | Participants familiarized with treadmill walking before conducting four random trials: walking at preferred speed with and without VR | Ground reaction force motion data 3D kinematics and kinetics Joint and segment angles Walking speed, stride length, stride time, step width, and stance percentage | The gait pattern; the ankle peak power; and work for the hip, knee, and ankle | No drop-out | The study suggests self-regulated and treadmill-induced walking, with or without VR, are interchangeable for gait analysis, with potential benefits such as increased walking speed variability during self-paced walking and VR’s motivational aspect akin to surface walking, providing feedback or challenges. |
| Booth et al. [35] | Clinical Study | 22 children with spastic CP, SMI level I–II (age between 5 and 16 years old) | Double-belt instrumented treadmill Camera system with 26 retro-reflective markersHuman body model | 1 session | Ankle power generation during pushing, knee extension, stride length, and biofeedback on aspects of gait | Stride length, knee extension, and ankle power | 3 children | There were significant increases in ankle power and notable improvements in knee extension and stride length, which are clinically significant. |
| Saussez et al. [36] | RCT | 40 children with hemiparetic CP-MACS I–III and GMFCS I–II (age 5–18 years): EG = 20 children CG = 20 children | EG: therapy with HABIT-ILE and SEMI IVR (Reatouch) CG: therapy with HABIT-ILE | EG: 53 h of HABIT-ILE and 37 h of REAtouch over two weeks, while the CG underwent 90 h of HABIT-ILE over the same duration | AHA | JTHFT BBT 6MWT ACTIVLIM-CP PEDI COPM | 2 children | Both groups demonstrated significant improvements in most measures, with REAtouch showing efficacy in the less affected hand and HABIT-ILE in the more affected hand, suggesting REAtouch’s non-inferiority during HABIT-ILE compared to conventional intervention in children with unilateral cerebral palsy. |
| Bortone et al. [37] | Clinical Study | 20 children EG: 3 children with CP (MACS I-III and GMFCS I–II) and 5 children with DD (age 7–14 years) CG: 8 children with TD (age 8–16 years) and 4 adults (age 24) | Two wearable haptic interfaces for cutaneous feedback, two dedicated immersive serious games for upper limbs | Each of the 4 levels were performed 3 times, for a total of 12 repetitions | Zoia’s protocol 9-HPT | A kinematic evaluation | 1 child | The findings indicate the system’s compatibility with diverse motor skill levels, enabling patients to complete the experimental rehabilitation session, with performance varying according to the expected motor skills of distinct groups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, M.G.; Valeri, M.C.; De Luca, R.; Di Iulio, F.; Ciancarelli, I.; De Francesco, M.; Calabrò, R.S.; Morone, G. The Role of Immersive Virtual Reality Interventions in Pediatric Cerebral Palsy: A Systematic Review across Motor and Cognitive Domains. Brain Sci. 2024, 14, 490. https://doi.org/10.3390/brainsci14050490
Maggio MG, Valeri MC, De Luca R, Di Iulio F, Ciancarelli I, De Francesco M, Calabrò RS, Morone G. The Role of Immersive Virtual Reality Interventions in Pediatric Cerebral Palsy: A Systematic Review across Motor and Cognitive Domains. Brain Sciences. 2024; 14(5):490. https://doi.org/10.3390/brainsci14050490
Chicago/Turabian StyleMaggio, Maria Grazia, Maria Chiara Valeri, Rosaria De Luca, Fulvia Di Iulio, Irene Ciancarelli, Morena De Francesco, Rocco Salvatore Calabrò, and Giovanni Morone. 2024. "The Role of Immersive Virtual Reality Interventions in Pediatric Cerebral Palsy: A Systematic Review across Motor and Cognitive Domains" Brain Sciences 14, no. 5: 490. https://doi.org/10.3390/brainsci14050490
APA StyleMaggio, M. G., Valeri, M. C., De Luca, R., Di Iulio, F., Ciancarelli, I., De Francesco, M., Calabrò, R. S., & Morone, G. (2024). The Role of Immersive Virtual Reality Interventions in Pediatric Cerebral Palsy: A Systematic Review across Motor and Cognitive Domains. Brain Sciences, 14(5), 490. https://doi.org/10.3390/brainsci14050490









