A Systematic Review on Autism and Hyperserotonemia: State-of-the-Art, Limitations, and Future Directions
Abstract
1. Introduction
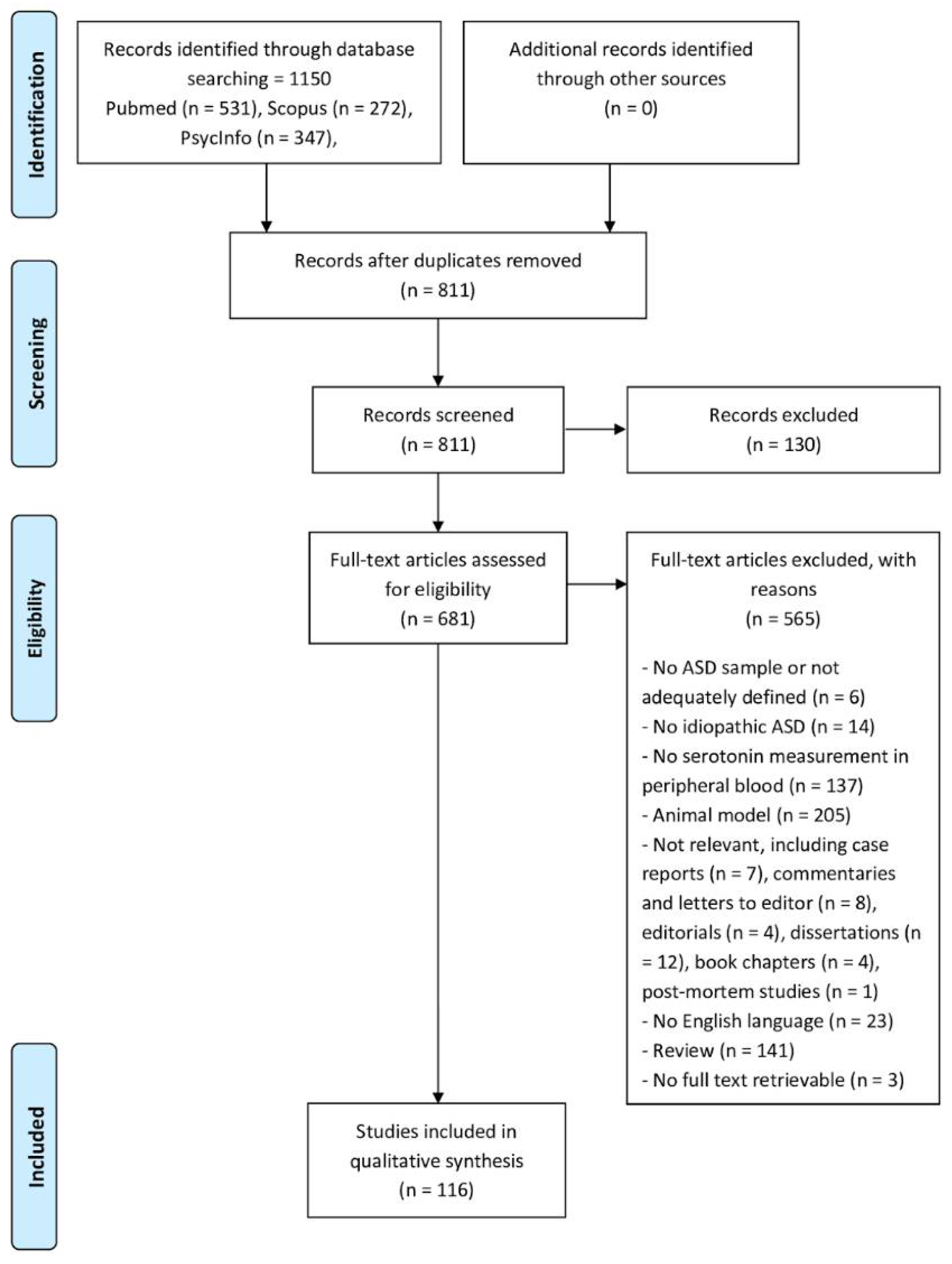
2. Materials and Methods
2.1. Database Search Strategy
2.2. Literature Search Strategy and Study Eligibility
2.3. Variables of Interest and Data Extraction
3. Results
3.1. Demographic Characteristics of the Studies
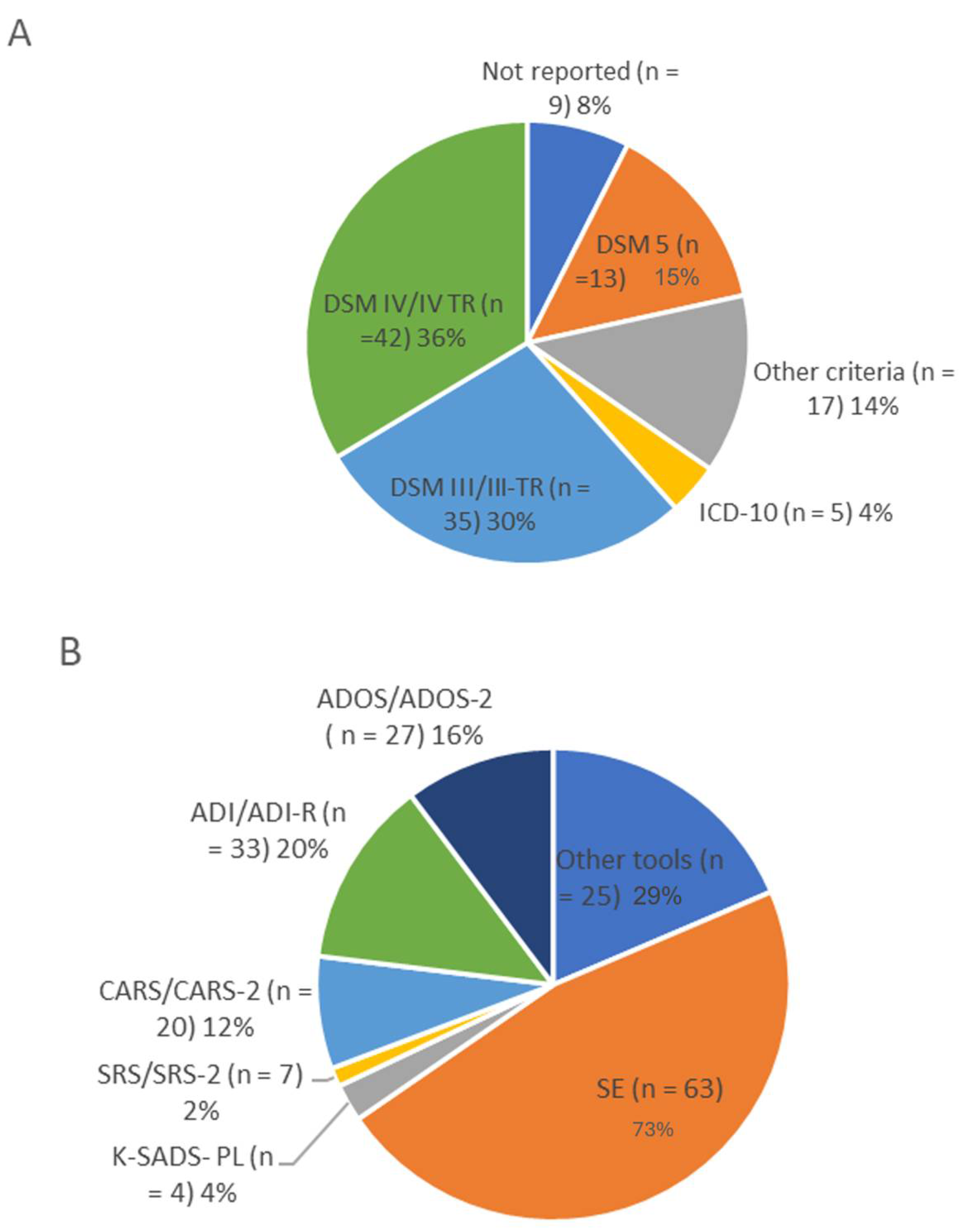
3.2. Diagnostic and Clinical Assessment
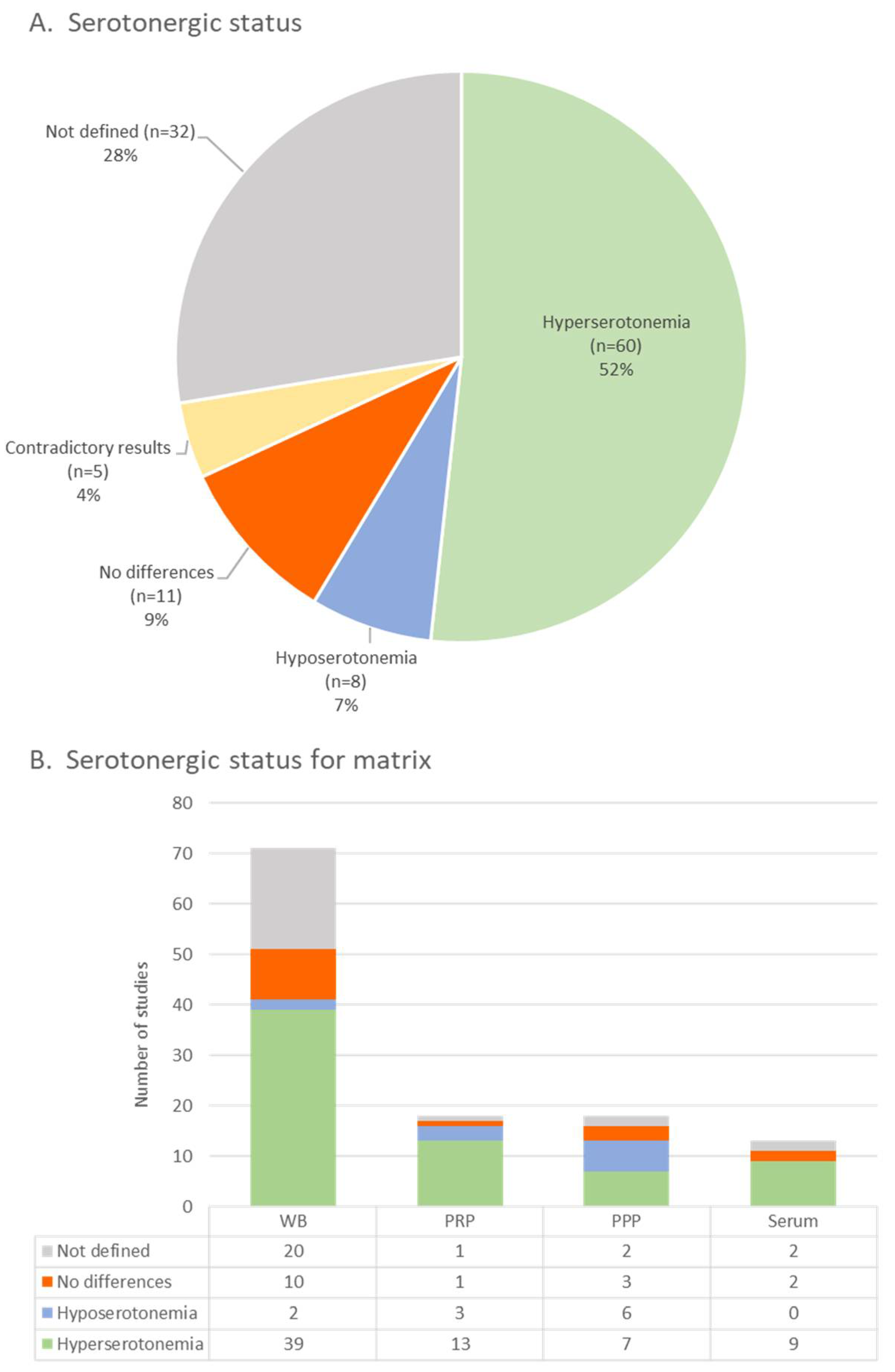
3.3. Serotonin Analysis
3.3.1. Pre-Study Treatment, Sampling, and Pre-Analytical Procedures
3.3.2. Analytical Methods
3.3.3. Matrices
3.3.4. Peripheral Serotonin Concentration
3.3.5. Statistical Analysis and Cut-Off Values of Hyperserotonemia
3.4. Serotonin Levels and Clinical Outcomes
3.4.1. ASD Core Symptoms and Neurocognitive Outcomes
3.4.2. Emotional–Behavioral Outcomes
3.4.3. Other Relevant Clinical Outcomes
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM Library: Des Moines, IA, USA, 2022; Available online: https://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425787 (accessed on 5 September 2023).
- Fakhoury, M. Autistic spectrum disorders: A review of clinical features, theories and diagnosis. Int. J. Dev. Neurosci. 2015, 43, 70–77. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR. Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Geschwind, D.H. Genetics of autism spectrum disorders. Trends Cogn. Sci. 2011, 15, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, B.; Sarkans, U.; Schumann, G.; Persico, A.M. Biomarkers in autism spectrum disorder: The old and the new. Psychopharmacology 2014, 231, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Baizer, J.S. Functional and Neuropathological Evidence for a Role of the Brainstem in Autism. Front. Integr. Neurosci. 2021, 15, 748977. [Google Scholar] [CrossRef]
- Lovato, D.V.; Herai, R.R.; Pignatari, G.C.; Beltrão-Braga, P.C. The Relevance of Variants With Unknown Significance for Autism Spectrum Disorder Considering the Genotype–Phenotype Interrelationship. Front. Psychiatry 2019, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; Casanova, E.L.; Frye, R.E.; Baeza-Velasco, C.; LaSalle, J.M.; Hagerman, R.J.; Scherer, S.W.; Natowicz, M.R. Editorial: Secondary vs. Idiopathic Autism. Front. Psychiatry 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- National Human Genome Research Institute. National Institute of Health about Autism. 2022. Available online: https://www.genome.gov/Genetic-Disorders/Autism (accessed on 15 May 2023).
- Kates, W.R.; Burnette, C.P.; Eliez, S.; Strunge, L.A.; Kaplan, D.; Landa, R.; Reiss, A.L.; Pearlson, G.D. Neuroanatomic Variation in Monozygotic Twin Pairs Discordant for the Narrow Phenotype for Autism. Am. J. Psychiatry 2004, 161, 539–546. [Google Scholar] [CrossRef]
- Huguet, G.; Benabou, M.; Bourgeron, T. The Genetics of Autism Spectrum Disorders. In A Time for Metabolism and Hormones (Research and Perspectives in Endocrine Interactions); Sassone-Corsi, P., Christen, Y., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 101–129. [Google Scholar] [CrossRef]
- Ho, A.; Towheed, A.; Luong, S.; Zucker, S.; Fethke, E. Clinical Discordance in Monozygotic Twins with Autism Spectrum Disorder. Cureus 2022, 14, e24813. [Google Scholar] [CrossRef]
- Mitchell, S.; Cardy, J.O.; Zwaigenbaum, L. Differentiating Autism Spectrum Disorder from Other Developmental Delays in the First Two Years of Life. Dev. Disabil. Res. Rev. 2011, 17, 130–140. [Google Scholar] [CrossRef]
- Gabriele, S.; Sacco, R.; Persico, A.M. Blood serotonin levels in autism spectrum disorder: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2014, 24, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Bridgemohan, C.; Cochran, D.M.; Howe, Y.J.; Pawlowski, K.; Zimmerman, A.W.; Anderson, G.M.; Choueiri, R.; Sices, L.; Miller, K.J.; Ultmann, M.; et al. Investigating Potential Biomarkers in Autism Spectrum Disorder. Front. Integr. Neurosci. 2019, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Padmakumar, M.; Van Raes, E.; Van Geet, C.; Freson, K. Blood platelet research in autism spectrum disorders: In search of biomarkers. Res. Pract. Thromb. Haemost. 2019, 3, 566–577. [Google Scholar] [CrossRef]
- Janšáková, K.; Kyselicová, K.; Ostatníková, D.; Repiská, G. Potential of Salivary Biomarkers in Autism Research: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10873. [Google Scholar] [CrossRef] [PubMed]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource. Food and Drug Administration (US). 2016. Available online: http://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 10 May 2023).
- Schain, R.J.; Freedman, D.X. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J. Pediatr. 1961, 58, 315–320. [Google Scholar] [CrossRef]
- Hanley, H.G.; Stahl, S.M.; Freedman, D.X. Hyperserotonemia and Amine Metabolites in Autistic and Retarded Children. Arch. Gen. Psychiatry 1977, 34, 521–531. [Google Scholar] [CrossRef]
- Anderson, G.M.; Horne, W.C.; Chatterjee, D.; Cohen, D.J. The Hyperserotonemia of Autism. Ann. New York Acad. Sci. 1990, 600, 331–340. [Google Scholar] [CrossRef]
- Veenstra-VanderWeele, J.; Muller, C.L.; Iwamoto, H.; Sauer, J.E.; Owens, W.A.; Shah, C.R.; Cohen, J.; Mannangatti, P.; Jessen, T.; Thompson, B.J.; et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc. Natl. Acad. Sci. USA 2012, 109, 5469–5474. [Google Scholar] [CrossRef]
- Pagan, C.; Benabou, M.; Leblond, C.; Cliquet, F.; Mathieu, A.; Lemière, N.; Goubran-Botros, H.; Delorme, R.; Leboyer, M.; Callebert, J.; et al. Decreased phenol sulfotransferase activities associated with hyperserotonemia in autism spectrum disorders. Transl. Psychiatry 2021, 11, 23. [Google Scholar] [CrossRef]
- Persico, A.M.; Sacco, R. Endophenotypes in Autism Spectrum Disorders. In Comprehensive Guide to Autism; Patel, V.B., Preedy, V.R., Martin, C.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 77–95. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S. Epilepsy, status epilepticus, and refractory status epilepticus. In Refractory Status Epilepticus: Diagnosis and Treatment; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–41. [Google Scholar] [CrossRef]
- Gottesman, I.I.; Gould, T.D.; Zulueta, J.; Leow, A.D.; Ajilore, O.; Goldsmith, D.R.; Crooks, C.L.; Walker, E.F.; Cotes, R.O.; Rush, A.J.; et al. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Israelyan, N.; Margolis, K.G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 2018, 132, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The ever-changing roles of serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef]
- Mercado, C.P.; Kilic, F. Molecular Mechanisms of SERT in Platelets: Regulation of Plasma Serotonin Levels. Mol. Interv. 2010, 10, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Persico, A.M. Developmental roles for the serotonin transporter. In Experimental Models in Serotonin Transporter Research; Kalueff, A.V., LaPorte, J.L., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 78–104. [Google Scholar] [CrossRef]
- Marazziti, D.; Muratori, F.; Cesari, A.; Masala, I.; Baroni, S.; Giannaccini, G.; Dell’Osso, L.; Cosenza, A.; Pfanner, P.; Cassano, G.B. Increased Density of the Platelet Serotonin Transporter in Autism. Pharmacopsychiatry 2000, 33, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Mohanakumar, K.P.; Rajamma, U. Serotonin mediated immunoregulation and neural functions: Complicity in the aetiology of autism spectrum disorders. Neurosci. Biobehav. Rev. 2015, 55, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.M.; Oliveira, G.; Morgadinho, T.; Fesel, C.; Macedo, T.R.; Bento, C.; Marques, C.; Ataíde, A.; Miguel, T.; Borges, L.; et al. Variants of the serotonin transporter gene (SLC6A4) significantly contribute to hyperserotonemia in autism. Mol. Psychiatry 2004, 9, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Devlin, B.; Cook, E.H.; Coon, H.; Dawson, G.; Grigorenko, E.L.; McMahon, W.; Minshew, N.; Pauls, D.; Smith, M.; A Spence, M.; et al. Autism and the serotonin transporter: The long and short of it. Mol. Psychiatry 2005, 10, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Napolioni, V.; Lombardi, F.; Sacco, R.; Curatolo, P.; Manzi, B.; Alessandrelli, R.; Militerni, R.; Bravaccio, C.; Lenti, C.; Saccani, M.; et al. Family-based association study of ITGB3 in autism spectrum disorder and its endophenotypes. Eur. J. Hum. Genet. 2011, 19, 353–359. [Google Scholar] [CrossRef]
- Gabriele, S.; Canali, M.; Lintas, C.; Sacco, R.; Tirindelli, M.C.; Ricciardello, A.; Persico, A.M. Evidence that ITGB3 promoter variants increase serotonin blood levels by regulating platelet serotonin transporter trafficking. Hum. Mol. Genet. 2019, 28, 1153–1161. [Google Scholar] [CrossRef]
- Pino, G.; Moessner, R.; Lesch, K.P.; Lauder, J.M.; Persico, A.M. Roles for Serotonin in Neurodevelopment: More than just Neural Transmission. Curr. Neuropharmacol. 2004, 2, 403–417. [Google Scholar] [CrossRef]
- Miyazaki, K.; Narita, N.; Narita, M. Maternal administration of thalidomide or valproic acid causes abnormal serotonergic neurons in the offspring: Implication for pathogenesis of autism. Int. J. Dev. Neurosci. 2005, 23, 287–297. [Google Scholar] [CrossRef]
- Dufour-Rainfray, D.; Vourc’h, P.; Tourlet, S.; Guilloteau, D.; Chalon, S.; Andres, C.R. Fetal exposure to teratogens: Evidence of genes involved in autism. Neurosci. Biobehav. Rev. 2011, 35, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 2009, 6, e1000097-269. [Google Scholar] [CrossRef] [PubMed]
- A Grimes, D.; Schulz, K.F. An overview of clinical research: The lay of the land. Lancet 2002, 359, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa ON, USA, 2014; Volume 7. [Google Scholar]
- Carpita, B.; Stagnari, R.; Palego, L.; Baroni, D.; Massimetti, G.; Nardi, B.; Cremone, I.M.; Betti, L.; Giannaccini, G.; Dell’Osso, L. Circulating Levels of 5-HT and BDNF in Adults with Autism Spectrum Conditions: An Investigation in a Sample of Subjects with Autism Spectrum Disorder, their First-degree Relatives and Controls. Curr. Med. Chem. 2024, 31, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Cremone, I.M.; Nardi, B.; Amatori, G.; Palego, L.; Baroni, D.; Casagrande, D.; Massimetti, E.; Betti, L.; Giannaccini, G.; Dell’Osso, L.; et al. Unlocking the Secrets: Exploring the Biochemical Correlates of Suicidal Thoughts and Behaviors in Adults with Autism Spectrum Conditions. Biomedicines 2023, 11, 1600. [Google Scholar] [CrossRef]
- Xiaoxue, L. Correlation between 5-HT, Hcy and the incidence and severity of autism in children. Cell. Mol. Biol. 2023, 69, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zuniga-Kennedy, M.; Davoren, M.; Shuffrey, L.C.; Luna, R.A.; Savidge, T.; Prasad, V.; Anderson, G.M.; Veenstra-VanderWeele, J.; Williams, K.C. Intestinal Predictors of Whole Blood Serotonin Levels in Children With or Without Autism. J. Autism Dev. Disord. 2022, 52, 3780–3789. [Google Scholar] [CrossRef]
- Mostafa, G.A.; Meguid, N.A.; Shehab, A.A.S.; Elsaeid, A.; Maher, M. Plasma levels of nerve growth factor in Egyptian autistic children: Relation to hyperserotonemia and autoimmunity. J. Neuroimmunol. 2021, 358, 577638. [Google Scholar] [CrossRef]
- Meyyazhagan, A.; Balasubramanian, B.; Easwaran, M.; Alagamuthu, K.K.; Shanmugam, S.; Bhotla, H.K.; Pappusamy, M.; Arumugam, V.A.; Thangaraj, A.; Kaul, T.; et al. Biomarker study of the biological parameter and neurotransmitter levels in autistics. Mol. Cell. Biochem. 2020, 474, 277–284. [Google Scholar] [CrossRef]
- Ali, L.B.M.; Alasadi, I.J.B.; Zearah, S.A. Determination of Some Biomarkers that affect in Behaviors of Autism Spectrum Disorder Individuals in Iraq. Indian J. Forensic Med. Toxicol. 2020, 14, 1681–1686. [Google Scholar] [CrossRef]
- Javadfar, Z.; Abdollahzad, H.; Moludi, J.; Rezaeian, S.; Amirian, H.; Foroughi, A.A.; Nachvak, S.M.; Goharmehr, N.; Mostafai, R. Effects of vitamin D supplementation on core symptoms, serum serotonin, and interleukin-6 in children with autism spectrum disorders: A randomized clinical trial. Nutrition 2020, 79–80, 110986. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, B.; Verma, D.; Guhathakurta, S.; Jaiswal, P.; Singh, A.S.; Sinha, S.; Ghosh, S.; Mukhopadhyay, K.; Mohanakumar, K.P.; Rajamma, U. Gender-Specific Effect of 5-HT and 5-HIAA on Threshold Level of Behavioral Symptoms and Sex-Bias in Prevalence of Autism Spectrum Disorder. Front. Neurosci. 2020, 13, 1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, N.; Yang, J.-J.; Zhao, D.-M.; Chen, B.; Zhang, G.-Q.; Chen, S.; Cao, R.-F.; Yu, H.; Zhao, C.-Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Zhu, J.; Yang, T.; Guo, M.; Li, Q.; Chen, J.; Li, T. The Gut Microbiota and Associated Metabolites Are Altered in Sleep Disorder of Children With Autism Spectrum Disorders. Front. Psychiatry 2020, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Wichers, R.H.; Findon, J.L.; Jelsma, A.; Giampietro, V.; Stoencheva, V.; Robertson, D.M.; Murphy, C.M.; McAlonan, G.; Ecker, C.; Rubia, K.; et al. Modulation of brain activation during executive functioning in autism with citalopram. Transl. Psychiatry 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Aaron, E.; Montgomery, A.; Ren, X.; Guter, S.; Anderson, G.; Carneiro, A.M.D.; Jacob, S.; Mosconi, M.; Pandey, G.N.; Cook, E.; et al. Whole Blood Serotonin Levels and Platelet 5-HT2A Binding in Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 2417–2425. [Google Scholar] [CrossRef]
- Montgomery, A.K.; Shuffrey, L.C.; Guter, S.J.; Anderson, G.M.; Jacob, S.; Mosconi, M.W.; Sweeney, J.A.; Turner, J.B.; Sutcliffe, J.S.; Cook, E.H.; et al. Maternal Serotonin Levels Are Associated With Cognitive Ability and Core Symptoms in Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2018, 57, 867–875. [Google Scholar] [CrossRef]
- Lefevre, A.; Mottolese, R.; Redouté, J.; Costes, N.; Le Bars, D.; Geoffray, M.-M.; Leboyer, M.; Sirigu, A. Oxytocin Fails to Recruit Serotonergic Neurotransmission in the Autistic Brain. Cereb. Cortex 2018, 28, 4169–4178. [Google Scholar] [CrossRef]
- Abdulamir, H.A.; Abdul-Rasheed, O.F.; Abdulghani, E.A. Serotonin and serotonin transporter levels in autistic children. SciVee 2018, 39, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhu, J.; Yang, T.; Lai, X.; Liu, X.; Liu, J.; Chen, J.; Li, T. Vitamin A improves the symptoms of autism spectrum disorders and decreases 5-hydroxytryptamine (5-HT): A pilot study. Brain Res. Bull. 2018, 137, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ormstad, H.; Bryn, V.; Verkerk, R.; Skjeldal, O.H.; Halvorsen, B.; Saugstad, O.D.; Isaksen, J.; Maes, M. Serum Tryptophan, Tryptophan Catabolites and Brain-derived Neurotrophic Factor in Subgroups of Youngsters with Autism Spectrum Disorders. CNS Neurol. Disord. Drug Targets 2018, 17, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Shuffrey, L.C.; Guter, S.J.; Delaney, S.; Jacob, S.; Anderson, G.M.; Sutcliffe, J.S.; Cook, E.H.; Veenstra-VanderWeele, J. Is There Sexual Dimorphism of Hyperserotonemia in Autism Spectrum Disorder? Autism Res 2017, 10, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Pagan, C.; Goubran-Botros, H.; Delorme, R.; Benabou, M.; Lemière, N.; Murray, K.; Amsellem, F.; Callebert, J.; Chaste, P.; Jamain, S.; et al. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci. Rep. 2017, 7, 2096. [Google Scholar] [CrossRef] [PubMed]
- Benabou, M.; Rolland, T.; Leblond, C.S.; Millot, G.A.; Huguet, G.; Delorme, R.; Leboyer, M.; Pagan, C.; Callebert, J.; Maronde, E.; et al. Heritability of the melatonin synthesis variability in autism spectrum disorders. Sci. Rep. 2017, 7, 17746. [Google Scholar] [CrossRef]
- Marler, S.; Ferguson, B.J.; Lee, E.B.; Peters, B.; Williams, K.C.; McDonnell, E.; Macklin, E.A.; Levitt, P.; Gillespie, C.H.; Anderson, G.M.; et al. Brief Report: Whole Blood Serotonin Levels and Gastrointestinal Symptoms in Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 1124–1130. [Google Scholar] [CrossRef]
- Chakraborti, B.; Verma, D.; Karmakar, A.; Jaiswal, P.; Sanyal, A.; Paul, D.; Sinha, S.; Singh, A.S.; Guhathakurta, S.; Roychowdhury, A.; et al. Genetic variants of MAOB affect serotonin level and specific behavioral attributes to increase autism spectrum disorder (ASD) susceptibility in males. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 123–136. [Google Scholar] [CrossRef]
- Kheirouri, S.; Kalejahi, P.; Noorazar, S.G. Plasma levels of serotonin, gastrointestinal symptoms,and sleep problems in children with autism. Turk. J. Med. Sci. 2016, 46, 1765–1772. [Google Scholar] [CrossRef]
- Francis, S.M.; Kistner-Griffin, E.; Yan, Z.; Guter, S.; Cook, E.H.; Jacob, S. Variants in Adjacent Oxytocin/Vasopressin Gene Region and Associations with ASD Diagnosis and Other Autism Related Endophenotypes. Front. Neurosci. 2016, 10, 195. [Google Scholar] [CrossRef]
- Chugani, D.C.; Chugani, H.T.; Wiznitzer, M.; Parikh, S.; Evans, P.A.; Hansen, R.L.; Nass, R.; Janisse, J.J.; Dixon-Thomas, P.; Behen, M.; et al. Efficacy of Low-Dose Buspirone for Restricted and Repetitive Behavior in Young Children with Autism Spectrum Disorder: A Randomized Trial. J. Pediatr. 2016, 170, 45–53.e4. [Google Scholar] [CrossRef]
- Gebril, O.H.; Meguid, N.; Khalil, R. A study of blood serotonin and serotonin transporter promoter variant (5-HTTLPR) polymorphism in Egyptian autistic children. Adv. Biomed. Res. 2015, 4, 94. [Google Scholar] [CrossRef]
- Bijl, N.; Thys, C.; Wittevrongel, C.; De la Marche, W.; Devriendt, K.; Peeters, H.; Van Geet, C.; Freson, K. Platelet studies in autism spectrum disorder patients and first-degree relatives. Mol. Autism 2015, 6, 57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, C.-J.; Tan, H.-P.; Yang, F.-Y.; Wang, H.-P.; Liu, C.-L.; He, H.-Z.; Sang, B.; Zhu, X.-M.; Du, Y.-J. The cortisol, serotonin and oxytocin are associated with repetitive behavior in autism spectrum disorder. Res. Autism Spectr. Disord. 2015, 18, 12–20. [Google Scholar] [CrossRef]
- Yang, C.-J.; Liu, C.-L.; Sang, B.; Zhu, X.-M.; Du, Y.-J. The combined role of serotonin and interleukin-6 as biomarker for autism. Neuroscience 2015, 284, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Alabdali, A.; Al-Ayadhi, L.; El-Ansary, A. Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J. Neuroinflammation 2014, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Kolevzon, A.; Lim, T.; Schmeidler, J.; Martello, T.; Cook, E.H., Jr.; Silverman, J.M. Self-injury in autism spectrum disorder: An effect of serotonin transporter gene promoter variants. Psychiatry Res. 2014, 220, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, S.; Lombardi, F.; Sacco, R.; Napolioni, V.; Altieri, L.; Tirindelli, M.C.; Gregorj, C.; Bravaccio, C.; Rousseau, F.; Persico, A.M. The GLO1 C332 (Ala111) allele confers autism vulnerability: Family-based genetic association and functional correlates. J. Psychiatr. Res. 2014, 59, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Pagan, C.; Delorme, R.; Callebert, J.; Goubran-Botros, H.; Amsellem, F.; Drouot, X.; Boudebesse, C.; Le Dudal, K.; Ngo-Nguyen, N.; Laouamri, H.; et al. The serotonin-N-acetylserotonin–melatonin pathway as a biomarker for autism spectrum disorders. Transl. Psychiatry 2014, 4, e479. [Google Scholar] [CrossRef] [PubMed]
- Levin-Decanini, T.; Maltman, N.; Francis, S.M.; Guter, S.; Anderson, G.M.; Cook, E.H.; Jacob, S. Parental Broader Autism Subphenotypes in ASD Affected Families: Relationship to Gender, Child’s Symptoms, SSRI Treatment, and Platelet Serotonin. Autism Res. 2013, 6, 621–630. [Google Scholar] [CrossRef][Green Version]
- Anderson, G.M.; Hertzig, M.E.; McBride, P.A. Brief Report: Platelet-Poor Plasma Serotonin in Autism. J. Autism Dev. Disord. 2012, 42, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.; Al-Ayadhi, L.Y. A lack of association between hyperserotonemia and the increased frequency of serum anti-myelin basic protein auto-antibodies in autistic children. J. Neuroinflammation 2011, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.K.; Ben Bacha, A.; Ayahdi, L.Y.A. Relationship between chronic lead toxicity and plasma neurotransmitters in autistic patients from Saudi Arabia. Clin. Biochem. 2011, 44, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Curatolo, P.; Manzi, B.; Militerni, R.; Bravaccio, C.; Frolli, A.; Lenti, C.; Saccani, M.; Elia, M.; Reichelt, K.; et al. Principal pathogenetic components and biological endophenotypes in autism spectrum disorders. Autism Res. 2010, 3, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Kazek, B.; Huzarska, M.; Grzybowska-Chlebowczyk, U.; Kajor, M.; Ciupińska-Kajor, M.; Woś, H.; Marszał, E. Platelet and intestinal 5-HT2A receptor mRNA in autistic spectrum disorders—Results of a pilot study. Acta Neurobiol. Exp. 2010, 70, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Kolevzon, A.; Newcorn, J.H.; Kryzak, L.; Chaplin, W.; Watner, D.; Hollander, E.; Smith, C.J.; Cook, E.H.; Silverman, J.M. Relationship between whole blood serotonin and repetitive behaviors in autism. Psychiatry Res. 2010, 175, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Mulder, E.J.; Anderson, G.M.; Kemperman, R.F.; Oosterloo-Duinkerken, A.; Minderaa, R.B.; Kema, I.P. Urinary Excretion of 5-Hydroxyindoleacetic Acid, Serotonin and 6-Sulphatoxymelatonin in Normoserotonemic and Hyperserotonemic Autistic Individuals. Neuropsychobiology 2009, 61, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.F.J.; Muskiet, F.D.; Boutier, A.I.; Kema, I.P.; Muskiet, F.A.J. Brief Report: Normal Intestinal Permeability at Elevated Platelet Serotonin Levels in a Subgroup of Children with Pervasive Developmental Disorders in Curaçao (The Netherlands Antilles). J. Autism Dev. Disord. 2008, 38, 401–406. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, D.F.; Hamza, R.T.; AL Shehab, A.; Mostafa, G.A. Hyperserotonemia in Egyptian autistic children: Relation to allergic manifestations. J. Pediatr. Neurol. 2008, 06, 227–236. [Google Scholar] [CrossRef]
- Warren, R.; Singh, V. Elevated Serotonin Levels in Autism: Association with the Major Histocompatibility Complex. Neuropsychobiology 2008, 34, 72–75. [Google Scholar] [CrossRef]
- Sacco, R.; Papaleo, V.; Hager, J.; Rousseau, F.; Moessner, R.; Militerni, R.; Bravaccio, C.; Trillo, S.; Schneider, C.; Melmed, R.; et al. Case-control and family-based association studies of candidate genes in autistic disorder and its endophenotypes: TPH2 and GLO1. BMC Med. Genet. 2007, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Militerni, R.; Frolli, A.; Bravaccio, C.; Gritti, A.; Elia, M.; Curatolo, P.; Manzi, B.; Trillo, S.; Lenti, C.; et al. Clinical, Morphological, and Biochemical Correlates of Head Circumference in Autism. Biol. Psychiatry 2007, 62, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.M.; Sousa, I.; Martins, M.; Correia, C.; Morgadinho, T.; Bento, C.; Marques, C.; Ataíde, A.; Miguel, T.S.; Moore, J.H.; et al. Evidence for epistasis between SLC6A4 and ITGB3 in autism etiology and in the determination of platelet serotonin levels. Hum. Genet. 2007, 121, 243–256. [Google Scholar] [CrossRef]
- Hranilovic, D.; Bujas-Petkovic, Z.; Vragovic, R.; Vuk, T.; Hock, K.; Jernej, B. Hyperserotonemia in Adults with Autistic Disorder. J. Autism Dev. Disord. 2007, 37, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Connors, S.L.; Matteson, K.J.; Sega, G.A.; Lozzio, C.B.; Carroll, R.C.; Zimmerman, A.W. Plasma Serotonin in Autism. Pediatr. Neurol. 2006, 35, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Kosova, G.; Delahanty, R.J.; Jiang, L.; Cook, E.H.; Ober, C.; Sutcliffe, J.S. Variation in ITGB3 is associated with whole-blood serotonin level and autism susceptibility. Eur. J. Hum. Genet. 2006, 14, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Croonenberghs, J.; Verkerk, R.; Scharpe, S.; Deboutte, D.; Maes, M. Serotonergic disturbances in autistic disorder: L-5-hydroxytryptophan administration to autistic youngsters increases the blood concentrations of serotonin in patients but not in controls. Life Sci. 2005, 76, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Spivak, B.; Golubchik, P.; Mozes, T.; Vered, Y.; Nechmad, A.; Weizman, A.; Strous, R.D. Low Platelet-Poor Plasma Levels of Serotonin in Adult Autistic Patients. Neuropsychobiology 2004, 50, 157–160. [Google Scholar] [CrossRef]
- Mulder, E.J.; Anderson, G.M.; Kema, I.P.; de Bildt, A.; van Lang, N.D.; den Boer, J.A.; Minderaa, R.B. Platelet Serotonin Levels in Pervasive Developmental Disorders and Mental Retardation: Diagnostic Group Differences, Within-Group Distribution, and Behavioral Correlates. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 491–499. [Google Scholar] [CrossRef]
- Martin, A.; Koenig, K.; Anderson, G.M.; Scahill, L. Low-dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: A prospective, open-label study. J. Autism Dev. Disord. 2003, 33, 77–85. [Google Scholar] [CrossRef]
- Vered, Y.; Golubchik, P.; Mozes, T.; Strous, R.; Nechmad, A.; Mester, R.; Weizman, A.; Spivak, B. The platelet-poor plasma 5-HT response to carbohydrate rich meal administration in adult autistic patients compared with normal controls. Hum. Psychopharmacol. Clin. Exp. 2003, 18, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Betancur, C.; Corbex, M.; Spielewoy, C.; Philippe, A.; Laplanche, J.-L.; Launay, J.-M.; Gillberg, C.; Mouren-Siméoni, M.-C.; Hamon, M.; Giros, B.; et al. Serotonin transporter gene polymorphisms and hyperserotonemia in autistic disorder. Mol. Psychiatry 2002, 7, 67–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Persico, A.M.; Pascucci, T.; Puglisi-Allegra, S.; Militerni, R.; Bravaccio, C.; Schneider, C.; Melmed, R.; Trillo, S.; Montecchi, F.; Palermo, M.; et al. Serotonin transporter gene promoter variants do not explain the hyperserotoninemia in autistic children. Mol. Psychiatry 2002, 7, 795–800. [Google Scholar] [CrossRef][Green Version]
- Croonenberghs, J.; Delmeire, L.; Verkerk, R.; Lin, A.H.; Meskal, A.; Neels, H.; Van der Planken, M.; Scharpe, S.; Deboutte, D.; Pison, G.; et al. Peripheral Markers of Serotonergic and Noradrenergic Function in Post-Pubertal, Caucasian Males with Autistic Disorder. Neuropsychopharmacology 2000, 22, 275–283. [Google Scholar] [CrossRef]
- Leboyer, M.; Philippe, A.; Bouvard, M.; Guilloud-Bataille, M.; Bondoux, D.; Tabuteau, F.; Feingold, J.; Mouren-Simeoni, M.-C.; Launay, J.-M. Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives. Biol. Psychiatry 1999, 45, 158–163. [Google Scholar] [CrossRef]
- Mcbride, P.A.; Anderson, G.M.; Hertzig, M.E.; Snow, M.E.; Thompson, S.M.; Khait, V.D.; Shapiro, T.; Cohen, D.J. Effects of Diagnosis, Race, and Puberty on Platelet Serotonin Levels in Autism and Mental Retardation. J. Am. Acad. Child Adolesc. Psychiatry 1998, 37, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Singh, E.A.; Warren, R.P. Hyperserotoninemia and serotonin receptor antibodies in children with autism but not mental retardation. Biol. Psychiatry 1997, 41, 753–755. [Google Scholar] [CrossRef]
- Hérault, J.; Petit, E.; Martineau, J.; Cherpi, C.; Perrot, A.; Barthélémy, C.; Lelord, G.; Müh, J.P. Serotonin and autism: Biochemical and molecular biology features. Psychiatry Res. 1996, 65, 33–43. [Google Scholar] [CrossRef]
- Bouvard, M.P.; Leboyer, M.; Launay, J.-M.; Recasens, C.; Plumet, M.-H.; Waller-Perotte, D.; Tabuteau, F.; Bondoux, D.; Dugas, M.; Lensing, P.; et al. Low-dose naltrexone effects on plasma chemistries and clinical symptoms in autism: A double-blind, placebo-controlled study. Psychiatry Res. 1995, 58, 191–201. [Google Scholar] [CrossRef]
- Tordjman, S.; Anderson, G.M.; McBride, P.A.; Hertzig, M.E.; Snow, M.E.; Hall, L.M.; Ferrari, P.; Cohen, D.J. Plasma androgens in autism. J. Autism Dev. Disord. 1995, 25, 295–304. [Google Scholar] [CrossRef]
- László, A.; Horváth, E.; Eck, E.; Fekete, M. Serum serotonin, lactate and pyruvate levels in infantile autistic children. Clin. Chim. Acta 1994, 229, 205–207. [Google Scholar] [CrossRef]
- Rolf, L.H.; Haarmann, F.Y.; Grotemeyer, K.; Kehrer, H. Serotonin and amino acid content in platelets of autistic children. Acta Psychiatr. Scand. 1993, 87, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Naffah-Mazzacoratti, M.D.G.; Rosenberg, R.; Fernandes, M.J.S.; Draque, C.M.; Silvestrini, W.; Calderazzo, L.; Cavalheiro, E.A. Serum serotonin levels of normal and autistic children. Braz. J. Med. Biol. Res. 1993, 26, 309–317. [Google Scholar] [PubMed]
- Cuccaro, M.L.; Wright, H.H.; Abramson, R.K.; A Marsteller, F.; Valentine, J. Whole-blood serotonin and cognitive functioning in autistic individuals and their first-degree relatives. J. Neuropsychiatry 1993, 5, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, B.L.; Cook, E.H.; Morford, M.; Ravitz, A.J.; Heller, W.; Freedman, D.X. Clinical and neurochemical effects of fenfluramine in children with autism. J. Neuropsychiatry 1993, 5, 307–315. [Google Scholar] [CrossRef]
- Hérault, J.; Martineau, J.; Perrot-Beaugerie, A.; Jouve, J.; Tournade, H.; Barthelemy, C.; Lelord, G.; Muh, J.-P. Investigation of whole blood and urine monoamines in autism. Eur. Child Adolesc. Psychiatry 1993, 2, 211–220. [Google Scholar] [CrossRef]
- Yuwiler, A.; Shih, J.C.; Chen, C.-H.; Ritvo, E.R.; Hanna, G.; Ellison, G.W.; King, B.H. Hyperserotoninemia and antiserotonin antibodies in autism and other disorders. J. Autism Dev. Disord. 1992, 22, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Duker, P.C.; Welles, K.; Seys, D.; Rensen, H.; Vis, A.; Berg, G.v.D. Brief report: Effects of fenfluramine on communicative, stereotypic, and inappropriate behaviors of autistic-type mentally handicapped individuals. J. Autism Dev. Disord. 1991, 21, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.D.; Cook, E.H.; Leventhal, B.L.; Wainwright, M.S.; Freedman, D.X. Platelet 5-HT2 serotonin receptor binding sites in autistic children and their first-degree relatives. Biol. Psychiatry 1991, 30, 121–130. [Google Scholar] [CrossRef]
- Piven, J.; Tsai, G.; Nehme, E.; Coyle, J.T.; Chase, G.A.; Folstein, S.E. Platelet serotonin, a possible marker for familial autism. J. Autism Dev. Disord. 1991, 21, 51–59. [Google Scholar] [CrossRef]
- Stern, L.M.; Walker, M.K.; Sawyer, M.G.; Oades, R.D.; Badcock, N.R.; Spence, J.G. A Controlled Crossover Trial of Fenfluramine in Autism. J. Child Psychol. Psychiatry 1990, 31, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.H.; Leventhal, B.L.; Heller, W.; Metz, J.; Wainwright, M.; Freedman, D.X. Autistic children and their first-degree relatives: Relationships between serotonin and norepinephrine levels and intelligence. J. Neuropsychiatry 1990, 2, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Oades, R.; Stern, L.; Walker, M.; Clark, C.; Kapoor, V. Event-related potentials and monoamines in autistic children on a clinical trial of fenfluramine. Int. J. Psychophysiol. Off. J. Int. Organ Psychophysiol. 1990, 8, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, B.L.; Cook, E.H.; Morford, M.; Ravitz, A.; Freedman, D.X. Relationships of whole blood serotonin and plasma norepinephrine within families. J. Autism Dev. Disord. 1990, 20, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Abramson, R.K.; Wright, H.H.; Carpenter, R.; Brennan, W.; Lumpuy, O.; Cole, E.; Young, S.R. Elevated blood serotonin in autistic probands and their first-degree relatives. J. Autism Dev. Disord. 1989, 19, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Ekman, G.; Miranda-Linné, F.; Gillberg, C.; Garle, M.; Wetterberg, L. Fenfluramine treatment of twenty children with autism. J. Autism Dev. Disord. 1989, 19, 511–532. [Google Scholar] [CrossRef] [PubMed]
- McBride, P.A.; Anderson, G.M.; Hertzig, M.E.; Sweeney, J.A.; Kream, J.; Cohen, D.J.; Mann, J.J. Serotonergic Responsivity in Male Young Adults With Autistic Disorder. Results of a pilot study. Arch. Gen. Psychiatry 1989, 46, 213–221. [Google Scholar] [CrossRef]
- Sherman, J.; Factor, D.C.; Swinson, R.; Darjes, R.W. The effects of fenfluramine (Hydrochloride) on the behaviors of fifteen autistic children. J. Autism Dev. Disord. 1989, 19, 533–543. [Google Scholar] [CrossRef]
- Minderaa, R.B.; Anderson, G.M.; Volkmar, F.R.; Harcherick, D.; Akkerhuis, G.W.; Cohen, D.J. Whole blood serotonin and tryptophan in autism: Temporal stability and the effects of medication. J. Autism Dev. Disord. 1989, 19, 129–136. [Google Scholar] [CrossRef]
- Coggins, T.E.; Morisset, C.; Krasney, L.; Frederickson, R.; Holm, V.A.; Raisys, V.A. Brief report: Does fenfluramine treatment enhance the cognitive and communicative functioning of autistic children? J. Autism Dev. Disord. 1988, 18, 425–434. [Google Scholar] [CrossRef]
- Cook, E.H.; Leventhal, B.L.; Freedman, D.X. Free serotonin in plasma: Autistic children and their first-degree relatives. Biol. Psychiatry 1988, 24, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Geller, E.; Yuwiler, A.; Freeman, B.J.; Ritvo, E. Platelet size, number, and serotonin content in blood of autistic, childhood schizophrenic, and normal children. J. Autism Dev. Disord. 1988, 18, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Launay, J.; Ferrari, P.; Haimart, M.; Bursztejn, C.; Tabuteau, F.; Braconnier, A.; Pasques-Bondoux, D.; Luong, C.; Dreux, C. Serotonin Metabolism and Other Biochemical Parameters in Infantile Autism. Neuropsychobiology 1988, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, S.; Beeghly, J.; Burns, T.; Tsai, L. Association of serotonin concentration to behavior and IQ in autistic children. J. Autism Dev. Disord. 1987, 17, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Badcock, N.R.; Spence, J.G.; Stern, L.M. Blood Serotonin Levels in Adults, Autistic and Non-Autistic Children—With a Comparison of Different Methodologies. Ann. Clin. Biochem. 1987, 24 Pt 6, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Launay, J.-M.; Bursztejn, C.; Ferrari, P.; Dreux, C.; Braconnier, A.; Zarifian, E.; Lancrenon, S.; Fermanian, J. Catecholamines metabolism in infantile autism: A controlled study of 22 autistic children. J. Autism Dev. Disord. 1987, 17, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Minderaa, R.B.; Anderson, G.M.; Volkmar, F.R.; Akkerhuis, G.W.; Cohen, D.J. Urinary 5-hydroxyindoleacetic acid and whole blood serotonin and tryptophan in autistic and normal subjects. Biol. Psychiatry 1987, 22, 933–940. [Google Scholar] [CrossRef]
- Anderson, G.M.; Freedman, D.X.; Cohen, D.J.; Volkmar, F.R.; Hoder, E.L.; McPhedran, P.; Minderaa, R.B.; Hansen, C.R.; Young, J.G. Whole blood serotonin in autistic and normal subjects. J. Child Psychol. Psychiatry 1987, 28, 885–900. [Google Scholar] [CrossRef]
- Ho, H.H.; Lockitch, G.; Eaves, L.; Jacobson, B. Blood serotonin concentrations and fenfluramine therapy in autistic children. J. Pediatr. 1986, 108, 465–469. [Google Scholar] [CrossRef]
- Stubbs, E.G.; Budden, S.S.; Jackson, R.H.; Terdal, L.G.; Ritvo, E.R. Effects of fenfluramine on eight outpatients with the syndrome of autism. Dev. Med. Child Neurol. 1986, 28, 229–235. [Google Scholar] [CrossRef]
- Israngkun, P.P.; Newman, H.A.I.; Patel, S.T.; Duruibe, V.A.; Abou-Issa, H. Potential biochemical markers for infantile autism. Neurochem. Pathol. 1986, 5, 51–70. [Google Scholar] [CrossRef][Green Version]
- Piggott, L.R.; Gdowski, C.L.; Villanueva, D.; Fischhoff, J.; Frohman, C.F. Side Effects of Fenfluramine in Autistic Children. J. Am. Acad. Child Psychiatry 1986, 25, 287–289. [Google Scholar] [CrossRef] [PubMed]
- August, G.J.; Raz, N.; Baird, T.D. Brief report: Effects of fenfluramine on behavioral, cognitive, and affective disturbances in autistic children. J. Autism Dev. Disord. 1985, 15, 97–107. [Google Scholar] [CrossRef]
- Kuperman, S.; Beeghly, J.H.; Burns, T.L.; Tsai, L.Y. Serotonin Relationships of Autistic Probands and Their First-Degree Relatives. J. Am. Acad. Child Psychiatry 1985, 24, 186–190. [Google Scholar] [CrossRef]
- Hoshino, Y.; Yamamoto, T.; Kaneko, M.; Tachibana, R.; Watanabe, M.; Ono, Y.; Kumashiro, H. Blood Serotonin and Free Tryptophan Concentration in Autistic Children. Neuropsychobiology 1984, 11, 22–27. [Google Scholar] [CrossRef]
- August, G.J.; Raz, N.; Papanicolaou, A.C.; Baird, T.D.; Hirsh, S.L.; Hsu, L.L. Fenfluramine treatment in infantile autism. Neurochemical, electrophysiological, and behavioral effects. J. Nerv. Ment. Dis. 1984, 172, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Ritvo, E.R.; Freeman, B.; Yuwiler, A.; Geller, E.; Yokota, A.; Schroth, P.; Novak, P. Study of fenfluramine in outpatients with the syndrome of autism. J. Pediatr. 1984, 105, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Ritvo, E.R.; Freeman, B.; Geller, E.; Yuwiler, A. Effects of Fenfluramine on 14 Outpatients with the Syndrome of Autism. J. Am. Acad. Child Psychiatry 1983, 22, 549–558. [Google Scholar] [CrossRef]
- Rotman, A.; Caplan, R.; Szekely, G.A. Platelet uptake of serotonin in psychotic children. Psychopharmacology 1980, 67, 245–248. [Google Scholar] [CrossRef]
- Takahashi, S.; Kanai, H.; Miyamoto, Y. Monoamine Oxidase Activity in Blood-Platelets From Autistic Children. Folia Psychiatr Neurol Jpn. 1977, 31, 597–603. [Google Scholar] [CrossRef]
- Takahashi, S.; Kanai, H.; Miyamoto, Y. Reassessment of elevated serotonin levels in blood platelets in early infantile autism. J. Autism Child. Schizophr. 1976, 6, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Yuwiler, A.; Ritvo, E.; Geller, E.; Glousman, R.; Schneiderman, G.; Matsuno, D. Uptake and efflux of serotonin from platelets of autistic and nonautistic children. J. Autism Child Schizophr. 1975, 5, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Ritvo, E.R.; Yuwiler, A.; Geller, E.; Kales, A.; Rashkis, S.; Schicor, A.; Plotkin, S.; Axelrod, R.; Howard, C. Effects of L-dopa in Autism. J. Autism Child. Schizophr. 1971, 1, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Yuwiler, A.; Ritvo, E.R.; Bald, D.; Kipper, D.; Koper, A. Examination of circadian rhythmicity of blood serotonin and platelets in autistic and non-autistic children. J. Autism Child. Schizophr. 1971, 1, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Ritvo, E.R.; Yuwiler, A.; Geller, E.; Ornitz, E.M.; Saeger, K.; Plotkin, S. Increased Blood Serotonin and Platelets in Early Infantile Autism. Arch. Gen. Psychiatry 1970, 23, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Lord, C. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Modules 1–4. Published Online 2015. Available online: https://cir.nii.ac.jp/crid/1370285712602755200 (accessed on 28 May 2023).
- Anderson, G.M.; Young, J.G.; Cohen, D.J.; Schlicht, K.R.; Patel, N. Liquid-chromatographic determination of serotonin and tryptophan in whole blood and plasma. Clin. Chem. 1981, 27, 775–776. [Google Scholar] [CrossRef]
- Yuwiler, A.; Plotkin, S.; Geller, E.; Ritvo, E.R. A rapid accurate procedure for the determination of serotonin in whole human blood. Biochem. Med. 1970, 3, 426–431. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Segal, J.B.; Moliterno, A.R. Platelet Counts Differ by Sex, Ethnicity, and Age in the United States. Ann. Epidemiol. 2006, 16, 123–130. [Google Scholar] [CrossRef]
- Balduini, C.L.; Noris, P. Platelet count and aging. Haematologica 2014, 99, 953–955. [Google Scholar] [CrossRef]
- Wu, P.I.-K.; Diaz, R.; Borg-Stein, J. Platelet-Rich Plasma. Phys. Med. Rehabilitation Clin. North Am. 2016, 27, 825–853. [Google Scholar] [CrossRef]
- Friedman, L.; Sterling, A. A Review of Language, Executive Function, and Intervention in Autism Spectrum Disorder. Semin. Speech Lang. 2019, 40, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, A.; Vasaghi-Gharamaleki, B.; Samadi, S.A.; Amiri-Shavaki, Y.; Alaghband-Rad, J. Working Memory Deficits and its Relationship to Autism Spectrum Disorders. Iran. J. Med. Sci. 2020, 45, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Sucksmith, E.; Roth, I.; Hoekstra, R.A. Autistic Traits Below the Clinical Threshold: Re-examining the Broader Autism Phenotype in the 21st Century. Neuropsychol. Rev. 2011, 21, 360–389. [Google Scholar] [CrossRef] [PubMed]
- Yousefvand, S.; Dadgar, H.; Mohammadi, M.R.; Maroufizadeh, S.; Yousefvand, S. Broader Autism Phenotype and Communication Skills in Parents of Children with Autism. J. Mod. Rehabil. 2022, 16, 338–346. [Google Scholar] [CrossRef]
- Schopler, E.; Reichler, R.J.; DeVellis, R.F.; Daly, K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J. Autism Dev. Disord. 1980, 10, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Schopler, E.; Van Bourgondien, M.E.; Wellman, G.J.; Love, S.R. Childhood Autism Rating Scale (CARS-2), 2nd ed.; Western Psychological Services: Torrance, CA, USA, 2010. [Google Scholar]
- Muller, C.; Anacker, A.; Veenstra-VanderWeele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G. A role for the serotonin reuptake transporter in the brain and intestinal features of autism spectrum disorders and developmental antidepressant exposure. J. Chem. Neuroanat. 2017, 83–84, 36–40. [Google Scholar] [CrossRef]
- Young, L.W.; Darios, E.S.; Watts, S.W. An immunohistochemical analysis of SERT in the blood–brain barrier of the male rat brain. Histochem. 2015, 144, 321–329. [Google Scholar] [CrossRef]
- Briguglio, M.; Dell’osso, B.; Panzica, G.; Malgaroli, A.; Banfi, G.; Dina, C.Z.; Galentino, R.; Porta, M. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients 2018, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Chung, H.; Park, J. Choking dermatitis: A neologism alerting the nature of factitious dermatitis acquired from self-asphyxial behaviors. J. Dermatol. 2018, 45, e29–e30. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.M.; Kloek, J.; Elliott, J.M. Parallel changes in serotonin levels in brain and blood following acute administration of MDMA. J. Psychopharmacol. 2013, 27, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Pennington, B.F.; McGrath, L.; Peterson, R.L. Diagnosing Learning Disorders: Third Edition: From Science to Practice; Guilford Press: New York, NY, USA, 2019. [Google Scholar]
- Risi, S.; Lord, C.; Gotham, K.; Corsello, C.; Chrysler, C.; Szatmari, P.; Cook, E.H.; Leventhal, B.L.; Pickles, A. Combining Information From Multiple Sources in the Diagnosis of Autism Spectrum Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, N.K. Rethinking “gold standards” and “best practices” in the assessment of autism. Appl. Neuropsychol. Child 2022, 11, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Hwang, J.S.; Shin, A.L.; Kim, J.Y.; Bae, S.M.; Sheehy-Knight, J.; Kim, J.W. Accuracy of the Childhood Autism Rating Scale: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2019, 61, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Ventola, P.E.; Kleinman, J.; Pandey, J.; Barton, M.; Allen, S.; Green, J.; Robins, D.; Fein, D. Agreement Among Four Diagnostic Instruments for Autism Spectrum Disorders in Toddlers. J. Autism Dev. Disord. 2006, 36, 839–847. [Google Scholar] [CrossRef]
- Russell, G.; Stapley, S.; Newlove-Delgado, T.; Salmon, A.; White, R.; Warren, F.; Pearson, A.; Ford, T. Time trends in autism diagnosis over 20 years: A UK population-based cohort study. J. Child Psychol. Psychiatry 2022, 63, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Parner, E.T.; Schendel, D.E.; Thorsen, P. Autism Prevalence Trends Over Time in Denmark. Arch. Pediatr. Adolesc. Med. 2008, 162, 1150–1156. [Google Scholar] [CrossRef]
- Keyes, K.M.; Susser, E.; Cheslack-Postava, K.; Fountain, C.; Liu, K.; Bearman, P.S. Cohort effects explain the increase in autism diagnosis among children born from 1992 to 2003 in California. Leuk. Res. 2012, 41, 495–503. [Google Scholar] [CrossRef]
- Mathew, J.; Sankar, P.; Varacallo, M. Physiology, Blood Plasma. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK531504/ (accessed on 8 August 2023).
- Brand, T.; Anderson, G.M. The Measurement of Platelet-Poor Plasma Serotonin: A Systematic Review of Prior Reports and Recommendations for Improved Analysis. Clin. Chem. 2011, 57, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Loth, E.; Charman, T.; Mason, L.; Tillmann, J.; Jones, E.J.H.; Wooldridge, C.; Ahmad, J.; Auyeung, B.; Brogna, C.; Ambrosino, S.; et al. The EU-AIMS Longitudinal European Autism Project (LEAP): Design and methodologies to identify and validate stratification biomarkers for autism spectrum disorders. Mol. Autism 2017, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.C.; Hagerman, R.J. Serotonin dysregulation in Fragile X Syndrome: Implications for treatment. Intractable Rare Dis. Res. 2014, 3, 110–117. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Reference (Quality Score) [Ref.] | Country | ASD Samples N (Mean Age ± SD), N Females (F) | Criteria/Tools for ASD Diagnosis | Controls N (Mean Age ± SD), N Females (F), [Confirmation Tools] |
|---|---|---|---|---|
| Carpita et al., 2023 (6) [44] | Italy | 24 (27.75 ± 6.97), 7 F | DSM-5/AdAS, RAADS | 24 Unaffected relatives (55.42 ± 10.25) 21 F; 24 HC (33.29 ± 8.05), 15 F [AdAS, RAADS] |
| Cremone et al., 2023 (6) [45] | Italy | 24 (27.75 ± 6.97), 7 F | DSM-5/SE | 24 Unaffected relatives (55.42 ± 10.25) 21 F; 24 HC (33.29 ± 8.05), 15 F |
| Xiaoxue, 2023 (5) [46] | China | 240 divided into 2 groups: early psychological intervention group N = 120 (4.14 ± 1.15), 50 F; late psychological intervention group N = 120 (4.24 ± 1.24), 51 F | DSM-5/SE | 120 HC (4.13 ± 1.12), 60 F |
| Zuniga-Kennedy et al., 2022 (7) [47] | US (OH) | 12 (median: 8, range 4–13), 0 F | DSM-5/ADOS-2 | 14 HC with gastrointestinal disorder group (median 7.5; range 3–18), 0 F 5 HC without gastrointestinal disorder group (median 13; range 8–18), 0 F [SRS] |
| Pagan et al., 2021 (5) [24] | France | 97 (age and sex not defined) | DSM-IV TR/K-SADS, SRS, ADI-R, ADOS-2 | 138 PAs (age and sex not defined); 56 USs (age and sex not defined); 106 HC (age and sex not defined) [SRS] |
| Mostafa et al., 2021 (6) [48] | Egypt | 22 (6.11 ± 2.23), 6 F | DSM-5/CARS | 22 HC (6.21 ± 2.41), 5 F |
| Meyyazhagan et al., 2020 (5) [49] | India | 98 divided into 2 age groups: Group 1 N = 62 (7.81 ± 2.36), 21 F; Group 2 N = 36 (16.82 ± 3.19) 6 F | DSM-IV TR/SE | 98 divided into 2 age groups: Group 1 N = 62 HC (7.76 ± 2.50), 21 F Group 2 N = 36 HC (16.67 ± 1.99), 6 F |
| Ali et al., 2020 (1) [50] | Iraq | 60 (range: 3–8 years), 18 F | NR/SE | 28 HC (adults, age not defined), 11 F |
| Javadfar et al., 2020 (2) [51] | Iran | 43 divided into 2 groups: Vitamin D group N = 22 (8.88 ± 2.45), 2 F; Placebo group N = 21 (8.95 ± 3.31) 5 F | DSM-5/CARS, ABC-C, ATEC | - |
| Chakraborti et al., 2020 (7) [52] | India | 104 (5.59 ± 0.37), 17 F | DSM-5/CARS | 26 HC (5.13 ± 0.82), 11 F |
| Wang et al., 2020 (7) [53] | China | 26 (4.3; SD not defined), 2 F | DSM-5/SE | 24 HC (4.5; SD not defined), 2 F |
| Hua et al., 2020 (6) [54] | China | 120 divided into 2 groups: Sleep disorder group N = 60 (3.99 ± 0.17), 12 F; No sleep disorder group N = 60 (3.93 ± 0.14), 8 F | DSM-5/CARS, ABC, SRS | 60 HC (age and sex not defined) |
| Bridgemohan et al., 2019 (3) [16] | US (MA) | 83 (7.4 ± 1.6), 15 F | NR/SE | - |
| Wichers et al., 2019 (7) [55] | UK | 19 (30 ± 11), 0 F | ICD-10/ADI-R, ADOS-2 | 19 HC (27 ± 9), 0 F |
| Aaron et al., 2019 (7) [56] | US (IL, TX) | 110 (140.7 months ± 73.9), 21 F | DSM-IV TR/ADI-R, ADOS-2 | 18 HC (201.3 months ± 100.7), 4 F |
| Montgomery et al., 2018 (5) [57] | US (IL, TX) | 188 (9.7 ± 5.2), 31 F | DSM-IV TR/ADI-R, ADOS-2 | 119 mothers (41.4 ± 6.8), 99 fathers (43.3 ± 7.5) |
| Lefevre et al., 2018 (6) [58] | France | 18 (34.3 ± 7.6), 0 F | DSM-IV TR/ADI | 24 HC (26.3 ± 6.3), 0 F |
| Abdulamir et al., 2018 (6) [59] | Iraq | 60 (7.28 ± 2.89), 0 F | DSM-V; ICD-10/SE | 26 HC (6.92 ± 2.59), 0 F |
| Guo et al., 2018 (8) [60] | China | 33 (5.14 ± 1.33), 5 F | DSM-5/CARS, ABC | 32 HC (5.18 ± 0.87), 6 F |
| Ormstad et al., 2018 (4) [61] | Norway | 65 (11.2, SD not defined), 13 F | ICD-10/ADI-R, ADOS-2 | 30 HC (10.9, SD not defined), 16 F |
| Shuffrey et al., 2017 (3) [62] | US (IL) | 264 divided into 2 groups: Pre-pubertal group N = 182 (7.56 ± 2.41), 23 F; Post-pubertal group N = 82 (16.72 ± 4.72), 14 F. | DSM-IV TR/ADI-R, ADOS-2 | - |
| Pagan et al., 2017 (5) [63] | France | 239 (age and sex not defined) | DSM-IV TR/K-SADS, SRS, ADI-R, ADOS-2 | 303 PAs (age and sex not defined), 78 USs (age and sex not defined), 278 HC (age and sex not defined) [SRS] |
| Benabou et al., 2017 (5) [64] | France | 213 (14.4 ± 9.1), 39 F | DSM-IV TR/K-SADS, SRS, ADI-R, ADOS-2 | 364 PAs (46.5 ± 9.5), 128 USs (14.8 ± 8.0), 185 F [SRS] |
| Chen et al., 2017 (4) [26] | US (TN) | 116 (9.67 ± 5.93), 18 F | DSM-IV TR/ADI-R, ADOS-2 | 63 mothers (age not defined); 72 fathers (age not defined) [BAPQ] |
| Marler et al., 2016 (3) [65] | US (MO) | 82 divided into 2 groups: normoserotonemia group N = 63 (11.2 ± 4.1), 6 F; hyperserotonemia group N = 19 (11.7 ± 3.6), 2 F. | DSM-IV/ADOS-2 | - |
| Chakraborti et al., 2016 (5) [66] | India | 203 (range 1.9–14), 33 F | DSM-IV TR or DSM-5/CARS | 236 HC (range 4–31), 111 F |
| Kheirouri et al., 2016 (7) [67] | Iran | 35 (8.1 ± 4.0), 11 F | DSM-IV TR/GARS | 31 HC (7.3 ± 2.6), 13 F [SDQ] |
| Francis et al., 2016 (3) [68] | US (IL) | 207 (9.88 ± 5.48), 37 F | DSM-IV TR/ADI-R, ADOS-2 | - |
| Chugani et al., 2016 (4) [69] | US (MI, OH, TX, NY) | 166 divided into 3 groups: 2.5 mg buspirone group N = 54 (age range 2–6), 8 F; 5.0 mg buspirone group N = 55 (age range 2–6), 11 F, Placebo group N = 57 (age range 2–6), 10 F | DSM-IV TR/ADI-R, ADOS-2 | - |
| Gebril et al., 2015 (7) [70] | Egypt | 20 (7.4 ± 2.6), 0 F | DSM-IV/CARS | 20 HC (9 ± 1.6). 0 F |
| Bijl et al., 2015 (7) [71] | Belgium | 159 (11.9 ± 3.8), 34 F | DSM-IV TR/SRS | 186 PAs (43.0 ± 4.4), 99 F; 103 USs (13.0 ± 4.7), 69 F; 65 pediatric HC (15.6 ± 3.9), 34 F; 45 adult HC (36.5 ± 10.9), 23 F |
| Jaiswal et al., 2015 (7) [33] | India | 169 (5.86 ± 0.24), 28 F | DSM-IV TR/CARS | 317 PAs (25.10 ± 1.04), 164 F; 168 HC (21.92 ± 1.15), 87 F |
| Yang et al., 2015 (6) [72] | China | 33 (12.21 ± 2.67), 6 F | DSM-IV/CARS | 31 HC (12.52 ± 2.14), 7 F |
| Yang et al., 2015b (8) [73] | China | 43 (7.51 ± 1.47), 8 F | DSM-5/CARS | 40 HC (7.83 ± 1.63), 10 F |
| Alabdali et al., 2014 (7) [74] | Saudi Arabia | 52 (7.0 ± 2.34), 0 F | DSM-IV/CARS, SRS | 30 HC (7.2 ± 2.14), 0 F |
| Kolevzon et al., 2014 (3) [75] | US (NY) | 64 (6.85 ± 2.75), 19 F | DSM-IV/ADI-R | - |
| Gabriele, Lombardi, et al., 2014 (6) [76] | Italy | 428 (8.86 ± 0.29), 57 F | DSM-IV/ADI-R, ADOS-2 | 809 PAs (age and sex not defined); 158 USs (age and sex not defined) |
| Pagan et al., 2014 (8) [77] | France | 278 (N = 135 < 16 years; N = 143 > 16 years), 53 F | DSM-IV TR/K-SADS, SRS, ADI-R, ADOS-2 | 377 PAs (N = 377 > 16 y), 199 F; 129 USs (N = 72 < 16 y, N = 57 > 16 y), 67 F; 416 HC (N = 111 < 16 y, N = 305 > 16 y), 182 F [SRS] |
| Levin-Decanini et al., 2013 (5) [78] | US (IL) | 197 (240 ± 67.32 months), 35 F | DSM-IV TR/ADI-R, ADOS-2 | 196 mothers (492 ± 84 months); 161 fathers (532 ± 96 months) [BAPQ] |
| Anderson et al., 2012 (7) [79] | US (NY) | 18 (10.1 ± 5.5), 0 F | DSM-III-R/SE | 24 HC (14.2 ± 8.5), 4 F |
| Mostafa & Al-Ayadhi, 2011 (8) [80] | Saudi Arabia | 50 (8.22 ± 2.28), 9 F | DSM-IV/CARS | 30 HC (8.23 ± 2.36), 5 F |
| El-Ansary et al., 2011 (6) [81] | Saudi Arabia | 25 (range 4–12), sex not defined | NR/ADI-R, ADOS, 3DI | 16 HC (range 4–11), sex not defined |
| Sacco et al., 2010 (3) [82] | Italy | 245 (8.82 ± 5.62), 29 F | DSM-IV/ADI-R, ADOS | - |
| Kazek et al., 2010 (8) [83] | Poland | 51 (8,1, SD not defined), 12 F | NR/SE | 28 HC (7.9, SD not defined), 8 F |
| Kolevzon et al., 2010 (3) [84] | US (NY) | 78 (6.77 ± 2.93), 13 F | ICD-10, DSM-IV/ADI-R | - |
| Mulder et al., 2009 (7) [85] | The Netherlands | 19 divided into 2 groups: normoserotonemic N = 10 (15.3 ± 4.0), 0 F; Hyperserotonemic N = 9 (15.3 ± 4.4), 0 F | DSM-IV TR/ADI-R, ADOS | - |
| Kemperman et al., 2008 (3) [86] | The Netherlands | 24 (9.9 ± 3.9), 6 F | DSM-IV TR/SE | - |
| El-Sherif et al., 2008 (8) [87] | Egypt | 40 (7.35 ± 2.6), 8 F | DSM-IV/CARS | 40 HC (7.68 ± 2.5), 8 F |
| Warren & Singh, 2008 (7) [88] | US (UT) | 20 (10.1, SD not defined), 4 F | DSM-III-R/SE | 13 HC (8.8, SD not defined), 3 F |
| Sacco, Papaleo, et al., 2007 (6) [89] | Italy | 371 (age and sex not defined) | DSM-IV/ADI-R, ADOS | 156 USs (age and sex not defined); 180 HC (age and sex not defined) |
| Sacco, Militerni, et al., 2007 (3) [90] | Italy | 241 (7.10 ± 2.78), 36 F | DSM-IV/ADI-R, ADOS | - |
| Coutinho et al., 2007 (7) [91] | Portugal | 186 (6.8, SD not defined), sex not defined | DSM-IV/ADI-R, CARS | 181 adult HC, (age and sex not defined) |
| Hranilovic et al., 2007 (7) [92] | Croatia | 53 (26.1 ± 6.6), 15 F | DSM-IV/CARS | 45 HC (39.2 ± 9.2), 1 F |
| Connors et al., 2006 (7) [93] | US (TN) | 17 (age range 2–18), 0 F | DSM-IV/CARS | 17 mothers (range 25–40); 12 fathers (range 27–45); 7 USs (range 6–15, sex not specified); 8 mothers of HC (age range 25–40) |
| Weiss et al., 2006 (6) [94] | US (IL, TN) | 50 (5.9 ± 3.3), sex not defined | DSM-IV/ADI-R, ADOS | 567 Hutterites (age and sex not defined); 392 HC adults (age and sex not defined) |
| Croonenberghs et al., 2005 (7) [95] | Belgium | 18 (16.2 ± 1.7), 0 F | DSM-IV/ADI-R, Consensus | 22 HC (16.0 ± 1.8), 0 F |
| Spivak et al., 2004 (6) [96] | Israel | 10 (24.3 ± 4.5), 4 F | DSM-IV/CARS, RLRS | 12 HC (30.0 ± 3.6), 6 F |
| Mulder et al., 2004 (8) [97] | The Netherlands | 81 divided into 3 groups: autism N = 33 (11.7 ± 4.0), 4 F; Asperger’s N = 5 (13.2 ± 3.3), 1 F; PDD-NOS N = 43 (13.1 ± 4.2), 6 F | DSM-IV-TR/ADI-R, ADOS, ABC, PDD-MRS | 54 Cognitively impaired (13.0 ± 3.3), 11 F; 60 HC (11.5 ± 3.9), 31 F [ABC, PDD-MRS] |
| Coutinho et al., 2004 (5) [34] | Portugal | 105 (7.14, SD not defined), sex not defined | DSM-IV/ADI-R, CARS | 52 HC (7.27, SD not defined), sex not defined |
| Martin et al., 2003 (3) [98] | US (CT) | 18 (11.3 ± 3.6), 4 F | NR/ADI-R, ADOS | - |
| Vered et al., 2003 (7) [99] | Israel | 7 (25.4 ± 4.8), 3 F | DSM-IV/Consensus | 10 HC (30.3 ± 4.6), 5 F |
| Betancur et al., 2002 (5) [100] | France | 150 (14.5, SD not defined), 41 F | DSM-IV/ADI-R | PA and USs (N, age and sex not defined) |
| Persico et al., 2002 (6) [101] | Italy | 134 (age and sex not defined) | DSM-IV/ADI-R, ADOS | 244 PAs (age and sex not defined), 49 USs (age and sex not defined) |
| Croonenberghs et al., 2000 (7) [102] | Belgium | 13 (14.5 ± 1.8), 0 F | DSM-IV/Consensus | 13 HC (15.1 ± 1.5), 0 F |
| Leboyer et al., 1999 (8) [103] | France | 62 (9.2 ± 4.2), 20 F | DSM-III-R, ICD-10/ADI | 61 mothers (age not defined), 42 fathers (age not defined), 11 sisters (age not defined); 9 brothers (age not defined), 91 young HC (range 2–16; sex not defined), 118 adult HC (age and sex not defined) |
| McBride et al., 1998 (8) [104] | US (NY) | 7 (23.1 ± 3.8), 0 F | DSM-III-R/ODI, ABC, ADOS | 8 HC (25 ± 2.8), 0 F |
| Singh et al., 1997 (8) [105] | US (UT) | 23 (6.3, SD not defined), 5 F | DSM-III-R/Consensus | 10 Cognitively impaired (6.5, SD not reported), 4 F; 23 HC (6.8, SD not reported), 11 F |
| Hérault et al., 1996 (3) [106] | France | 65 (7.0, SD not defined), 25 F | DSM-III-R/Consensus | - |
| Bouvard et al., 1995 (3) [107] | France | 10 (9.5, SD not defined), 5 F | DSM-III-R/ADI | - |
| Tordjman et al., 1995 (9) [108] | US (NY) | 38 (prepubertal 5.5 ± 2.49; postpubertal 22.9 ± 4.8), 0 F | DSM-III-R/ODI, ABC, ADOS | 12 Cognitively impaired (6.00 ± 2.45), 0 F; 21 HC (prepubertal 7.70 ± 2.45, postpubertal 22.1 ± 5.8), 0 F |
| László et al., 1994 (5) [109] | Hungary | 46 (5.4, SD not defined), 9 F | DSM-III/SE | 20 HC (range 2–10), sex not defined |
| Rolf et al., 1993 (7) [110] | Germany | 18 (9.9 ± 2.8), 2 F | DSM-III/SE | 14 HC (11.5 ± 2.0), 6 F |
| Naffah-Mazzacoratti et al., 1993 (6) [111] | Brasil | 19 (range 1–12), sex not defined | DSM-III-R/SE | 46 HC (range 1–12), sex not defined |
| Cuccaro et al., 1993 (6) [112] | US (SC) | 18 (18 ± 9), 5 F | DSM-III-R/SE | 21 PAs (45 ± 12), 13 F; 13 USs (24 ± 13),4 F |
| Leventhal et al., 1993 (3) [113] | US (IL) | 15 (7.6 ± 2.6), 2 F | DSM-III/ODI, Consensus | - |
| Hérault et al., 1993 (7) [114] | France | 23 (5.9, SD not defined), 7 F | DSM-III-R/SE | 59 HC (6.0, SD not defined), 25 F |
| Yuwiler et al., 1992 (6) [115] | US (CA) | N not defined (10, SD not defined), sex not defined | DSM-III-R, NSAC/SE | Obsessive-compulsive patients (N not defined, mean age 13, SD not defined), sex not defined; multiple sclerosis patients (N not defined, mean age 41, SD not defined), sex not defined; HC (N not defined, mean age 33, SD not defined), sex not defined |
| Duker et al., 1991 (2) [116] | The Netherlands | 11 (18.36 ± 8.38), 4 F | DSM-III-R/consensus | - |
| Perry et al., 1991 (8) [117] | US (IL) | 12 (7.5 ± 2.9), 0 F | DSM-III-R/consensus | 22 PAs (35.2 ± 4.1), 11 F; 6 USs (8.3 ± 1.8), 2 F; 7 adult HC (24, SD not reported), 0 F; 10 child HC (11.0 ± 2.7), 0 F. |
| Piven et al., 1991 (7) [118] | US (MD) | 28 divided into 2 groups: Simplex N = 23 (21.5, SD not defined), 4 F; Multiplex N = 5 (26.4, SD not defined), 0 F | DSM-III-R/ADI | 10 HC (25.1, SD not reported), 5 F |
| Stern et al., 1990 (3) [119] | Australia | 20 (mean age 10.0, SD not defined), 6 F | DSM-III/SE | - |
| Cook et al., 1990 (7) [120] | US (IL) | 16 (9.0 ± 3.5), 0 F | DSM-III-R/consensus | 53 PAs (37.3 ± 5.1), 29 F; 21 USs (11.9 ± 5.4), 9 F |
| Oades et al., 1990 (2) [121] | Australia | 7 (11.3 ± 4.0), 1 F | DSM-III/SE | - |
| Leventhal et al., 1990 (6) [122] | US (IL) | 39 (8.99 ± 4.37), 7 F | DSM-III-R/SE | 78 PAs (38.71 ± 6.52), 42 F; 32 USs (11.22 ± 5.27), 18 F |
| Abramson et al., 1989 (8) [123] | US (SC) | 57 (13.9 ± 5), 4 F | DSM-III/SE | 17 HC (13.3 ± 6.5), 14 F |
| Ekman et al., 1989 (3) [124] | Sweden | 20 (6.25, SD not defined), 2 F | DSM-III-R; Rutter (1978)/ABC, RLRS | - |
| McBride et al., 1989 (8) [125] | US (NY) | 7 (23.1 ± 3.8), 0 F | DSM-III-R/ABC, ODI, consensus | 8 HC (25.0 ± 2.8), 0 F |
| Sherman et al., 1989 (2) [126] | Canada | 15 (11.4, SD not defined), 2 F | DSM-III; NSAC (1981)/RLRS | - |
| Minderaa et al., 1989 (8) [127] | The Netherlands | 40 (19.4 ± 4.9), 12 F | DSM-III/SE | 20 HC (22.0 ± 7.5), 5 F |
| Coggins et al., 1988 (1) [128] | US (CA) | 5 (age not defined), 1 F | NR/consensus | - |
| Cook et al., 1988 (4) [129] | US (IL) | 22 (10.7 ± 3.4), 1 F | NR/SE | 21 mothers (37.7 ± 4.5), 14 fathers (41.2 ± 5.2), 9 brothers (10.5 ± 5.1), 8 sisters (15.0 ± 6.3) |
| Geller et al., 1988 (8) [130] | US (CA) | 19 (range 20–140 months), sex not defined | DSM-III/Consensus | 6 with schizophrenic reaction in childhood (range 20–140 months), sex not defined; 26 HC (range 20–140 months), sex not defined |
| Launay et al., 1988 (7) [131] | France | 22 (10.5, SD not defined), 6 F | DSM-III/SE | 22 HC (age and sex not defined) |
| Kuperman et al., 1987 (3) [132] | US (IA) | 25 (126.2 ± 52.6 months), 0 F | DSM-III/ABC | - |
| Badcock et al., 1987 (7) [133] | Australia | 30 (10.2, SD not defined), sex not defined | DSM-III; NSAC (1981)/SE | 11 developmental dysphasia (5.5, SD not defined), sex not defined; 106 HC (9.7, SD not defined), sex not defined |
| Launay et al., 1987 (7) [134] | France | 22 (10.5, SD not defined), sex not defined | DSM-III/SE | HC (N, age and sex not defined) |
| Minderaa et al., 1987 (8) [135] | The Netherlands | 36 divided into 2 groups: unmedicated N = 16 (20.6 ± 4.6), 5 F; medicated N = 20 (19.4 ± 4.1), 4 F | DSM-III/SE | 27 HC (20.3 ± 6.9), 8 F [self-report questionnaire] |
| Anderson et al., 1987 (7) [136] | US (CT) | 40 (16.8 ± 6.04), 11 F | DSM-III/SE | 87 HC (14.6 ± 7.47), 42 F [pediatrician evaluation and parental questionnaires] |
| Ho et al., 1986 (6) [137] | Canada | 31 (8, SD not defined), sex not defined | DSM-III/SE | 10 with cognitive impairment (7, SD not defined), sex not defined; 18 Down syndrome (9, SD not defined), sex not defined; 23 HC (10, SD not defined), sex not defined |
| Stubbs et al., 1986 (1) [138] | US (OR) | 8 (7.88 ± 4.26), 4 F | DSM-III/SE | - |
| Israngkun et al., 1986 (8) [139] | US (OH) | 14 (14.57 ± 5.64), 3 F | DSM-III, Rimland (1968)/CARS, E-2 | 10 HC (14.6 ± 5.08), 2 F |
| Piggott et al., 1986 (1) [140] | US (MI) | 8 (range 4–15), sex not defined | DSM-III; NSAC/SE | - |
| August et al., 1985 (1) [141] | US (TX) | 9 (8.44 ± 2.40), 1 F | NR | - |
| Kuperman et al., 1985 (6) [142] | US (IA) | 25 males (10.5 ± 4.3), and 5 F (9.9 ± 5.0) | DSM-III/SE | 30 mothers (36.2 ± 7.2); 24 fathers (38.3 ± 8.3); 11 brothers (9.0 ± 4.9); 10 sisters (13.2 ± 9.8) |
| Hoshino et al., 1984 (6) [143] | Japan | 37 (4.7, SD not defined), 3 F | Kanner (1943), Rutter (1972)/E-2 | 12 young HC (10.5, SD not defined), 6 F; 28 adult HC (26.8, SD not defined), 17 F |
| August et al., 1984 (7) [144] | US (TX) | 10 (range 5–12), sex not defined | DSM III; NSAC/SE | 8 HC (age and sex not defined) |
| Ritvo et al., 1984 (3) [145] | US (CA) | 14 (range 2–18), 3 F | DSM-III; NSAC/RLRS | - |
| Ritvo et al., 1983 (3) [146] | US (CA) | 14 (range 2–12), 3 F | DSM-III; NSAC/RLRS | - |
| Rotman et al., 1980 (1) [147] | Israel | 4 (range 8–14), sex not defined | NR/SE | - |
| Hanley et al., 1977 (6) [21] | US (IL) | 27 (age and sex not defined) | Schain and Freedman criteria/SE | 25 severe cognitive impairment (age and sex not defined); 23 mild cognitive impairment (age and sex not defined); 6 HC (age and sex not defined) |
| Takahashi et al., 1977 (7) [148] | Japan | 20 (4.5 ± 2.9), 1 F | Kanner (1943)/consensus | 39 psychiatric and neurological patients (7.5 ± 3.4), 17 F; 30 HC (5.4 ± 3.2), 7 F |
| Takahashi et al., 1976 (8) [149] | Japan | 30 (4.8 ± 2.9), 3 F | Kanner (1943)/consensus | 45 psychiatric and neurological patients (7.1 ± 3.3), 20 F; 30 HC (5.4 ± 3.2), 7 F |
| Yuwiler et al., 1975 (6) [150] | US (CA) | 12 (60.2 ± 31.1 months), 1 F | Kanner (1943)/consensus | 15 hospitalized patients (69.4 ± 20.6 months), 7 F; HC (N not defined, 97.5 ± 25.9 months), sex not defined |
| Ritvo, 1971 (4) [151] | US (CA) | 4 (range 3–13), 0 F | Rimland (1968)/consensus | 4 non-autistic patients (age and sex not defined) |
| Yuwiler et al., 1971 (3) [152] | US (CA) | 7 (60.6 ± 24.2 months), 0 F | Rimland (1968)/consensus | 4 non-autistic patients (71.5 ± 36.9 months), 1 F |
| Ritvo et al., 1970 (6) [153] | US (CA) | 24 (58.2 ± 15.8 months), 6 F | Kanner (1943)/consensus | HC (N, age and sex not defined) |
| Correlation | References (QA) |
|---|---|
| Autism severity | |
| ↑ | Leventhal et al., 1993 [113] (3); Mostafa et al., 2011 [80] (8); Alabdali et al. 2014 [74] (7); Yang et al., 2015 [72] (6); Yang et al., 2015 [73] (8); Marler et al., 2016 [65] (3); Abdulamir et al., 2018 [59] (6); Guo et al.,2018 [60] (8); Chakraborti et al., 2020 [52] (7); Pagan et al., 2021 [24] (5); Mostafa et al., 2021 [48] (8) |
| ↓ | Cook et al., 1990 [120] (7); Sacco et al., 2010 [82] (3); Jaiswal et al. 2015 [33] (7); Kheirouri et al., 2016 [67] (7); Chakraborti et al., 2016 [66] (5); Ormstad 2018 [61] (4); Pagan et al., 2021 [24] (5) |
| Intellectual disability | |
| ↑ | Pagan et al., 2021 [24] (5) |
| Repetitive behaviors | |
| ↑ | Sacco et al., 2010 [82] (3); Yang et al., 2015 [72] (8); Marler et al., 2016 [65] (3). |
| ↓ | Pagan et al., 2021 [24] (5) |
| Verbal communication | |
| ↓ | Hranilovic et al., 2007 [92] (7) |
| Memory | |
| ↓ | Cuccaro et al., 1993 [112] (6) |
| Self-injurious behaviors | |
| ↑ | Yang et al., 2015 [73] (8) |
| ↓ | Sacco et al., 2010 [82] (3); Kolevzon et al., 2010 [84] (3) and 2014 [75] (3) |
| Hetero-aggressive behaviors | |
| ↑ | Spivak et al., 2004 [96] (6) |
| Gender differences in emotion expression | |
| ↑ | Chakraborti et al., 2020 [52] (7) |
| Sleep problems | |
| ↑ | Hua et al., 2020 [54] (6) |
| ↓ | Kheirouri et al., 2016 [67] (7) |
| Gastrointestinal problems | |
| ↑ | Marler et al., 2016 [65] (3); Bridgemohan et al., 2019 [16] (3) |
| Allergies and immune disorders | |
| ↑ | Sacco et al., 2010 [82] (3); Mostafa et al., 2021 [48] (8) |
| Patients: Report demographic and epidemiological characteristics (ethnicity, gender, age, and pubertal stage). Pay attention to medications, drugs, and diets that can affect 5-HT metabolism. Evaluate and report comorbidities:
Clinical outcome:
|
Controls:
|
Peripheral 5-HT assay:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, D.; Cruciani, G.; Zaccaro, L.; Di Carlo, E.; Spitoni, G.F.; Manti, F.; Carducci, C.; Fiori, E.; Leuzzi, V.; Pascucci, T. A Systematic Review on Autism and Hyperserotonemia: State-of-the-Art, Limitations, and Future Directions. Brain Sci. 2024, 14, 481. https://doi.org/10.3390/brainsci14050481
Esposito D, Cruciani G, Zaccaro L, Di Carlo E, Spitoni GF, Manti F, Carducci C, Fiori E, Leuzzi V, Pascucci T. A Systematic Review on Autism and Hyperserotonemia: State-of-the-Art, Limitations, and Future Directions. Brain Sciences. 2024; 14(5):481. https://doi.org/10.3390/brainsci14050481
Chicago/Turabian StyleEsposito, Dario, Gianluca Cruciani, Laura Zaccaro, Emanuele Di Carlo, Grazia Fernanda Spitoni, Filippo Manti, Claudia Carducci, Elena Fiori, Vincenzo Leuzzi, and Tiziana Pascucci. 2024. "A Systematic Review on Autism and Hyperserotonemia: State-of-the-Art, Limitations, and Future Directions" Brain Sciences 14, no. 5: 481. https://doi.org/10.3390/brainsci14050481
APA StyleEsposito, D., Cruciani, G., Zaccaro, L., Di Carlo, E., Spitoni, G. F., Manti, F., Carducci, C., Fiori, E., Leuzzi, V., & Pascucci, T. (2024). A Systematic Review on Autism and Hyperserotonemia: State-of-the-Art, Limitations, and Future Directions. Brain Sciences, 14(5), 481. https://doi.org/10.3390/brainsci14050481







